Early control of short hepatic portal veins in isolated or combined hepatic caudate lobectomy
Zhi-Quan Qiu, Wei-Feng Tan, Pei-Ning Yan, Xiang-Ji Luo, Bai-He Zhang, Meng-Chao Wu, Xiao-Qing Jiang and Wan-Yee Lau
Shanghai, China
Early control of short hepatic portal veins in isolated or combined hepatic caudate lobectomy
Zhi-Quan Qiu, Wei-Feng Tan, Pei-Ning Yan, Xiang-Ji Luo, Bai-He Zhang, Meng-Chao Wu, Xiao-Qing Jiang and Wan-Yee Lau
Shanghai, China
BACKGROUND:Caudate lobectomy has long been considered technically difficult. This study aimed to elaborate the significance of early control of short hepatic portal veins (SHPVs) in isolated hepatic caudate lobectomy or in hepatic caudate lobectomy combined with major partial hepatectomy, and to describe the anatomical characteristics of SHPVs.
METHODS:The data of 117 patients who underwent either isolated or combined caudate lobectomy by the same team of surgeons from 2005 to 2009 were retrospectively analyzed. From 2005 to 2007 (group A,n=55), we carried out early control of short hepatic veins (SHVs) only; from 2008 to 2009 (group B,n=62), we carried out early control of both SHVs and SHPVs. The two groups were compared to evaluate which surgical procedure was better. A detailed anatomical study was then carried out on the last 25 consecutive patients in group B to study the number and distribution of SHPVs during surgery.
RESULTS:Patients in group B had less intra-operative blood loss, less impairment of liver function, shorter postoperative hospital stay, fewer postoperative complications and required less blood transfusion (P<0.05). The number of SHPVs in the 25 patients was 183, with 7.3±2.7 per patient. The diameters of SHPVs were 1 to 4 mm. On average, 3.4 SHPVs/patient came from the left portal vein, 2.2 from the bifurcation, 1.4 from the right portal vein, and 0.3 from the main portal vein. On average, 3.3 SHPVs/patient supplied segmentiof the liver, 0.4 for segment II, 2.1 for segment IV, 1.4 for segment V and 0.1 for segment VI.
CONCLUSION:Early control of SHPVs in isolated or combined hepatic caudate lobectomy may be a useful method to decrease surgical risk and improve postoperative recovery.
(Hepatobiliary Pancreat Dis Int 2012;11:377-382)
short hepatic portal vein; caudate lobe; anatomy
Introduction
The liver is a large organ with complicated vascular structures and a dual inflow blood supply. The caudate lobe is the dorsal portion of the liver, lying posteriorly and embracing the retrohepatic inferior vena cava (IVC) in a semicircumferential fashion. The caudate lobe lies between major vascular structures with the IVC posteriorly, the portal triad inferiorly, and the hepatic veins superiorly.[1]It can be divided into three parts, the Spiegel lobe, the paracaval portion and the caudate process.[2]Caudate lobectomy has long been considered technically difficult. The deep location of this lobe and its proximity to great vessels leads to high operative risks and excessive intraoperative blood loss.[3-5]Progress in medical knowledge and technology has made caudate lobectomy safe.[6-8]
Various techniques have been suggested to minimize bleeding during caudate lobectomy. Zhou et al[9]suggested early control of short hepatic veins (SHVs) and complete separation of the caudate lobe from the IVC, thus avoiding uncontrolled bleeding from the IVC. These SHVs were hepatic veins which came from the liver (mainly the caudate lobe) and drained directly and mainly into the IVC, and occasionally into the left, middle or right hepatic veins. Ortale et al[7]reported the number of these SHVs to range from 1-3 to 7-9, with a diameter ranging from 1 to 7 mm (average 3). AbdelWahab et al[3]reported on 54 patients with caudate lobectomy and 1-3 SHVs had diameters of 3 to 8 mm. The biggest SHVs usually drained into the anterior wall of the IVC.
"Early control of SHVs" means dissecting their branches before hepatectomy, and this reduces the technical difficulty of caudate lobectomy. However, occasional damage to small branches of the portal vein which supply the caudate lobe can result in excessive bleeding. These small branches arise from the portal venous system in the porta hepatis and enter directly into adjacent liver tissues to supply segmenti(caudate lobe), and parts of segments II, IV, V and VI. The authors referred to these small branches as short hepatic portal veins (SHPVs). This historical comparative study was conducted to determine whether dissecting the branches of both SHVs and SHPVs before hepatectomy is better than early control of SHVs alone. This study also aimed to study and record the anatomy of SHPVs in 25 consecutive patients.
Methods
Patients
All 117 patients (72 males and 45 females) were subjected to operations from 2005 to 2009. Their age ranged from 23 to 75 years (average 45). Their liver function was Child A (n=85) or Child B (n=32). Patients who had obstructive jaundice with a total bilirubin >85 µmol/L were endoscopically or percutaneously drained before liver resection until the total bilirubin came down to <85 µmol/L. The histopathological diagnoses were hilar cholangiocarcinoma (58 patients) [Bismuth-Corlette classification[10]types II (11), III (33) and IV (14)], hepatocellular carcinoma (30), intrahepatic cholangiocarcinoma (13), gallbladder cancer (11), and hepatic hemangioma (5). The operations included isolated caudate lobectomy (7 patients), caudate lobectomy combined with left hepatectomy (25), caudate lobectomy combined with right hepatectomy (33), caudate lobectomy combined with expanded left hepatectomy (16), and caudate lobectomy combined with expanded right hepatectomy (36), and no patients were given hepatic portal occlusion because we treated blood vessels before liver resection.
Comparison between the two groups
From 2005 to 2007 (group A,n=55), we carried out early control of short hepatic veins (SHVs) only; from 2008 to 2009 (group B,n=62), we carried out early control of both SHVs and SHPVs. We retrospectively analyzed and compared the operation time, blood loss, postoperative complications, postoperative liver function, postoperative hospital stay and total blood transfusion between the two groups. The last consecutive 25 patients in group B were studied for details on the anatomy of SHPVs during the operation.
Statistical analysis
All data were analyzed using SPSS 15.0. Data were presented as mean±standard deviation (SD) or median and range as appropriate. Comparison between the two groups was done using Student'sttest for parametric data and the Mann-WhitneyUtest for non-parametric data. The Chi-square test was used for categorical data. APvalue <0.05 was considered statistically significant.
Results
Patient characteristics
The clinical variables including gender, age, histopathology, surgical procedures, total bilirubin and ALT were not significantly different between the two groups (Table 1). Apart from operation time, there were significantdifferences in blood loss, postoperative complications, postoperative liver function, postoperative hospital stay, and total blood transfusion between the two groups (Table 2).

Table 1. Preoperative data in the two groups
ALT levels on postoperative days 1, 3 and 7 in group A were 877.3±385.3, 473.6±125.8, and 339.1±79.7 U/L respectively, and compared with the corresponding levels in group B of 715.9±348.4, 323.4±120.0, and 145.7±61.2 U/L, significant differences were found (Table 2).
Postoperative complications
The overall incidence of postoperative complication in group A was higher than that in group B (P=0.026). The incidence of ascites and abdominal sepsis differed between the two groups (P=0.033 andP=0.041, respectively). Other complications such as postoperative hemorrhage, bile leakage and mortality were not different between the two groups (Table 3). The postoperative hospital stay was 19.4±8.4 days in group A and 14.3±6.8 days in group B (P=0.006). The intraoperative and postoperative blood transfusion in groups A and B were 700 mL (range 0-3000) and 400 mL (range 0-1800), respectively (P<0.001).

Table 2. Intra-operative and postoperative data
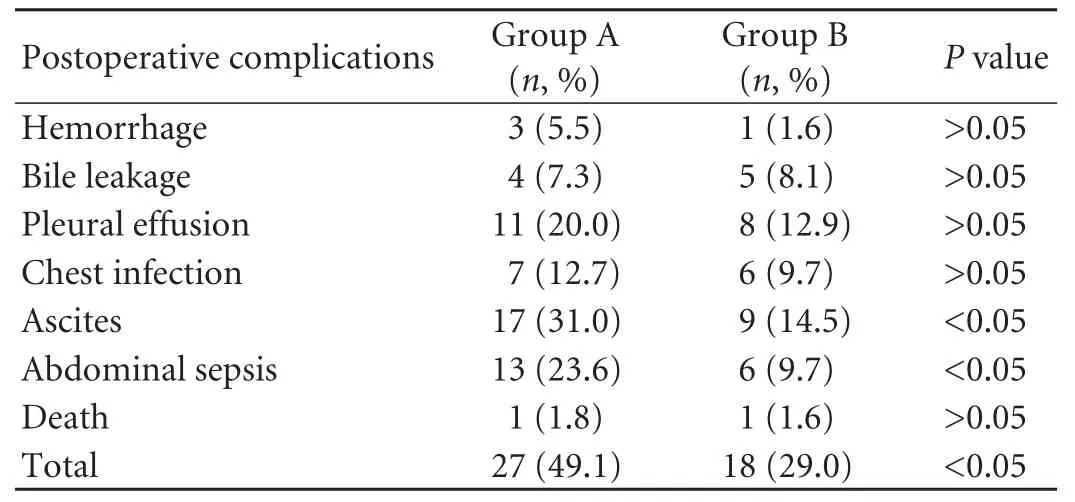
Table 3. Postoperative complications in the two groups
Number and distribution of SHPVs
The anatomy of SHPVs was studied in detail in 25 patients in group B (Fig. 1). There were 183 SHPVs in 25 patients with 7.3±2.7 branches per patient. The diameters of SHPV were 1 to 4 mm. On average, 3.4 SHPVs per patient came from the left portal vein, 2.2 from the bifurcation, 1.4 from the right portal vein, and 0.3 from the main portal vein. On average, 3.3 SHPVs per patient supplied for segmentiof the liver, 0.4 for segment II, 2.1 for segment IV, 1.4 for segment V, and 0.1 for segment VI (Tables 4 and 5). The distribution of these SHPVs in geometric space is shown in Fig. 2.
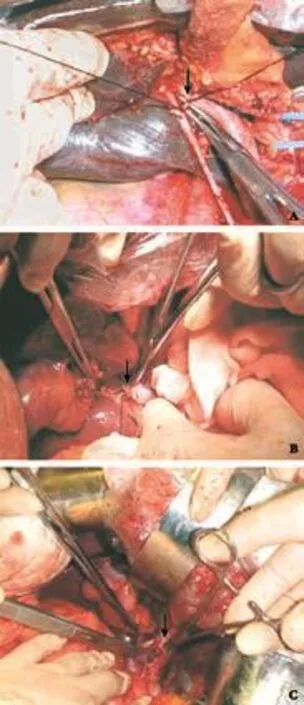
Fig. 1. SHPV marked by arrows. A: Arising from the right branch of the portal vein to the caudate lobe; B: arising from bifurcation of the portal vein to segment IV; C: arising from the left branch of the portal vein to segment II.

Table 4. Distribution of the origin of SHPVs in 25 patients

Table 5. Area of blood supply of SHPVs in 25 patients

Fig. 2. Geometry of anatomy of SHPVs. The hilum of the liver is along the a' a axis and the main portal vein along the b' b axis. Vertical to the a' a axis and inside the visceral surface of the liver is the c' c axis. These three axes meet at point "o". The visceral surface of the liver can be divided into 4 quadrants, α, β, γ and δ, with their corresponding locations at top left, top right, bottom left and bottom right. SHPVs are located in these 4 quadrants as cluster-like structures.
Discussion
Malignant tumors in the caudate lobe are not uncommon and they are technically difficult to treat. As the caudate lobe is supplied by a number of small arteries, transcatheter arterial chemo-embolization is not satisfactory.[11,12]The proximity of the caudate lobe to the IVC and major hepatic veins makes radiofrequency ablation and percutaneous ethanol injection potentially hazardous and technically difficult.[13,14]Surgery remains the best option to treat caudate lobe tumors.[15]Since the caudate lobe is adjacent to the liver pedicle and IVC, reducing blood loss during surgery is the key to success in caudate lobectomy. With improvements in surgical techniques such as early control of SHVs, caudate lobectomy has become technically less challenging.
Because of its hard-to-approach anatomical location, patients with caudate lobe tumors who have good liver function can undergo caudate lobe resection along with removal of adjacent portions of the liver, i.e., right or left lobectomy, and anterior or posterior segmentectomy.[16]In contrast, patients concurrently having chronic liver disease should undergo isolated, complete caudate lobectomy (segmentectomy 1) to improve curability.[17]
The choice of approach is essential to the success of caudate lobectomy. Approaches are dependent largely on the size, the location of the lesion, and the severity of cirrhosis. In this series, four approaches were used for various types of caudate lobectomy: 1) the left side approach, suitable for small tumors situated in the Spiegel lobe or when the caudate lobe is to be resected combined with the left liver; 2) the right side approach, suitable for a tumor located in the caudate process or when the caudate lobe is resected together with the right liver, mostly right hemihepatectomy; 3) the bilateral approach, a combination of left and right side approaches that the caudate lobe may be approached mainly from the right or left side although dissection from both sides is necessary in many cases; and 4) the anterior transhepatic approach, suitable for cases when isolated complete resection of the caudate lobe is indicated and non-cancerous liver parenchyma should be preserved due to cirrhosis: the characteristic of this approach is that the liver is split through the mid-plane into two halves, so as to fully expose the caudate lobe. In addition, when the tumor is closely attached to the hepatic vein, the anterior approach is also the best indication.[18]
The clinical data from 117 patients show that it is better to use early control of both SHVs and SHPVs than SHVs alone in isolated or combined hepatic caudate lobectomy. Many problems can occur because of injury to SHPVs. First, it leads to more blood loss and reduces the blood-supply to the liver, so more blood transfusion is necessary. The two factors result in ischemic-reperfusion injury, intestinal mucosal barrier damage, and eventually lead to bacterial translocation and endotoxemia. Second, laceration of SHPVs results in more exudation and ascites. Third, these combined synergic effects, such as intestinal bacterial translocation, decreased blood leukocytes by blood transfusion and ascites, result in a higher incidence ofpostoperative abdominal septic complications. Finally, all these factors result in a delay in recovery, increased hospital stay, medical expenses, postoperative morbidity. The potential damage of tearing SHPVs is summarized in Fig. 3.

Fig. 3. Potential damage of tearing SHPVs.
ALT was used as an indicator of postoperative liver function. For patients in group B, ALT was highest on postoperative day 1 and decreased significantly on day 3. It further declined, though more slowly, by day 7. For patients in group A, the rate of decline of ALT from postoperative days 1 to 3 was similar to group B, but slower from days 3 to 7. There was ischemicreperfusion injury to the liver after operation, ensuring the importance of early control of SHPVs.
To control SHPVs, adequate knowledge of the anatomy is required. Peng[19]described an anatomical structure called the caudate portal triad or the caudate pedicle. Inside this pedicle are the branches of the portal vein, hepatic artery and bile duct. Lau[1]pointed out that "while the caudate lobe artery and bile duct branches do not form a Glisson's triad immediately after branching within the liver, the portal vein branches do". Thus, branches of the portal vein supplying the caudate lobe are separated from the artery and bile duct branches for a short distance before they reach the liver. As a consequence, these SHPVs can be controlled extrahepatically.
Reports on the portal veins of the caudate lobe are rare. Ortale et al[7]reported 1-3 portal branches for the caudate lobe in 40 cases according to the vascularization bed. In 15/40 (37.5%) cases there was a single portal branch, and in 25/40 (62.5%), there were two or three caudate portal branches, with the occurrence of four types of branches, denominated posterior, anterior, left, and right. The results of our study are similar to those of Ortale et al. But they focused on the portal vein branches that went directly into the caudate lobe, while we summarized both short portal veins that went into the caudate lobe and into the hepatic segments adjacent to this lobe. When caudate lobectomy surgery is performed, it might not be enough to treat only the caudate branches, and all SHPVs should be known well.
In regard to how to find and transect SHPVs, our experience was as follows: first, we dissociate the ligaments around the liver, and then open the Glisson sheath, separating the portal vein from the common bile duct and hepatic artery along the anterior wall of the portal vein, until the left and right stems of the portal vein are revealed, then the portal vein and its left and right branches were pull up while ligating SHPVs at the side wall and posterior wall of the portal vein. According to our experience, there were no SHPVs at the anterior wall of the portal vein, and they were chiefly distributed along the side and posterior walls. SHPVs entering the caudate lobe were chiefly from the posterior wall, while the others entering segments II, IV, V, and VI were chiefly from the side wall. We pretreated SHPVs mainly according to the range of hepatectomy and how many SHPVs needed to be controlled are shown with geometric description in Fig. 2. For isolated caudate lobectomy, branches in the γ and δ quadrants needed to be controlled; for lobectomy combined with left hepatectomy, branches in the β, γ and δ quadrants needed to be controlled; for lobectomy combined with right hepatectomy, branches in the α, γ and δ quadrants needed to be controlled; and for lobectomy combined with expanded right or left hepatectomy, all SHPVs in the α, β, γ and δ quadrants needed to be controlled. In this way, as many SHPVs as possible were preserved for the remnant liver, with a resultant decrease in liver injury and better postoperative recovery.
Acknowledgement:We thank Prof. Chen Liu, Prof. Bin Yi, Dr. Bin Li and Dr. Fei-Ling Feng for their valuable advice.
Contributors:TWF, JXQ and LWY designed the study. QZQ performed the research and wrote the first draft. QZQ and TWF collected and analyzed the data. QZQ, TWF and YPN contributed equally to this article. All authors contributed to the design and interpretation of the study and to further drafts. JXQ is the guarantor.
Funding:This study was supported by grants from the Research Fund of Shanghai Science and Technology Commission (10495810400) and the Youth Research Fund of Shanghai Health Bureau (2009Y065).
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Lau WY. Applied anatomy in liver resection and liver transplantation. People's Medical Publishing House Publishing; 2010.
2 Vàn Minh T, Galizia G, Lieto E. Anatomy of the caudate lobe of the liver. New aspects and surgical applications. Ann Chir 1992;46:309-318.
3 Abdel Wahab M, Lawal AR, EL Hanafy E, Salah T, Hamdy E, Sultan AM. Caudate lobe resection: an Egyptian center experience. Langenbecks Arch Surg 2009;394:1057-1063.
4 Sakoda M, Ueno S, Kubo F, Hiwatashi K, Tateno T, Kurahara H, et al. Surgery for hepatocellular carcinoma located in the caudate lobe. World J Surg 2009;33:1922-1926.
5 Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, et al. Complete resection of the caudate lobe of the liver with tumor: technique and experience. Hepatogastroenterology 2001;48:808-811.
6 She ZF, Luo YZ, Wang JM. Research on the applied anatomy of the hepatic caudate lode. Gan Dan Yi Wai Ke Za Zhi 2003; 15:21-25.
7 Ortale JR, Borges Keiralla LC. Anatomy of the portal branches and the hepatic veins in the caudate lobe of the liver. Surg Radiol Anat 2004;26:384-391.
8 Hou DS, Zhong SZ, Ding ZH, Yan DH, Ye JS, Zhu J, et al. Research on the applied anatomy of hepatic caudate lobectomy. Zhongguo Lin Chuang Jie Pou Xue Za Zhi 2006; 24:612-615.
9 Zhou WP, Li AJ, Fu SY, Pan ZY, Shi JJ, Wu BW, et al. Resection of hepatic caudate lobe tumors: a summary of 76 cases. Zhongguo Shi Yong Wai Ke Za Zhi 2006;26:618-620.
10 Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992; 215:31-38.
11 Yoon CJ, Chung JW, Cho BH, Jae HJ, Kang SG, Kim HC, et al. Hepatocellular carcinoma in the caudate lobe of the liver: angiographic analysis of tumor-feeding arteries according to subsegmental location. J Vasc Interv Radiol 2008;19:1543-1550.
12 Terayama N, Miyayama S, Tatsu H, Yamamoto T, Toya D, Tanaka N, et al. Subsegmental transcatheter arterial embolization for hepatocellular carcinoma in the caudate lobe. J Vasc Interv Radiol 1998;9:501-508.
13 Seror O, Haddar D, N'Kontchou G, Ajavon Y, Trinchet JC, Beaugrand M, et al. Radiofrequency ablation for the treatment of liver tumors in the caudate lobe. J Vasc Interv Radiol 2005;16:981-990.
14 Shibata T, Kubo S, Tabuchi T, Maetani Y, Ametani F, Itoh K, et al. Percutaneous ethanol injection for hepatocellular carcinoma originating in the caudate lobe. Hepatogastroenterology 2000; 47:824-827.
15 Hawkins WG, DeMatteo RP, Cohen MS, Jarnagin WR, Fong Y, D'Angelica M, et al. Caudate hepatectomy for cancer: a single institution experience with 150 patients. J Am Coll Surg 2005;200:345-352.
16 Takayama T, Makuuchi M, Kosuge T, Yamazaki S, Hasegawa H, Takayama J, et al. A hepatoblastoma originating in the caudate lobe radically resected with the inferior vena cava. Surgery 1991;109:208-213.
17 Midorikawa Y, Takayama T. Caudate lobectomy (segmentectomy 1) (with video). J Hepatobiliary Pancreat Sci 2012;19:48-53.
18 Peng SY, Li JT, Liu YB, Cai XJ, Mou YP, Feng XD, et al. Surgical treatment of hepatocellular carcinoma originating from caudate lobe--a report of 39 cases. J Gastrointest Surg 2006;10:371-378.
19 Peng SY. Hepatic caudate lobe resection. Zhejiang University Press Publishing; 2009.
January 30, 2012
Accepted after revision May 2, 2012
Author Affiliations: First Department of Biliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China (Qiu ZQ, Tan WF, Yan PN, Luo XJ, Zhang BH, Wu MC and Jiang XQ); Faculty of Medicine, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong SAR, China (Lau WY)
Xiao-Qing Jiang, MD, PhD, First Department of Biliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China (Tel: 86-21-81875283; Fax: 86-21-81875281; Email: jiangxq423@126.com)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60195-7
 Hepatobiliary & Pancreatic Diseases International2012年4期
Hepatobiliary & Pancreatic Diseases International2012年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Muscarinic acetylcholine receptor M3 in proliferation and perineural invasion of cholangiocarcinoma cells
- Expression of SOCS3 throughout liver regeneration is not regulated by DNA methylation
- Early changes of hepatic hemodynamics measured by functional CT perfusion in a rabbit model of liver tumor
- A common variant in the precursor miR-146a sequence does not predispose to cholangiocarcinoma in a large European cohort
- Hepatic veins anatomy and piggy-back liver transplantation
- Hepatocyte differentiation of mesenchymal stem cells
