CuCl2/Al2O3催化氮杂环的N-芳基化反应
施介华,王炯杰
(1.浙江工业大学药学院,浙江杭州310032;2.浙江工业大学绿色化学合成技术国家重点实验室培育基地,浙江杭州310032)
CuCl2/Al2O3催化氮杂环的N-芳基化反应
施介华1,2,王炯杰1
(1.浙江工业大学药学院,浙江杭州310032;2.浙江工业大学绿色化学合成技术国家重点实验室培育基地,浙江杭州310032)
研究了以CuCl2/Al2O3作为金属铜催化剂高效催化氮杂环与卤代芳烃的N-芳基化反应制备N-芳基杂环化合物.实验结果表明:在无有机配体存在下CuCl2/Al2O3催化剂具有良好的催化活性和重复使用性.以咪唑与碘苯的反应作为模板反应优化反应条件,考察了反应物配比、催化剂种类、碱的种类、溶剂的选择、反应温度以及反应时间等因素对反应收率的影响,确定了最佳反应条件,在此条件下制备N-苯基咪唑的收率达99%.而且,所选择的催化反应体系可应用于合成一系列N-芳基杂环化合物.
CuCl2/Al2O3;氮杂环;卤代芳烃;N-芳基化
有机合成反应中C-N偶联反应占有非常重要的地位,其合成的芳基吲哚,芳基咪唑,芳基吡咯,芳基咔唑等氮杂环芳烃化合物广泛应用于制药和材料科学领域[1-3].C-N偶联反应通常使用过渡金属作催化剂,如钯、镍、铜、铁、铟等.在过去几十年,国内外有大量研究关于金属钯催化剂应用于C-N偶联反应,结果表明钯催化体系在C-N偶联反应中具有非常高的催化活性[4-6].但是,钯催化剂的一些缺点,如价格昂贵,毒性较高和反应时条件苛刻等,限制了其工业化应用.相比金属钯催化剂,金属铜催化剂存在着价格低廉和性质稳定的优点.传统的无有机配体铜催化的C-N偶联反应需要较高的温度,而且反应活性不是很高,但加入一定量的配体后,其反应条件变得更温和,反应时间也大大缩短了.近几年来,国内外对金属铜催化的C-N偶联反应研究越来越多,特别是对一价铜盐配体的研究,目前一价铜盐的配体主要有二氮类7-17、氮氧类18-26、二氧类[27-33]二齿类配体,它们对于提高一价铜盐的催化活性起到了重要的作用.
相比一价铜盐,同样条件下二价铜盐的催化活性较低.但是二价铜盐具有在空气中更稳定、价格更低廉等优点,而且在一定条件下也能获得较高的催化活性,因此对二价铜盐的深入研究是具有深远的意义的.国内外对于二价铜盐催化C-N偶联反应的研究,有研究其配体的[34-40],有研究其络合物的[41-46],也有研究其负载的[47-48]等[49].到目前为止,氧化铝负载二价铜盐作为氮杂环与卤代芳烃C-N偶联反应的催化剂未被报道过.众所周知,氧化铝是一种常用的固体催化剂载体.Bhadra等报道了中性氧化铝负载硫酸铜高效催化硫醇与卤代芳烃的S-芳烃化反应[50]以及有机硼烷、有机硅烷和有机锡烷与PhSeBr的亲电取代反应[51].Kantam等通过假设Y型离子沸石能活化C-Cl,C-Br和C-I键,报道了Y型离子沸石负载二价铜盐高效催化氮杂环与卤代芳烃的C-N偶联反应[8].设想碱性Al2O3也能活化C-Cl,C-Br和C-I键,笔者报道了以CuCl2/Al2O3作为可回收的铜催化剂,在无配体存在下,高效催化氮杂环与卤代芳烃的C-N偶联反应.
1 实验方法
1.1 催化剂的制备
将CuCl2.2H2O(3.8 g)溶于10 m L蒸馏水中,加入碱性Al2O3(5.1 g),室温下搅拌1 h,60℃减压蒸馏至干,残余的固体在100℃干燥8 h,制得37% CuCl2/Al2O3催化剂.
1.2 C-N偶联反应的经典反应过程
在反应器中,氮气保护下,加入氮杂环化合物(3 mmol)、卤代芳烃(1 mmol)、K3PO4(2 mmol)、37%CuCl2/Al2O3(0.2 mmol)和2 m L DMSO,135℃搅拌反应23 h.自然冷却后,用20 m L水洗,再用Et OAc(3次每次10 m L)萃取,无水硫酸钠干燥,过滤,脱干溶剂,然后柱层析分离得到产物.
2 实验结果与讨论
2.1 C-N偶联反应的反应条件优化
以碘苯和咪唑的C-N偶联反应作为模板反应,其反应式为
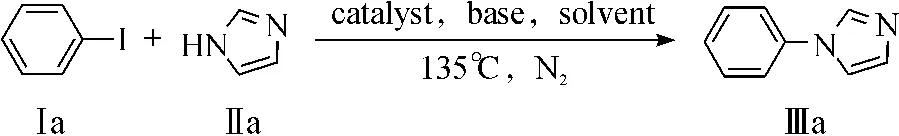
对反应条件进行优化改进(表1).首先对两种催化剂CuCl2与CuCl2/Al2O3进行比较(表1,编号1,2),发现Al2O3负载的CuCl2催化剂的活性显著提高.接着,考察了溶剂对催化剂活性影响,发现极性溶剂能更好地促进反应进行(表1,编号3-5),作者认为催化剂能更好地亲极性溶剂,使催化剂与溶剂及反应物的有效接触面积增大,从而更好地催化反应进行.研究结果还发现碱的种类对C-N偶合反应的影响不大(表1,编号3,6-10),但是不加碱的情况下(表1,编号11),反应不能进行.另外,实验结果还表明了咪唑投料比增加,合成收率随之增加(表1,编号12-14),当咪唑/碘苯比例大于3/1,偶联反应收率达99%.而且,从表还可发现偶联反应收率随反应温度的升高而增加,当反应温度大于135℃,收率趋于平稳.
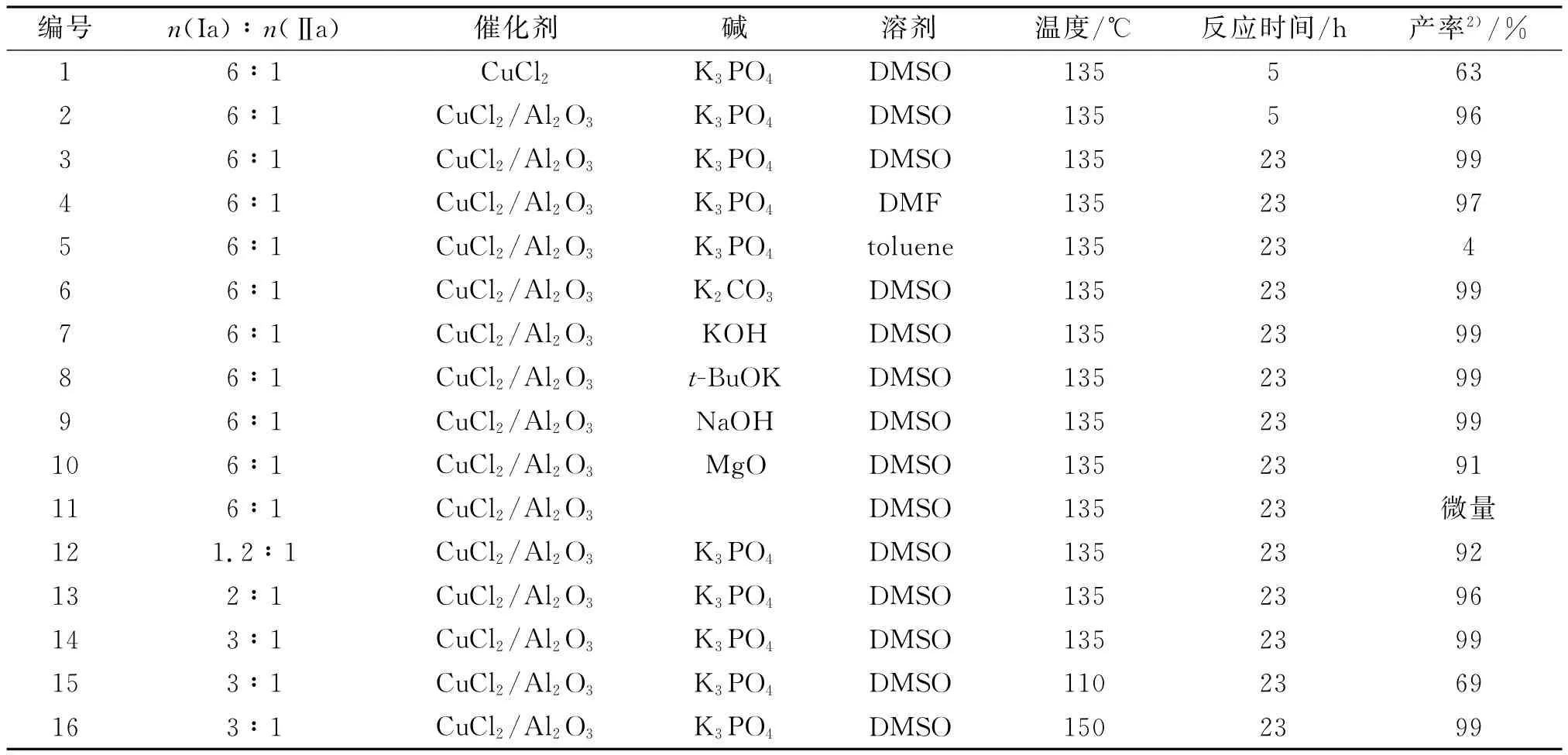
表1 咪唑与碘苯的C-N偶联反应1)Table 1 N-arylation of imidazole with iodobenzene
2.2 咪唑与卤代芳烃的C-N偶联反应
在上述优化后的反应条件下,进一步考察了咪唑和一系列卤代芳烃的偶联反应,反应式为
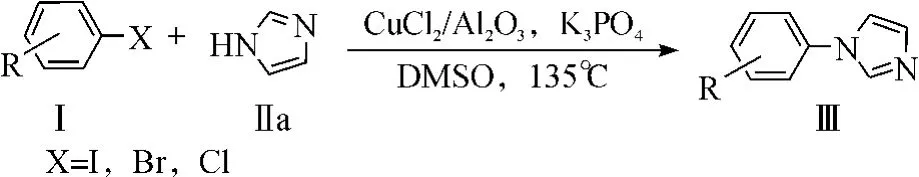
其结果如表2所示.结果发现碘代芳烃和溴代芳烃(表2,编号1,2,4-9)与咪唑反应收率较高;取代溴代芳烃与咪唑偶联反应的活性的大小与取代基种类密切相关,当吸电子基团取代的与咪唑的偶联反应的活性明显高于供电子基团取代的溴代芳烃(表2,编号4-7).同样,溴代噻吩与咪唑偶合反应的活性也较高,收率达95%(编号9).但间溴苯乙酮(表2,编号8)与咪唑反应时其收率较低,这可能是由于副反应发生比较多.
从表2中还可以看出:氯代芳烃相对于溴代和碘代芳烃,其反应活性比较低.氯苯作为底物时,没有检测到有产物生成.然而,对氯硝基苯(表2,编号10)和邻氯硝基苯(表2,编号11)为底物时,反应产率分别为81%和63%.这进一步说明了卤代芳烃上有其它吸电子基团存在可活化其活性.另外,还发现吸电子取代基位于卤素原子对位或间位时的反应活性优于邻位取代,这可能是因为邻位的位阻比较大,阻碍了其偶联的进行.
2.3 其他氮杂环与卤代芳烃的C-N偶联反应
为了阐明Al2O3负载的CuCl2催化剂催化CN偶合反应的适用范围,进一步考察了吲哚、咔唑、1,2,4-三氮唑与卤代芳烃的C-N偶联反应,反应式为
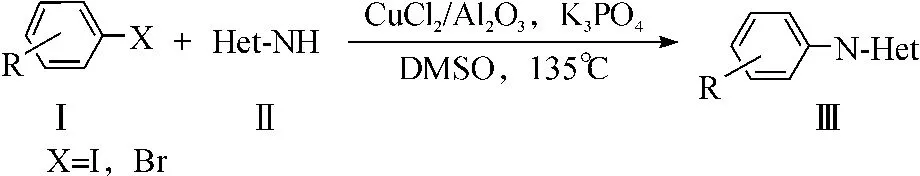
其结果如表3所示.从表3中可以看出:吲哚和一系列卤代芳烃的反应产率(表3,编号1-4)与咪唑相比较低,产率在19%~81%之间.咔唑与三氮唑(表3,编号6)的产率分别为94%和44%.这说明了该催化体系在C-N偶合反应的适用面较宽.
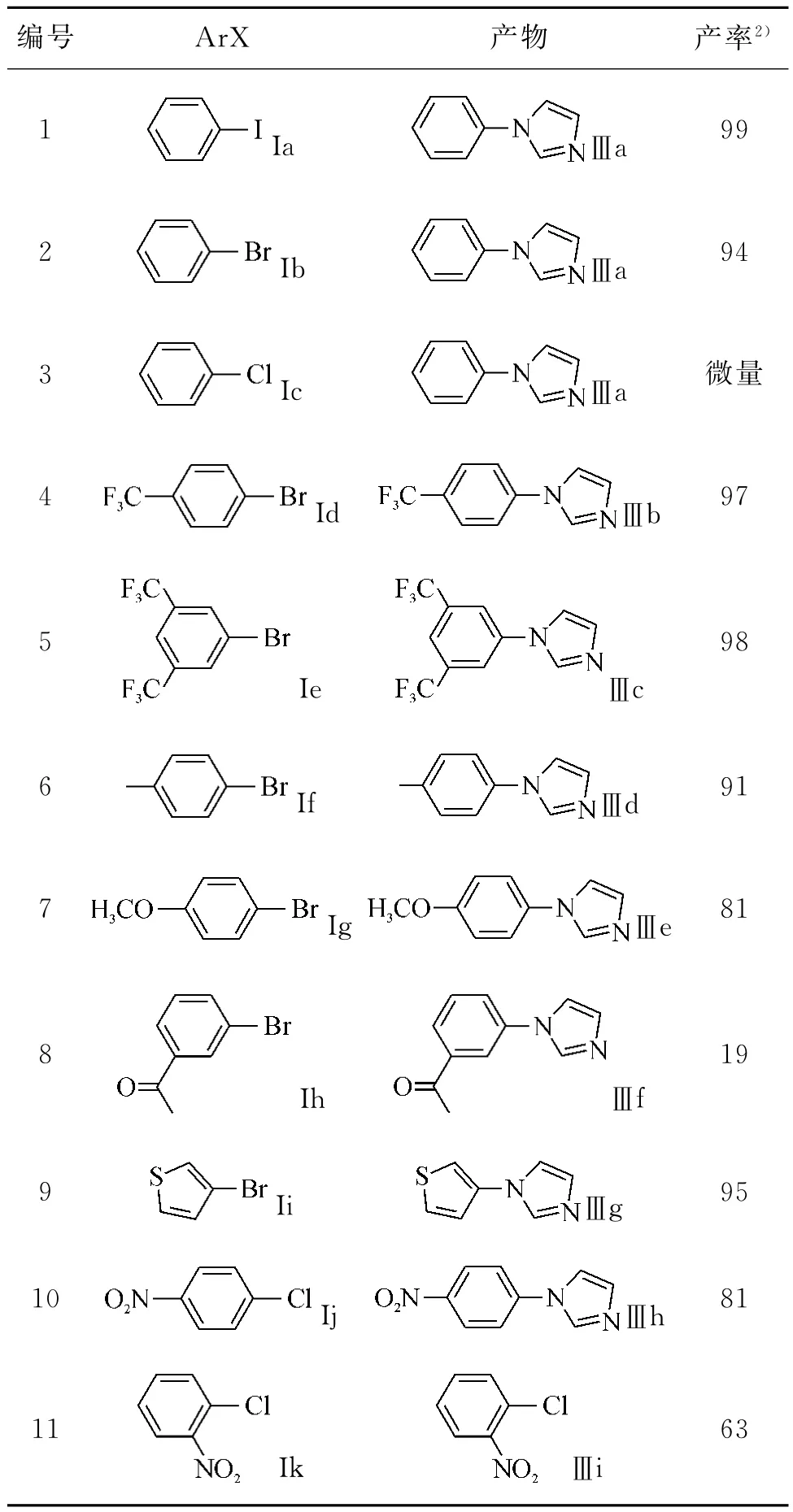
表2 咪唑与卤代芳烃的C-N偶联反应Tabel 2 N-arylation of imidazole with Aryl Halides %
2.4 催化剂的使用寿命
在反应介质中固相催化剂活性中心的稳定性已引起人们的高度重视,负载型催化剂尤为重要,因此负载型催化剂的重复使用性已成为考察这类催化剂的重要指标之一.为此,我们以碘苯和咪唑的C-N偶合反应为模板反应,考察了CuCl2/Al2O3催化剂的稳定性和催化性能的重复性,其实验结果如表4所示.从表4中可以看出:CuCl2/Al2O3催化剂经过滤、烘干处理后,其催化活性几乎没有变化,表明了该催化剂具有较好的重复使用性.
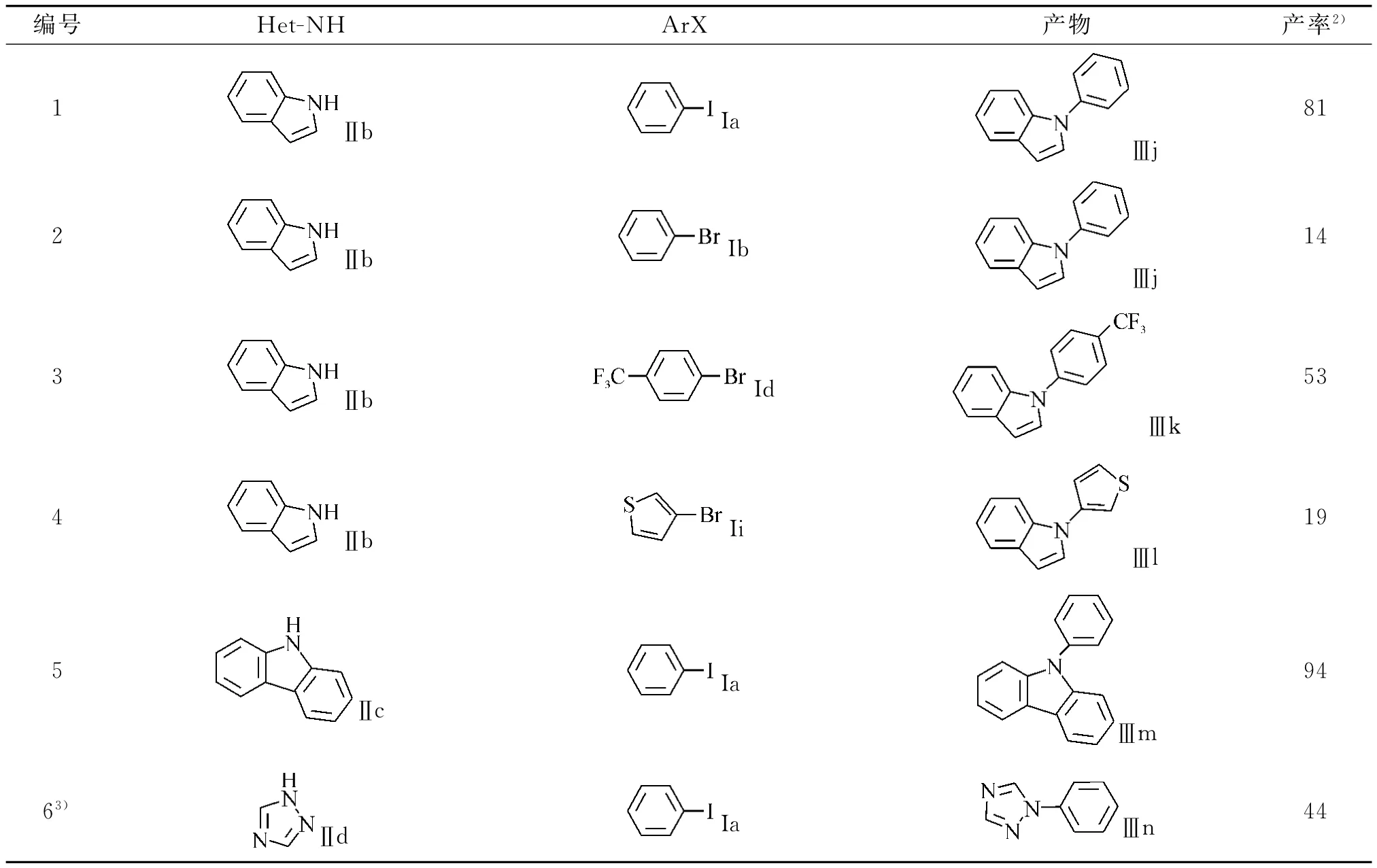
表3 其他氮杂环与卤代芳烃的C-N偶联反应Tabel 3 N-arylation of Various Nitrogen Hetrocycles with Aryl Halides %
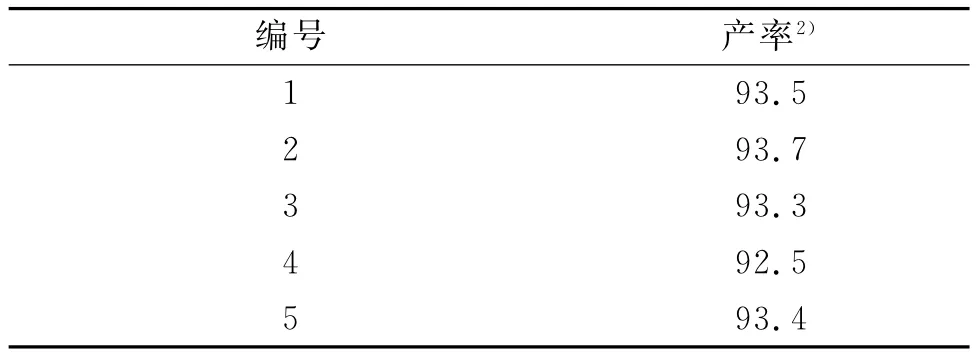
表4 催化剂体系重复套用的研究1)Table 4 Recycle studies of CuCl2/Al2O3catalysts N-arylation of imidazole with iodobenzene %
2.5 CuCl2/Al2O3催化剂催化C-N偶联反应机理的探讨
在文献[51]报道基础上,我们认为CuCl2/ Al2O3催化剂催化C-N偶合反应的机理:CuCl2在载体表面形成1,一个卤代物被氧化加成到1上,并形成中间过渡态2.接着氮杂环发生亲核取代,形成中间过渡态3.在碱的作用下,脱去一分子的盐,最后还原消除,得到目标产物(图1).
2.6 产物的表征
苯基咪唑(Ⅲa):无色液体,1HNMR(500 MHz,CDCl3)δ:7.87(s,1H),7.50~7.47(m, 2H),7.40~7.36(m,3 H),7.30(s,1H),7.22(s,1H).
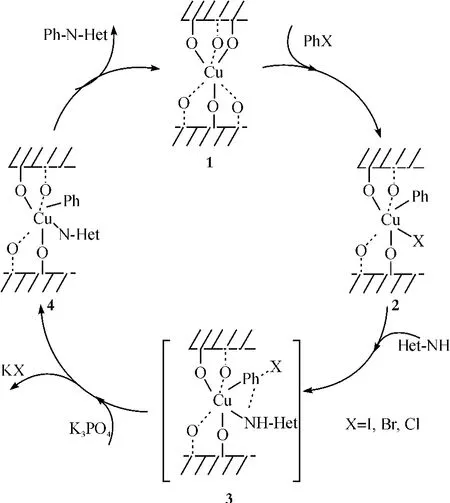
图1 C-N偶联反应可能的机理Fig.1 The possible mechanism of the C-N couplingreaction
N-咪唑基-4-三氟甲基苯(Ⅲb):黄色固体,m.p.63~65℃,1HNMR(500 MHz,CDCl3)δ:7.85(s,1H),7.65(d,J=8.4Hz,2H),7.44(d,J= 8.4 Hz,2 H),7.26(s,1H),7.14(s,1 H).
N-咪唑基-3,5-二三氟甲基苯(Ⅲc):淡黄色固体,m.p.120~121℃,1HNMR(500 MHz,CDCl3)δ:7.95(s,1H),7.85(s,2H),7.81(s,1H),7.37(s,1 H),7.21(s,1H).
N-4-甲基苯基咪唑(Ⅲd):淡黄色固体,m.p. 61~63℃,1HNMR(500 MHz,CDCl3)δ:7.72(s,1H),7.15(s,4H),7.14(s,1H),7.10(s,1H),2.29(s,3 H).
N-4-甲氧基苯基咪唑(Ⅲe):淡黄色液体,1HNMR(500 MHz,CDCl3)δ:7.71(s,1 H),7.27~7.22(m,2H),7.15(s,1H),7.12(s,1H),6.94~6.91(m,2H),3.78(s,3 H).
N-3-乙酰基苯基咪唑(Ⅲf):淡黄色固体,m.p. 70~72℃,1HNMR(500MHz,CDCl3)δ:7.97(d,J=1.0Hz,1H),7.96~7.90(m,2H),7.59~7.58(m,2 H),7.33(s,1H),7.21(s,1 H),2.64(s,3H).
N-3-噻吩基咪唑(Ⅲg):淡棕色固体,m.p.77~79℃,1HNMR(500 MHz,CDCl3)δ:7.63(s,1H),7.36(dd,J=5.1,3.3 Hz,1 H),7.18(s,1H),7.14(dd,J=5.6,4.1Hz,1H),7.11~7.10(m,2 H).
N-4硝基苯基咪唑(Ⅲh):白色荧光固体,m.p. 206~207℃,1HNMR(500 MHz,CDCl3)δ:8.41~8.38(m,2H),8.01(s,1H),7.61~7.58(m,2H),7.39(s,1 H),7.29(d,J=10.7 Hz,1 H).
N-2硝基苯基咪唑(Ⅲi):淡黄色晶体,m.p.93~95℃,1HNMR(500 MHz,CDCl3)δ:7.97(dd,J=8.1,1.3Hz,1H),7.72(td,J=7.7,1.4 Hz,1H),7.62~7.59(m,2H),7.46~7.44(m,1 H),7.17(s,1H),7.05(s,1H).
N-苯基吲哚(Ⅲj):淡黄色液体,1HNMR(500 MHz,CDCl3)δ:7.68~7.66(m,1H),7.55~7.53(m,1 H),7.46~7.45(m,4H),7.31~7.28(m,2H),7.21~7.12(m,2H),6.66(dd,J= 3.2,0.6 Hz,1 H).
N-4-三氟甲基苯基吲哚(Ⅲk):无色稠状液体,m.p.46~47℃,1HNMR(500 MHz,CDCl3)δ:7.84(d,J=8.4 Hz,2H),7.79(d,J=7.6 Hz,1H),7.67(d,J=8.3 Hz,2H),7.64(d,J=7.8 Hz,1H),7.40(d,J=3.6 Hz,1H),7.34(t,J =4.1 Hz,1 H),7.30(t,J=3.8 Hz,1H),6.81(d,J=3.5 Hz,1 H).
N-3-噻吩基吲哚(Ⅲl):黄色液体,1HNMR(500 MHz,CDCl3)δ:7.74~7.72(d,1H),7.63~7.61(d,1H),7.49~7.47(m,1H),7.36~7.24(m,4 H),7.23~7.21(m,1 H),6.71~6.70(d,1H).
N-苯基咔唑(Ⅲm):白色固体,m.p.85~86℃,1HNMR(500 MHz,CDCl3)δ:8.32(d,J= 7.8 Hz,2 H),7.71~7.70(m,4 H),7.58~7.57(m,5H),7.47~7.45(m,2H).
N-苯基-1,2,4三氮唑(Ⅲn):黄色液体,1HNMR(500 MHz,CDCl3)δ:8.55(s,1H),8.08(s,1 H),7.65(d,J=7.8 Hz,2 H),7.48(t,J= 8.0 Hz,2H),7.37(t,J=7.4 Hz,1H).
3 结 论
在无有机配体存在下,以CuCl2/Al2O3为催化剂催化一系列的氮杂环和卤代芳烃的C-N偶联反应,普遍得到了较高的产率.而且,CuCl2/Al2O3催化剂具有较好的重复使用性.开发了一种实用的氮杂环和卤代芳烃的C-N偶联反应的新方法.
[1] VISWANATHAN T,ALWORTH W L.Effects of l-arylpyrroles and naphthoflavones upon cytochrome p-450 dependent monooxygenase activities[J].J Med Chem,1981,24(7):822-830.
[2] MEAZZA G,BETTARINI F,PORTA P L,P,et al.Synthesis and herbicidal activity of novel heterocyclic protoporphyrinogen oxidase inhibitors[J].Pest Manag Sci,2004,60(12):1178-1188.
[3] ZHONG Chong-li,HE Jing-tao,XUE Chun-yu,et al.A QSAR study on inhibitory activities of 1-phenylbenzimidazoles against the platelet-derived growth factor receptor[J].Bioorg Med Chem,2004,12:4009-4015.
[4] BELLINA F,ROSSI R.Transition metal-catalyzed direct arylation of substrates with activated sp3-hybridized c-h bonds and some of their synthetic equivalents with aryl halides and pseudohalides[J].Chem Rev,2010,110(2):1082-1146.
[5] THANSANDOTE P,CHONG E,FELDMANN K O,et al. Palladium-catalyzed domino C-C/C-N coupling using a norbornene template:synthesis of substituted benzomorpholines,phenoxazines,and dihydrodibenzoxazepines[J].J Org Chem,2010,75(10):3495-3498.
[6] LUNDGREN R J,SAPPONG-KUMANKUMAH A,STRADIOTTO M A.Highly versatile catalyst system for the crosscoupling of aryl chlorides and amines[J].Chem Eur J,2010,16:1983-1991.
[7] YANG Hai-tao,XI Chao,MIAO Zhi-wei,et al.Cross-coupling reactions of aryl halides with amines,phenols,and thiols catalyzed by an N,N′-dioxide-copper(I)catalytic system[J]. Eur J Org Chem,2011:3353-3360.
[8] SWAPNA K,MURTHY S N,NAGESWAR Y V D.Copper iodide as a recyclable catalyst for buchwald n-arylation[J].Eur J Org Chem,2010,34:6678-6684.
[9] LI Fu-wei,ANDYHOR T S.Facile Synthesis of nitrogen tetradentate ligands and their applications in CuI-catalyzed N-arylation and azide-alkyne cycloaddition[J].Chem Eur J,2009,15:10585-10592.
[10] TANG Bo-xiao,GUO Sheng-mei,ZHANG Man-bo,et al. N-arylations of nitrogen-containing heterocycles with aryl and heteroaryl halides using a copper(I)oxide nanoparticle/1,10-phenanthroline catalytic system[J].Synthesis,2008,2008(11):1707-1716.
[11] MINO T,HARADA Y,SHINDO H,et al.Copper-catalyzed n-arylation of amides and azoles using phosphine-free hydrazone ligands[J].Synlett,2008,2008(4):614-620.
[12] MAHESWARAN H,KRISHNA G G,PRASANTH K L,et al.Bis(μ-iodo)bis((-)-sparteine)dicopper(I):versatile catalyst for direct N-arylation of diverse nitrogen heterocycles with haloarenes[J].Tetrahedron,2008,64:2471-2479.
[13] ALTMAN R A,KOVAL E D,BUCHWALD S L.Coppercatalyzed N-arylation of imidazoles and benzimidazoles[J].J Org Chem,2007,72:6190-6199.
[14] ZHU Liang-bo,CHENG Liang,ZHANG Yu-xi,et al. Highly efficient copper-catalyzed N-arylation of nitrogen-containing heterocycles with aryl and heteroaryl halides[J].J Org Chem,2007,72(16),2737-2743.
[15] HOSSEINZADEH R,TAJBAKHSH M,ALIKARAMI M. Highly efficient copper-catalyzed formation of N-aryl diazoles using KF/Al2O3[J].Synlett,2006,2006(13):2124-2126.
[16] ALTMAN R A,BUCHWALD S L.4,7-Dimethoxy-1,10-phenanthroline:an excellent ligand for the Cu-catalyzed N-arylation of imidazoles[J].Org Lett,2006,8(13):2779-2782.
[17] CRISTAU H J,CELLIER P P,SPINDLER J F,et al. Highly efficient and mild copper-catalyzed N-and C-arylations with aryl bromides and iodides[J].Chem Eur J,2004,10:5607-5622.
[18] CHEN Hua-ming,WANG De-ping,WANG Xian-yang,et al.Mild conditions for copper-catalyzed N-arylation of imidazoles[J].Synthesis,2010(9):1505-1511.
[19] YANG Chu-ting,FU Yao,HUANG Yao-bing,et al.Roomtemperature copper-catalyzed carbon-nitrogen coupling of aryl iodides and bromides promoted by organic ionic bases[J]. Angew Chem Int Ed,2009,48:7398-7401.
[20] CHOW W S,CHAN T H.Microwave-assisted solvent-free N-arylation of imidazole and pyrazole[J].Tetrahedron Lett,2009,50(12):1286-1289.
[21] WANG Hui-feng,LI Ya-ming,SUN Fang-fang,et al.1,2,3,4-Tetrahydro-8-hydroxyquinoline-promoted copper-catalyzed coupling of nitrogen nucleophiles and aryl bromides[J]. J Org Chem,2008,73(21):8639-8642.
[22] ZHU Zhi-qiang,XIANG Shao-ji.CHEN Qing-yun,et al. Novel low-melting salts with donor-acceptor substituents as targets for second-order nonlinear optical applications[J]. Chem Commun,2008,40:5016-5018.
[23] XUE Fei,CAI Cheng-yi,SUN Hong-mei,et al.β-Ketoimine as an efficient ligand for copper-catalyzed N-arylation of nitrogen-containing heterocycles with aryl halides[J].Tetrahedron Lett,2008,49:4386-4389.
[24] CHENG Dong-ping,GAN Feng-feng,QIAN Wei-xing,et al.D-Glucosamine-a natural ligand for the N-arylation of imidazoles with aryl and heteroaryl bromides catalyzed by CuI[J].Green Chem,2008,10:171-173.
[25] GUO Xun,RAO Hong-hua,FU Hua,et al.An inexpensive and efficient copper catalyst for N-arylation of amines,amides and nitrogen-containing heterocycles[J].Adv Synth Catal,2006,348:2197-2202.
[26] RAO Hong-hua,JIN Ying,FU Hua,et al.A versatile and efficient ligand for copper-catalyzed formation of C-N,C-O,and P-C bonds:pyrrolidine-2-phosphonic acid phenyl monoester[J].Chem Eur J,2006,12:3636-3646.
[27] CHEN H H,HUANG Hua-min,CHEN S C,et al.Highly efficient CuI-catalyzed N-arylation of azaheterocycles with aryl iodides using 1,1,1-tris(hydroxymethyl)ethane as a tridentate O-donor ligand:a shorter route to toloxatone and formal synthesis of linezolid[J].J Chin Chem Soc,2010,57(1):14-18.
[28] TAO Chuan-zhou,LIU Wei-wei,SUN Ji-you,et al.3-Acetylcoumarin as a practical ligand for copper-catalyzed C-N coupling reactions at room temperature[J].Synthesis,2010(8):1280-1284.
[29] XI Zhen-xing,LIU Feng-hui,ZHOU Yong-bo,et al.CuI/L(L=pyridine-functionalized 1,3-diketones)catalyzed C-N coupling reactions of aryl halides with NH-containing heterocycles[J].Tetrahedron,2008,64:4254-4259.
[30] HUANG Yi-zheng,GAO Jin,MA Hong,et al.Ninhydrin:an efficient ligand for the Cu-catalyzed N-arylation of nitrogen-containing heterocycles with aryl halides[J].Tetrahedron Lett,2008,49:948-951.
[31] YANG Ming-hua,LIU Fei.An Ullmann coupling of aryl iodides and amines using an air-stable diazaphospholane ligand[J].J Org Chem,2007,72(23):8969-8971.
[32] MA Heng-chang,JIANG Xuan-zhen.N-hydroxyimides as efficient ligands for the copper-catalyzed N-arylation of pyrrole,imidazole,and indole[J].J Org Chem,2007,72,8943-8946.
[33] LU Xin,Bao Wei-liang.Aβ-keto ester as a novel,efficient,and versatile ligand for copper(I)-catalyzed C-N,C-O,and CS coupling reactions[J].J Org Chem,2007,72(23):3863-3867.
[34] DE LANGE B,LAMBERS-VERSTAPPEN M H,SCHMIEDER-VAN L,et al.Aromatic amination of aryl bromides catalysed by copper/β-diketone catalysts:the effectof concentration[J].Synlett,2006(18):3105-3109.
[35] XIE Rui-long,FU Hua,LING Yun.Copper-catalyzed N-arylation of amines with part-per-million catalyst loadings under air at room temperature[J].Chem Commun,2011,47:8976-8978.
[36] MAO Jin-cheng,HUA Qiong-qiong,GUO Jun,et al.Natural alkaloids for copper-catalyzed N-arylation of amines and nitrogen-containing heterocycles[J].Catal Commun,2008,10:341-346.
[37] LIANG Lei,LI Zheng-kai,ZHOU Xiang-ge.Pyridine N-Oxides as ligands in Cu-catalyzed N-arylation of imidazoles in water[J].Org Lett,2009,11(15):3294-3297.
[38] HU Xin-hai,SU Li,HUANG Li-ye,et al.A facile and efficient oxalyldihydrazide/ketone-promoted copper-catalyzed amination of aryl halides in water[J].Eur J Org Chem,2009,5:635-642.
[39] HUANG He,YAN Xiu-hua,ZHU Wei-liang,et al.Efficient copper-promoted N-arylations of aryl halides with amines[J].J Comb Chem,2008,10(5):617-619.
[40] MAO Jin-cheng,GUO Jun,SONG Hong-liang.Copper-catalyzed amination of aryl halides with nitrogen-containing heterocycle using hippuric acid as the new ligand[J].Tetrahedron,2008,64:1383-1387.
[41] XU Zhong-lin,LI Hong-xi,REN Zhi-gang,et al.Cu(OAc)2.H2O-catalyzed N-arylation of nitrogen-containing heterocycles Tetrahedron[J].2011,67(29),5282-5288.
[42] WU Xiang-mei,WANG Yan.N-Arylation of azaheterocycles with aryl and heteroaryl halides catalyzed by iminodiacetic acid resin-chelated copper complex[J].Chin Chem Lett,2010,21:51-54.
[43] LIKHAR P R,ROY S,ROY M,et al.Silica immobilized copper complexes:Efficient and reusable catalysts for N-arylation of N(H)-heterocycles and benzyl amines with aryl halides and arylboronic acids[J].J Mol Catal A:Chem,2007,271:57-62.
[44] KANTAM M L,RAMANI T,CHAKRAPANI L.N-arylation of heterocycles with chloro-and fluoroarenes using resinsupported sulfonato-Cu(salen)complex[J].Synth Commun,2008,38:626-636.
[45] NANDURKAR N S,BHANUSHALI M J,BHOR M D,et al.N-Arylation of aliphatic,aromatic and heteroaromatic amines catalyzed by copper bis(2,2,6,6-tetramethyl-3,5-heptanedionate)[J].Tetrahedron Lett,2007,48:6573-6576.
[46] KANTAM M L,VENKANNA G T,SRIDHAR C,et al. Copper fluorapatite catalyzed N-arylation of heterocycles with bromo and iodoarenes[J].Tetrahedron Lett,2006,47:3897-3899.
[47] KIM A Y,LEE H J,PARK J C,et al.Highly efficient and reusable copper-catalyzed N-arylation of nitrogen-containing heterocycles with aryl halides[J].Molecules,2009,14(12):5169-5178.
[48] KANTAM M L,RAO B P C,CHOUDARY B M,et al.A mild and efficient method for N-arylation of nitrogen heterocycles with aryl halides catalyzed by Cu(Ⅱ)-Na Y zeolite[J]. Synlett,2006(14):2195-2198.
[49] ROUT L,JAMMI S,PUNNIYAMURTHY T.An efficient,inexpensive,and shelf-stable diazotransfer reagent:imidazole-1-sulfonyl azide hydrochloride[J].Org Lett,2007,9(19):3397-3399.
[50] BHADRA S,SREEDHAR B,RANU B C.Recyclable heterogeneous supported copper-catalyzed coupling of thiols with aryl halides:base-controlled differential arylthiolation of bromoiodobenzenes[J].Adv Synth Catal,2009,351(14):2369-2378.
[51] BHADRA S,SAHA A,RANU B C.Al2O3-supported Cucatalyzed electrophilic substitution by PhSeBr in organoboranes,organosilanes,and organostannanes:a protocol for the synthesis of unsymmetrical diaryl and alkyl aryl selenides[J].J Org Chem,2010,75(14):4864-4867.
(责任编辑:陈石平)
An efficient method for N-arylation of nitrogen-containing heterocycles catalyzed by CuCl2/Al2O3
SHI Jie-hua1,2,WANG Jiong-jie1
(1.College of Pharmaceutical Sciences,Zhejiang University of Technology,Hangzhou 310032,China;2.State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology,Zhejiang University of Technology,Hangzhou 310032,China)
An efficient method for N-arylation of nitrogen-containing heterocycles with aryl halides has been developed using CuCl2/Al2O3as an efficient catalyst to prepare a variety of N-arylheterocycles.The experimental results showed that the CuCl2/Al2O3catalyst has good catalytic activity and can be reused without any added organic ligand.The coupling reaction of iodobenzene with imidazole was used as the model reaction to optimize the reaction conditions,and the influences of molar ratio of reactants,catalyst,base,solvent,temperature and time were investigated.Under the selected reaction conditions,the yield for the preparation of N-phenyl imidazole is 99%.And,the selected catalytic reaction system can be applied in synthesis of a variety of N-arylheterocycles.
CuCl2/Al2O3;nitrogen hetercycles;aryl halides;N-arylation
O643.32+2
A
1006-4303(2013)02-0126-07
2012-03-07
施介华(1961-),男,浙江杭州人,教授,博士,主要从事药物合成和药物分析研究,E-mail:shijh@zjut.edu.cn.

