交替微波加热法对制备氧还原催化剂性能的影响
尹诗斌 罗 林 荆胜羽 朱强强 强颖怀,*
(1中国矿业大学低碳能源研究院,江苏徐州221116;2中国矿业大学材料科学与工程学院,江苏徐州221116; 3中国矿业大学信息与电气工程学院,江苏徐州221116)
交替微波加热法对制备氧还原催化剂性能的影响
尹诗斌1,2,*罗 林1,2荆胜羽3朱强强2强颖怀2,*
(1中国矿业大学低碳能源研究院,江苏徐州221116;2中国矿业大学材料科学与工程学院,江苏徐州221116;3中国矿业大学信息与电气工程学院,江苏徐州221116)
详细研究了交替微波加热法制备多壁碳纳米管负载Pt催化剂(Pt/MWCNTs)的过程中交替微波加热(5s-on/5s-off)次数对催化剂性能的影响.X射线粉末衍射(XRD)结果表明,Pt的晶粒尺寸在开始的加热阶段基本上没有发生变化,但是随着加热次数的增多,Pt的晶粒尺寸逐步增大.采用循环伏安法和旋转圆盘电极技术考察了催化剂的电化学活性.结果显示,以5s-on/5s-off加热20次时,催化剂显示出最佳的催化活性;在0.5 mol·L-1H2SO4饱和氧水溶液中催化剂的氧还原起峰电位接近1.0 V(vs RHE).交替微波加热法简单经济,在大批量制备催化剂等纳米材料方面显示出较好的应用前景.
催化剂;交替微波加热法;旋转圆盘电极;氧还原反应;多壁碳纳米管
1 Introduction
Platinum is widely used in proton exchange membrane fuel cells(PEMFCs)due to its high activity and superior stability.1,2It is still a challenge to rapidly synthesize the highly dispersed Pt with a uniform particle size distribution.Traditional methods,such as impregnation and colloid methods,are extensively used for the preparation of Pt-based catalysts.3-6However,impregnation cannot avoid the large particle sizes and broad size distributions,while colloid methods are always quite complex. It is well known that the particle size and the dispersion of catalysts are crucial to the catalytic reaction.Consequently,it is necessary to develop novel methods to prepare catalysts with a uniform size distribution.
The polyol method has been successfully used in the synthesis of highly active Pt/C catalysts with a high loading(40%, mass fraction)and narrow size distribution.7While,this process is time-consuming since it takes at least 3-6 h to reduce the metal completely.Therefore,it is necessary to develop an effective way for a time-saving preparation of the highly dispersed catalysts.Microwave irradiation has exhibited a remarkable effect on nanosized material preparation.8,9It has advantages of selective heating with high heating speed.The intermittent microwave heating(IMH)method is novel with respect to nanomaterial synthesis.10-13Tian et al.14,15prepared Pt/C catalysts with a Pt loading higher than 40%by adopting the IMH method.Song et al.16firstly combined the advantages of IMH and polyol methods to prepare high loading Pt/C catalysts,in which the metal reduction can be accomplished within 2 min. Moreover,the catalysts exhibited a comparable activity to the commercial ones for the oxygen reduction reaction(ORR). The influences of pH value and water content on the properties of catalysts prepared by polyol method were investigated.17,18However,the influence of microwave heating parameters on the performance of catalytic reaction is rarely reported.It is believed that,during the synthesis processing,the operating factors,such as the reduction time and temperature,will greatly affect the performance of catalysts.
In the present study,we prepared Pt particles supported on multi-walled carbon nanotubes(Pt/MWCNTs)by using the IMH polyol method,and the relationship between the number of pulse repetitions and the performance of catalysts was investigated.Their corresponding physicochemical and electrochemical properties were studied by employing X-ray diffraction (XRD),transmission electron microscopy(TEM),and rotating disk electrode(RDE)technologies.
2 Experimental
2.1 Carbon nanotube pretreatment
Multi-walled carbon nanotubes(MWCNTs)with diameters of 10-20 nm and purities of>95%(Shenzhen Nanotech.Co., Ltd.,China)were used as received.MWCNTs were treated by HF(AR,Sinopharm Chemical Regent Co.,Ltd.,China)as follows.19,20The MWCNTs(2.0 g)were added into 40%(mass fraction)HF aqueous solutions(12.5 g HF and 37.5 g water) under continuous stirring for 6 h.The treated MWCNTs were thoroughly rinsed with deionized water to neutral pH and dried at 80°C under vacuum for 24 h.
2.2 Catalyst preparation
The 20%Pt/MWCNTs catalysts were easily and rapidly prepared by the IMH polyol method as follows.16,21The chloroplatinic acid(AR,Sinopharm Chemical Regent Co.,Ltd.,China) as the starting precursor was well mixed with 20.0 mL ethylene glycol(EG,AR,Sinopharm Chemical Regent Co.,Ltd., China)in an ultrasonic bath,and then 200.0 mg MWCNTs were added into the mixture.After the pH value of the system was adjusted to be larger than 10.0 by NaOH/EG solution,the well-dispersed slurry was obtained with stirring and ultrasonication for 15 min.Thereafter,the slurry was microwave heated in a homemade program-controlled microwave oven(1000 W, 2.45 GHz)with pulses form of 5s-on/5s-off for 10,20,30,40, 50,60,70,and 100 repetitions.After re-acidification,the resulting black solid samples were filtered,washed,and dried at 80°C for 12 h in a vacuum.The catalysts are denoted as Pt/ MWCNTs-x,where x indicates the number of pulse repetitions.
2.3 Catalyst characterization
XRD measurements were carried out on a D/Max-III (Rigaku Co.,Japan)using Cu Kαradiation(λ=0.15406 nm), and operating at 40 kV and 30 mA.The 2θ angular regions between 20°and 90°were explored at a scan rate of 10(°)·min-1and the 64°-71°angle range was used to calculated the Pt crystal size according to the Scherrer formula.22,23TEM investigations were carried out in a JEOL JEM-2010(HR)at 200 kV to obtain the particle size distribution of Pt in the prepared catalysts.The histogram of the investigated catalysts was made by randomly measuring more than 300 particles.For Pt loadings, the catalysts were calcined in air at 700°C for 3 h,and then dissolved in freshly prepared aqua regia.Inductively coupled plasma atomic emission spectroscopy(ICP-AES,Perkin Elmer,Germany)was used to quantify the content of Pt in the prepared catalysts.
All electrochemical measurements were conducted on a PARETAT 2273(Princeton Applied Research,USA)instrument in a thermostat-controlled standard three-electrode cell at 30°C using a platinum foil(1.0 cm×1.0 cm)as the counter electrode.Reference electrode was saturated calomel electrode (SCE)and calibrated against a reversible hydrogen electrode (RHE).The specific steps were as follows:the SCE and the platinum foil were placed in 0.5 mol·L-1aqueous solutions together,and the platinum foil was bubbled with high-pure hydrogen gas,the voltage difference value between SCE and platinum foil was measured by multimeter and was recorded as RHE.A glass carbon(GC)disk electrode with a diameter of 5.0 mm was used as the substrate for the catalyst thin film in the electrochemical measurements.The thin film catalysts layer,as the working electrode,was prepared as follows:a mixture containing 5.0 mg catalysts,1.8 mL ethanol(AR,Sino-pharm Chemical Regent Co.,Ltd.,China),and 0.2 mL Nafion solution(5%Nafion®perfluorinated resin solution,AR,Sigma-Aldrich,USA)were dispersed in an ultrasonic bath for several minutes to obtain a well-dispersed ink.This catalysts ink was then quantitatively transferred onto the surface of the GC electrode by a micropipette,and dried under an infrared lamp to obtain the catalyst thin film.The estimated loading was 25.5µg· cm-2for each catalyst.An aqueous solution containing 0.5 mol· L-1H2SO4deaerated with high-pure nitrogen gas was used as the electrolyte.Rotating disk electrode tests were carried out in O2-saturated 0.5 mol·L-1H2SO4aqueous solutions with a potential range from 0.1 to 1.1 V at a rotating speed of 2500 r·min-1and a scan rate of 5 mV·s-1.
3 Results and discussion
Fig.1 presents the XRD results of the Pt/MWCNTs catalysts prepared by IMH with different numbers of pulse repetitions (x).As is clearly displayed,all the samples show the typical characteristics of a crystalline Pt face centered cubic(fcc) structure.The diffraction peaks at 2θ of 39.6°,46.3°,67.4°, and 81.6°are assigned to the Pt(111),Pt(200),Pt(220),and Pt(311)facets.The fitted(220)plane was used to calculate the Pt crystal size according to the Scherrer formula.22,23The corresponding Pt crystal sizes for Pt/WMCNTs-x are summarized in Table 1.It is obvious that the crystal sizes of Pt particles are hardly increased with the increment in the pulse repetitions at early stage(for example,10-60 times),while it becomes much larger after the further heating.
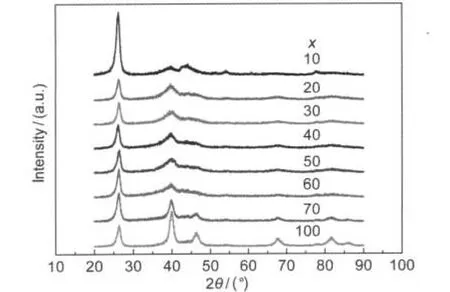
Fig.1 XRD patterns of Pt/MWCNTs microwave heated for different numbers of pulse repetitions(x)at a scan rate of 10(°)·min-1
From the TEM images given in Fig.2(a,b)at different magnifications,it can be clearly seen that the Pt particles are uniform and well distributed on the surface of MWCNTs.Based on the measurements of 300 randomly chosen particles,the average particle size is estimated to be 2.8 nm for Pt/MWCNTs-20.The corresponding histogram in Fig.2(c)reveals that the particle size distributions are narrow and approximately Gaussian type.
Fig.3 plots the cyclic voltammetry(CV)curves of all the investigated catalysts in the deaerated 0.5 mol·L-1H2SO4aqueous solutions at a scan rate of 20 mV·s-1.The electrochemical surface area(SESA,m2·g-1,Pt as reference)of the catalysts can be calculated from the integrated charge in the hydrogen desorption peak area in the CV curves.24,25The Pt poly-crystallite hydrogen adsorption constant is 210 μC·cm−2,and then the value of SESAcan be obtained from Eq.(1).
SESA=QH/(2.1×mPt) (1) where QH(in μC·cm-2)is the charge due to the hydrogen desorption in the hydrogen region of the CVs shown in Fig.3,mPtis the quality of Pt loaded on the surface of GC electrode with the unit of mg·cm-2.
SESAis one of the most important parameters for characterizing Pt catalysts.A higher SESAimplies a better electrode,as more catalytic sites are available for electrode reactions.The relationships of the size of Pt(d)or SESAversus the number of pulse repetitions is plotted in Fig.4.SESAdoes not change much from 20 to 50 pulse repetitions,but it changes dramatically initially and at larger pulse repetitions(>50 times).There aremany factors for this phenomenon.During the synthesis process,if only microwave heating for 10 pulse repetitions,the temperature and time may not be sufficient to reduce the chloroplatinic acid precursor.On the other hand,with 20 pulse repetitions,the chloroplatinic acid precursor could be completely reduced.As increased up to 50 pulse repetitions,the temperature of the whole system would not change so much,and the corresponding crystal size would almost have no change.With the pulse repetitions further increasing,the Pt particles that reduce in the EG solutions could move and agglomerate together.This conclusion is further verified by the Pt loading results obtained from the inductively coupled plasma atomic emission spectrometry.The Pt loading rate is the content of Pt quantified by ICP versus the default values.As displayed in Table 1,Pt/ MWCNTs-10 has the lowest Pt loading rate(only 15.4%), which means that the majority of the Pt did not adsorb on the surface of the MWCNTs.The reason could be attributed to the insufficient reduction of chloroplatinic acid at low IMH repetitions.While with the pulse repetitions further increasing,the Pt loading rate is significantly increased(Pt/MWCNTs-50 has the largest Pt loading rate of 88.7%).However,the Pt loading rate is reduced dramatically with the pulse repetitions further increased.It might due to the fact that the agglomerated Pt particles could not adsorb on the surface of MWCNTs during the re-acidification.

Table 1 Physico-chemical and electrochemical characterization for the Pt/MWCNTs microwave heated for different numbers of pulse repetitions

Fig.2 TEM images at two magnifications(a,b)and Pt particle size distribution histogram(c)of Pt/MWCNTs-20

Fig.3 CV curves of the Pt/MWCNTs microwave heated for different numbers of pulse repetitions in 0.5 mol·L-1H2SO4 aqueous solutions at 30°C with a scan rate of 20 mV·s-1

Fig.4 Relationship between the diameter or the electrochemical surface area of Pt/MWCNTs and the number of microwave heating pulse repetitions

Fig.5 Linear potential sweep curves of oxygen reduction reaction on Pt/MWCNTs microwave heated for different numbers of pulse repetitions in O2-saturated 0.5 mol·L-1H2SO4aqueous solutionsT=30°C;scan rate:5 mV·s-1;stirring:2500 r·min-1
The chemical specific surface area(SCSA,m2·g-1)of Pt particles can be calculated from Eq.(2),assuming that all particles are of spherical shape,16,25

where ρ is the density of Pt(21.4 g·cm-3)and d(in nm)is the diameter of the Pt particles in the catalysts calculated from the XRD results given in Table 1.
The Pt utilization efficiency(ηPt)for the catalysts is also a very important parameter,as it reflects the number of Pt particles that are active in electrochemical reactions.It is defined as the ratio of the SESAand SCSA.

For the cathode catalysts of PEMFCs,the capability for the oxygen reduction reaction is very important.Fig.5 shows the ORR polarization curves for all the investigated catalysts in oxygen saturated 0.5 mol·L-1H2SO4aqueous solutions as obtained by rotating the disk electrode at 2500 r·min-1and at a scan rate of 5 mV·s-1.The corresponding relationship between the activities towards ORR and the pulse repetitions is exhibited in Fig.6.As it is obviously displayed,the Pt/MWCNTs with 20 to 70 pulse repetitions show almost the same Pt utilization efficiency,which is significantly higher than those of the other catalysts.On the other hand,Pt/MWCNTs-20 shows a positive shift in the ORR onset potential indicating that the ORR is more favorable on Pt/MWCNTs-20 than on the other catalysts. We believe that such high performance predominantly origins from the better distribution of Pt particles on the prepared catalysts.

Fig.6 Relationship between the Pt utilization efficiency or the onset potential of Pt/MWCNTs and the number of microwave heating pulse repetitions
4 Conclusions
Pt/MWCNTs catalysts were prepared by using the intermittent microwave heating polyol method.The influence of pulse repetitions on the properties of catalysts was investigated.The Pt crystal size does not change much for low numbers of pulse repetitions(<50 times),but it changes dramatically at higher pulse repetitions.The electrochemical surface area and the Pt utilization reflect a similar tendency with number of pulse repetitions.The oxygen reduction reaction results show that the Pt/ MWCNTs-20 exhibits the most favorable activity towards oxygen reduction in the present study.The reason could be attributed to the uniformly distributed Pt particles on the surface of MWCNTs.The intermittent microwave heating method is simple and economical and can be potentially applied to scale-up for the mass production of nanosized materials.
Acknowledgments:The authors gratefully acknowledge the support by Prof.SHEN Pei-Kang in Sun Yat-Sen University.
(1) Zhang,S.S.;Yuan,X.Z.;Hin,J.N.C.;Wang,H.J.J.Power Sources 2009,194,588.
(2) Rao,G.S.;Cheng,M.Q.;Zhong,Y.;Deng,X.C.;Yi,F.;Chen, Z.R.;Zhong,Q.L.;Fan,F.R.;Ren,B.;Tian,Z.Q.Acta Phys.-Chim.Sin.2011,27,2373.[饶贵仕,程美琴,钟 艳,邓小聪,易 飞,陈治仁,钟起玲,范凤茹,任 斌,田中群.物理化学学报,2011,27,2373.]
(3) Fugane,K.;Mori,T.;Ou,D.R.;Suzuki,A.;Yoshikawa,H.; Masuda,T.;Uosaki,K.;Yamashita,Y.;Ueda,S.;Kobayashi,K.; Okazaki,N.;Matolinova,I.;Matolin,V.Electrochim.Acta 2011,56,3874.
(4)Hara,Y.;Minami,N.;Matsumoto,H.;Itagaki,H.Appl.Catal.A 2007,332,289.
(5) Keng,P.Y.;Bull,M.M.;Shim,I.B.;Nebesny,K.G.; Armstrong,N.R.;Sung,Y.;Char,K.;Pyun,J.Chem.Mater. 2011,23,1120.
(6)Yin,S.B.;Mu,S.C.;Lv,H.F.;Cheng,N.C.;Pan,M.;Fu,Z.Y. Appl.Catal.B 2010,93,233.
(7)Zhou,Z.H.;Wang,S.L.;Zhou,W.J.;Wang,G.X.;Jiang,L. H.;Li,W.Z.;Song,S.Q.;Liu,J.G.;Sun,G.Q.;Xin,Q.Chem. Commun.2003,394.
(8)Wang,X.Z.;Zheng,J.S.;Fu,R.;Ma,J.X.Acta Phys.-Chim. Sin.2011,27,85.[王喜照,郑俊生,符 蓉,马建新.物理化学学报,2011,27,85.]
(9) Shen,P.K.;Yin,S.B.;Li,Z.H.;Chen,C.Electrochim.Acta 2010,55,7969.
(10)Yin,S.B.;Luo,L.;Xu,C.;Zhao,Y.L.;Qiang,Y.H.;Mu,S.C. J.Power Sources 2012,198,1.
(11) Hu,Z.F.;Chen,C.;Meng,H.;Wang,R.H.;Shen,P.K.;Fu,H. G.Electrochem.Commun.2011,13,763.
(12)Yin,S.B.;Cai,M.;Wang,C.X.;Shen,P.K.Energy Environ. Sci.2011,4,558.
(13) Yin,S.B.;Shen,P.K.;Song,S.Q.;Jiang,S.P.Electrochim. Acta 2009,54,6954.
(14) Tian,Z.Q.;Xie,F.Y.;Shen,P.K.J.Mater.Sci.2004,39,1507.
(15) Tian,Z.Q.;Jiang,S.P.;Liang,Y.M.;Shen,P.K.J.Phys. Chem.B 2006,110,5343.
(16) Song,S.Q.;Wang,Y.;Shen,P.K.J.Power Sources 2007,170, 46.
(17)Li,X.;Chen,W.X.;Zhao,J.;Xing,W.;Xu,Z.D.Carbon 2005, 43,2168.
(18) Li,W.Z.;Liang,C.H.;Zhou,W.J.;Qiu,J.S.;Li,H.Q.;Sun, G.Q.;Xin,Q.Carbon 2004,42,436.
(19) Li,Y.L.;Hu,F.P.;Wang,X.;Shen,P.K.Electrochem. Commun.2008,10,1101.
(20) Hu,F.P.;Shen,P.K.;Li,Y.L.;Liang,J.Y.;Wu,J.;Bao,Q.L.; Li,C.M.;Wei,Z.D.Fuel Cells 2008,8,429.
(21)Song,S.Q.;Yin,S.B.;Li,Z.H.;Shen,P.K.;Fu,R.W.;Wu,D. C.J.Power Sources 2010,195,1946.
(22) Patterson,A.L.Phys.Rev.1939,56,978.
(23) Radmilovic,V.;Gasteiger,H.A.;Ross,P.N.J.Catal.1995, 154,98.
(24) Xing,Y.C.;Li,L.;Chusuei,C.C.;Hull,R.V.Langmuir 2005, 21,4185.
(25) Xing,Y.J.Phys.Chem.B 2004,108,19255.
October 9,2011;Revised:November 10,2011;Published on Web:November 15,2011.
Effect of Intermittent Microwave Heating on the Performance of Catalysts for Oxygen Reduction Reaction
YIN Shi-Bin1,2,*LUO Lin1,2JING Sheng-Yu3ZHU Qiang-Qiang2QIANG Ying-Huai2,*
(1Low Carbon Energy Institute,China University of Mining and Technology,Xuzhou 221116,Jiangsu Province,P.R.China;
2School of Materials Science and Engineering,China University of Mining and Technology,Xuzhou 221116,
Jiangsu Province,P.R.China;3School of Information and Electrical Engineering,China University of Mining and Technology, Xuzhou 221116,Jiangsu Province,P.R.China)
The influence of intermittent microwave heating(IMH)on the physicochemical and electrochemical properties of platinum loaded on multi-walled carbon nanotubes(Pt/MWCNTs)was investigated.X-ray diffraction results revealed that the crystal size of Pt particles hardly increased for smaller numbers of pulse repetitions,but became much larger as the number of pulse repetitions increased.Cyclic voltammetry(CV)and rotating disk electrode(RDE)results showed that the Pt/MWCNTs catalysts prepared by IMH in a repeated pulse form of 5s-on/5s-off for 20 pulse repetitions possessed the largest electrochemical surface area.An onset potential of approximately 1.0 V(vs RHE)was observed for the oxygen reduction reaction in oxygen-saturated 0.5 mol·L-1H2SO4aqueous solutions.The IMH method is simple,economical,and can potentially be scaled up for the mass production of nanomaterials.
Catalyst;Intermittent microwave heating method;Rotating disk electrode; Oxygen reduction reaction;Multi-walled carbon nanotube
10.3866/PKU.WHXB201111153www.whxb.pku.edu.cn
*Corresponding authors.YIN Shi-Bin,Email:shibinyin@126.com;Tel:+86-516-83883235;Fax:+86-516-83883501.
QIANG Ying-Huai,Email:yhqiang@cumt.edu.cn;Tel:+86-516-83591876;Fax:+86-516-83591870.
The project was supported by the National Natural Science Foundation of China(21106178),Postdoctoral Science Foundation of China (20110491480),Scientific Research Foundation of Xuzhou,China(XJ11B009),Polysilicon and Photovoltaic Energy Technology of Xuzhou,China (6AT102092),Open Fund of the State Key Laboratory ofAdvanced Technology for Materials Synthesis and Processing(Wuhan University of Technology)(2012-KF-13),and Scientific Research Foundation for Yong Teachers of the China University of Mining and Technology,China (2011QNA21,2009A026).
国家自然科学基金(21106178),中国博士后基金(20110491480),徐州市科技项目(XJ11B009),徐州市多晶硅与光伏能源技术专项(6AT102092),材料复合新技术国家重点实验室(武汉理工大学)开放基金(2012-KF-13)和中国矿业大学青年基金(2011QNA21,2009A026)资助
O646

