Adsorption and Desorption Behavior of Tannic Acid in Aqueous Solution on Polyaniline Adsorbent*
WANG Jiahong (王家宏), JI Yanfen (吉艳芬), DING Shaolan (丁绍兰), MA Hongrui (马宏瑞)and HAN Xiaojing (韩晓晶)
1College of Resource and Environment, Shaanxi University of Science & Technology, Xi’an 710021, China
2Key Laboratory of Auxiliary Chemistry and Technology for Chemical Industry, Ministry of Education, College of Chemistry and Chemical Engineering, Shaanxi University of Science & Technology, Xi’an 710021, China
Adsorption and Desorption Behavior of Tannic Acid in Aqueous Solution on Polyaniline Adsorbent*
WANG Jiahong (王家宏)1,2,**, JI Yanfen (吉艳芬)1, DING Shaolan (丁绍兰)1, MA Hongrui (马宏瑞)1and HAN Xiaojing (韩晓晶)2
1College of Resource and Environment, Shaanxi University of Science & Technology, Xi’an 710021, China
2Key Laboratory of Auxiliary Chemistry and Technology for Chemical Industry, Ministry of Education, College of Chemistry and Chemical Engineering, Shaanxi University of Science & Technology, Xi’an 710021, China
Tannic acid is generally considered as one of polyphenolic pollutants, which may cause severe threats to the environment. In this study, polyaniline adsorbent was synthesized by chemical oxidation to remove tannic acid in aqueous solutions. The adsorption amount of tannic acid varied greatly with pH of solution and strong adsorption was at pH 5.8-6.7. Coexisting cations, such as Na+, K+, and Ca2+, can enhance the adsorption of tannic acid on polyaniline, which may be contributed to the electrostatic interaction between tannic acid and polyaniline. The adsorption process could be well described by Langmuir model and the maximum adsorption capacity was 117.65 mg·g−1at 35 °C and pH 6.0. The thermodynamic parameters calculated from the adsorption isotherms indicate that the adsorption of tannic acid is spontaneous and endothermic process. The polyaniline saturated with tannic acid can be desorbed in alkaline solution and regenerated adsorbent can be used repeatedly with high adsorption capacity, which implies that polyaniline adsorbents have a great potential in water purification for the removal of tannic acid.
polyaniline, adsorption, desorption, tannic acid
1 INTRODUCTION
Tannic acid is one of phytic substances, from the decomposition of plant biomass. Tannic acid is present in most surface and ground water, which is also identified in industrial wastewater streams from coir and cork factories, paper and pulp board mills, tanneries, etc [1]. As a water soluble polyphenolic compound, tannic acid has been found to be toxic to the aquatic organism such as algae, fish and invertebrates [2, 3]. Moreover, tannic acid is a natural dissolved organic matter, which may react with chlorine disinfectants and form carcinogenic disinfection by-products during drinking water production [4, 5]. Hence, it is necessary to develop effective methods to minimize tannic acid in drinking water or other process waters.
Adsorption treatment is usually considered as an effective approach to remove polyphenolic compounds from aqueous solutions because of its simplicity and high efficiency. For example, it is found that activated carbon, clay, resin, metal-oxide and chitosan remove tannic acid from aqueous solutions effectively [6-12]. Among them, amino-adsorbents, such as aminated chitosan and polymeric resin, show high adsorption capacity, in which amino groups on the adsorbent matrix may play an important role on adsorption of tannic acid [11-13].
Polyaniline and its composites as adsorbents show strong affinity for organic and inorganic pollutants in aqueous solutions because of its large amount of amine and imine nitrogen. For example, heavy metals such as mercury, chromium, and arsenate can be efficiently removed from aqueous solutions by complexation, ion exchange and reduction process [14-19], and a few studies have been conducted for the removal of organic pollutants [20, 21]. However, no effort has been made to remove tannic acid from aqueous solutions by polyaniline and its composites.
In this study, polyaniline adsorbent is synthesized by chemical oxidation. Adsorption and desorption of tannic acid on polyaniline are studied by batch experiments. Effects of pH of solution and ionic strength on the adsorption are also investigated.
2 EX PERIMENTAL
2.1 Materials
Tannic acid of ACS reagent grade was purchased from Sigma Chemical Co. All other reagents of analytical grade were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China.
Polyaniline was prepared by conventional chemical oxidation according to the procedure described by Shimano and MacDiarmid [22]. Briefly, 0.11 mmol aniline was dissolved in 300 ml HCl (1.0 mol·L−1) and 0.11 mmol ammonium peroxodisulphate was dissolved in 200 ml HCl (1.0 mol·L−1). Both solutions were cooled to 273-277 K in an ice bath. Then, ammonium peroxodisulphate solution was added to the aniline solution with continuous stirring in an ice bath. After reaction for 2 h at temperature below 277 K under N2protection, the dark-green precipitate was filteredand washed with copious deionized water until the filtrate became colorless. The resulting material was dried in a vacuum desiccator.
2.2 Characterization of polyaniline
Fourier transform infrared (FTIR) spectrum of polyaniline was recorded on a Nicolet Nexus 870 FTIR spectrometer (Nicolet, USA) with the KBr pellet technique. BET surface area was calculated from N2adsorption-desorption isotherms collected on a Micromeritics ASAP 2200 instrument. The surface zeta potential of polyaniline was measured using Zeta Potential Analyzer (Brookhaven Instruments Co.) [23].
2.3 Adsorption experiment
A series of batch experiments were conducted to determine the adsorption isotherms, adsorption kinetics, and effects of solution pH and ionic strength on adsorption of tannic acid. In the preliminary tests, the adsorption reached equilibrium within 24 h. For determination of adsorption isotherms, 25 mg polyaniline was dispensed in 60 ml polytetrafluoroethylene-lined screw cap glass tubes containing 50 ml tannic acid solution at pH 6.0 and different initial concentrations in the range of 10-100 mg·L−1. The suspensions were mixed in incubator shaker at 15, 25, and 35 °C and shaken at 120 r·min−1for 24 h. After reaching adsorption equilibrium, the adsorbents were separated from solution by filtration and the residual concentrations of tannic acid in the aliquot were measured by a UV-vis spectrophotometer at wavelength 278 nm [10]. The adsorption amount of tannic acid on polyaniline was calculated as follows

where C0(mg·L−1) is the initial concentration of tannic acid, Ce(mg·L−1) is the equilibrium concentration, V (L) is the volume of solution, and W (g) is the mass of polyaniline.
In the experiments of adsorption kinetics, 250 mg polyaniline adsorbent was added into 500 ml flask containing 500 ml tannic acid solution at pH value of 6.0 and initial concentration of 25, 50, and 100 mg·L−1separately, with strongly stirred in an incubator at 25 °C. At time intervals, 4 ml of sample was withdrawn from the flask and filtrated. The residual tannic acid concentration in the solution was determined spectrophotometrically.
To study the effect of solution pH on the adsorption, a series of glass tubes with 25 mg polyaniline and 50 ml of 50 mg·L−1tannic acid solution were pre-adjusted to the desired pH value by 0.1 mol·L−1HCl or NaOH and shaken in an incubator at 120 r·min−1and 25 °C for 24 h. Effect of ionic strength on the adsorption was studied by dispersing 25 mg of adsorbents in 50 ml of NaCl, KCl, or CaCl2solution (2.5-25 mmol·L−1) containing 50 mg·L−1tannic acid at pH 6.0 and 25 °C.
2.4 Desorption and regeneration
To examine the desorption of tannic acid from polyaniline adsorbent, 25 mg polyaniline was placed into 50 ml of 50 mg·L−1tannic acid solution. After the adsorption in an incubator at pH 6.0 and 25 °C for 24 h, the loaded polyaniline was separated centrifugally and regenerated in 50 ml 0.1 mol·L−1NaOH solution for 2 h. Then regenerated polyaniline was separated centrifugally and washed with deionized water for four times to remove desorbed tannic acid. The adsorption and regeneration experiments were conducted for 4 cycles.
3 RESUL TS AND DISCUSSION
3.1 Characterization of adsorbent
BET analysis shows that the surface area of polyaniline is 29.97 m2·g−1. The FTIR spectrum of polyaniline is shown in Fig. 1. The characteristic vibration peaks of CC groups at 1575 and 1496 cm−1are ascribed to the quinine ring and benzene ring of polyaniline, respectively. The other characteristic peaks at 1301, 1245, 1105 and 804 cm−1are assigned to CN stretching vibration connected with benzene ring, CN+·stretching vibration, CN stretching vibration connected with quinine ring, and CH out of plane bending vibration, respectively. The observations suggest that as-synthesized polyaniline is in its emeraldine salt state [14].

Figure 1 FTIR spectrum of polyaniline adsorbent
3.2 Adsorption experiments
3.2.1Effect of solution pH on the adsorption
The effect of solution pH on the adsorption is shown in Fig. 2. The adsorption amount of tannic acid on polyaniline increases with pH in 3.0-5.8, which may be explained by the surface properties of adsorbent. Zeta potential of polyaniline is illustrated in Fig. 3. The isoelectric point (IEP) of polyaniline is about 5.8,so at pH below 5.8 the polyaniline adsorbent carries positive charges due to the protonation of imine and amine groups. When the IEP is about 4.5, tannic acid molecules exist as anions at pH 4.5-5.8, which may invoke the strong electrostatic interaction between positive polyaniline and negative tannic acid and enhance the adsorption of tannic acid [12]. In contrast, at pH below 4.5, tannic acid is in neutral form, and the weak hydrogen bonding interaction may be responsible for the less adsorption. At pH above 6.7, the adsorption amount of tannic acid decreases as solution pH increases due to the repulsive force between negative polyaniline and negative tannic acid. Moreover, higher solution pH improves the solubility of tannic acid, which may decrease its adsorption. The high adsorption amount at pH 5.8-6.7 suggest that in addition to electrostatic interactions, other driving forces such as the hydrogen bonding between tannic acid and uncharged polyaniline chain may contribute to the enhanced adsorption.
3.2.2Effect of ionic strength on the adsorption
Effect of different cations (Na+, K+, Ca2+) on the adsorption of tannic acid on polyaniline is shown in Fig. 4. The presence of Na+, K+, and Ca2+can improve the adsorption, and the adsorption amount increases with ionic concentration of three cations. The adsorption is enhanced in the order of Ca2+>K+≈Na+. It is notable that the adsorption amount increases markedly from 73.41 to 86.15 mg·g−1with Ca2+concentration from 0 to 10 mmol·L−1and gradually becomes constant as Ca2+concentration increases further, because the presence of Ca2+may weaken the repulsive forces between tannic acid molecules in the solution and adsorbed on polyaniline, which may create favorable adsorption sites and enhance the adsorption. Moreover, Ca2+adsorbed on polyaniline may form complex compound with tannic acid [24], which may be another reason for enhancing the adsorption. In addition, an increase in ionic strength decreases the solubility of tannic acid, which favors the transfer of tannic acid molecules from the solution to adsorbent surface, improving their adsorption [8]. The strong dependence of adsorption on ionic strength suggests that electrostatic interaction may play a significant role in the adsorption of tannic acid onto polyaniline [10].
3.2.3Adsorption isotherms
Adsorption isotherms of tannic acid on polyaniline adsorbent at 15, 25, and 35 °C are illustrated in Fig. 5. The adsorption amount increases with the increase of equilibrium concentration of tannic acid in the solution and reaches a plateau. To verify the adsorption mechanism, Langmuir and Freundlich models are used to fit the experimental data. The Langmuir model is given as
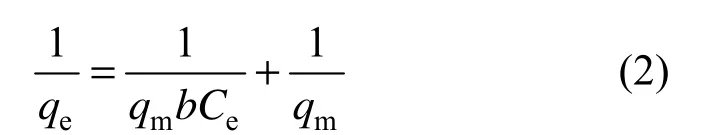
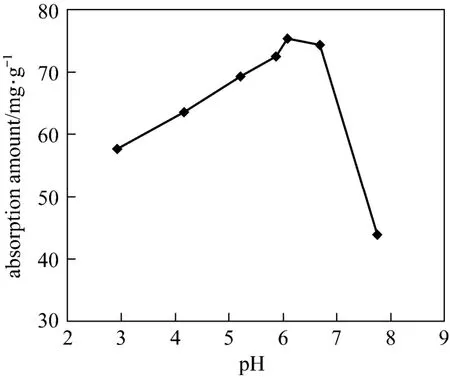
Figure 2 Effect of solution pH on the adsorption of tannic acid on polyaniline

Figure 3 Zeta potential of polyaniline as a function of pH

Figure 4 Effect of ionic strength on adsorption of tannic acid on polyaniline▲ Ca2+; ■ K+; ◆ Na+
and the Freundlich model can be expressed as
where qeis the equilibrium adsorption amount, qmis the theoretical maximum adsorption capacity, Ceis the equilibrium concentration of tannic acid, b is the affinity coefficient, Kfis the Freundlich constant, and 1/n is the heterogeneity factor.
Fitted parameters of adsorption isotherms by Langmuir and Freundlich models at 15, 25, and 35 °Care listed in Table 1. All the correlation factors (R2) with Langmuir model are larger than those with Freundlich model, suggesting that Langmuir model is preferable to Freundlich model.

Figure 5 Adsorption isotherms of tannic acid on polyaniline■ 35 °C; ◆ 25 °C; ▲ 15 °C

Table 1 Fitted parameters of adsorption isotherm by Freundlich and Langmuir model
Separation factor is the characteristic parameter of Langmuir isotherm, which is defined as

where C0is the initial concentration of tannic acid and b is the Langmuir isotherm constant [25]. The adsorption process is favorable at RL<1 and unfavorable at RL>1. The calculated RLvalues between 0.088 and 0.0096 at the initial concentration of 10-100 mg·L−1and 25 °C indicate that the adsorption of tannic acid on polyaniline is favorable.
Thermodynamic parameters for adsorption of tannic acid on polyaniline adsorbent, such as the change in standard free energy ΔGӨ, enthalpy ΔHӨ, and entropy ΔSӨ, can be obtained from adsorption isotherms with following equations

where R is the gas constant, T is the adsorption temperature, Kcis the equilibrium constant, and CAeand CSeare the equilibrium concentrations adsorbed on the adsorbent and in the solution, respectively.
The thermodynamic parameters for adsorption of tannic acid on polyaniline at different concentrations are calculated and illustrated in Table 2. The negative free energy values of ΔGӨreveal that the adsorption of tannic acid on polyaniline is spontaneous and thermodynamically favorable at tested temperatures. The free energy is more negative at higher temperature at all concentrations tested, suggesting that the spontaneity of adsorption process increases with temperature [26]. The positive value of enthalpy change ΔHӨmanifests that the adsorption is endothermic. In addition, the positive value of entropy ΔSӨshows the strong affinity of tannic acid molecules on polyaniline surface.

Table 2 Thermodynamic parameters for adsorption of tannic acid on polyaniline adsorbent
3.2.4Adsorption kinetics
The adsorption kinetics of tannic acid on polyaniline is shown in Fig. 6. The adsorption of tannic acid is quick in the first 2 h and reaches equilibrium within 6 h. To describe experimental data, the pseudofirst-order and pseudo-second-order models are used. The pseudo-first-order kinetics model is given as

The pseudo-second-order kinetics model is expressed as


Figure 6 Adsorption kinetics of tannic acid on polyaniline at different initial concentrations▲ 100 mg·L−1; ■ 50 mg·L−1; ◆ 25 mg·L−1

Table 3 Fitted parameters for adsorption of tannic acid on polyaniline
where qeis the equilibrium adsorption amount, qtis the adsorption amount at time t, k1and k2are pseudofirst-order and pseudo-second-order rate constants, respectively.
The fitted parameters based on the two models are tabulated in Table 3. With the pseudo-first-order kinetic model, the correlation coefficient (R2) is relatively low and the calculated equilibrium amount is not in agreement with the experimental data, so the model is not appropriate. With the pseudo-second-order kinetics, the correlation coefficient is satisfactory (R2>0.99) and the calculated equilibrium adsorption amount is almost identical to the experimental data, suggesting that the adsorption of tannic acid on polyaniline obeys the pseudo-second-order kinetics. In addition, the rate constants of adsorption at initial concentration of 25, 50, and 100 mg·L−1are 5.59×10−3, 2.08×10−3, and 1.12×10−3g·mg−1·min−1, respectively, indicating more rapid uptake rate at lower initial concentration of tannic acid, probably because tannic acid molecules in the solution can easily find available amino or imine groups of polyaniline and attach instantly to the adsorbent surface at lower initial concentrations. However, at higher initial concentrations, a larger number of tannic acid molecules are adsorbed on the surface of polyaniline and occupy most of active adsorption sites, so that tannic acid molecules to be further adsorbed must overcome the electrostatic repulsion with those in the solution and find available adsorption sites on the surface, leading to lower adsorption rate.
3.3 Desorption and regeneration
The suppressed adsorption amount of tannic acid on polyaniline at higher pH implies that the loaded polyaniline can be desorbed in alkaline solutions. The adsorption amount of tannic acid with regenerated adsorbent is shown in Fig. 7. The adsorption amount decreases by 12.1% and 21.7% in the first and second adsorption-regeneration cycle, respectively, but does not change obviously in the next two cycles, indicating that the regenerated polyaniline possesses relatively high adsorption capacity and can be recycled in removal of tannic acid in aqueous solutions.
4 CONCLU SIONS
The polyaniline adsorbent prepared by chemical oxidation is in its emeraldine salt state. Polyaniline adsorbents show high adsorption capacity for tannic acid in its aqueous solutions. The adsorption of tannic acid is highly dependent on solution pH and the maximum adsorption amount is at pH 5.8-6.7. Coexisting cations such as Na+, K+, and Ca2+may improve the electrostatic force between tannic acid and polyaniline and increase the adsorption of tannic acid onto polyaniline. The loaded polyaniline can be easily desorbed by NaOH solutions and the regenerated adsorbent possesses relatively high adsorption capacity. The results indicate that polyaniline may be used as an adsorbent in removal of tannic acid from water and wastewater.

Figure 7 Adsorption amount of tannic acid on virgin and regenerated p olyaniline in fou r s uccessive ad sorption-regeneration cycles
REFERENCES
1 Buso, A., Balbo, L., Giomo, M., Farnia, G., Sandona, G., “Electrochemical removal of tannins from aqueous solutions”, Ind. Eng. Chem. Res.,39(2), 494-499 (2000).
2 Lowry, J., McSweeney, C., Palmer, B., “Changing perceptions of the effect of plant phenolics on nutrient supply in the ruminant”, Aust. J. Agric. Res.,47(6), 829-842 (1996).
3 Liao, X.P., Lu, Z.B., Shi, B., “Selective adsorption of vegetable tannins onto collagen fibers”, Ind. Eng. Chem. Res.,42(14), 3397-3402 (2003).
4 Lu, Z.Y., Jiang, B.C., Li, A.M., Wang, X., Ma, Y.F., Sheng, X.P.,“Competitive adsorption of tannic acid and phenol onto a bi-functional polymeric adsorbent”, Acta. Chim. Sinica,68(5), 437-442 (2010).
5 Lin, Y.L., Chiang, P.C., Chang, E.E., “Removal of small trihalomethane precursors from aqueous solution by nanofiltration”, J. Hazard. Mater.,146(1-2), 20-29 (2007).
6 Rivera-Utrilla, J., Moreno-Castilla, C., Utrera-Hidalgo, E., Carrasco-Marin, F., “Removal of tannic acid from aqueous solutions by activated carbons”, Chem. Eng. J.,52(1), 37-39 (1993).
7 Sarici-Ozdemirnal, C., Onal, Y., “Equilibrium, kinetic and thermodynamic adsorptions of the environmental pollutant tannic acid onto activated carbon”, Desalination, 251 (1-3), 146-152 (2010).
8 Vinod, V., Anirudhan, T., “Sorption of tannic acid on zirconium pillared clay”, J. Chem. Technol. Biotechnol., 77 (1), 92-101 (2002).
9 Wang, J.N., Li, A.M., Xu, L., Zhou, Y., “Adsorption of tannic and gallic acids on a new polymeric adsorbent and the effect of Cu (II) on their removal”, J. Hazard. Mater., 169 (1-3), 794-800 (2009).
10 Anirudhan, T.S., Ramachandran, M., “Adsorptive removal of tannin from aqueous solutions by cationic surfactant-modified bentonite clay”, J. Colloid Interf. Sci., 299 (1), 116-124 (2006).
11 Chang, M.Y., Juang, R.S., “Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay”, J. Colloid Interf. Sci., 278 (1), 18-25 (2004).
12 An, J.H., Dultz, S., “Adsorption of tannic acid on chitosan-montmorillonite as a function of pH and surface charge properties”, Appl. Clay Sci., 36 (4), 256-264 (2007).
13 Sun, Y., Li, A.M., Zhang, Q.X., Chen, J.L., Fu, D.F., Wang, S.H.,“Adsorptive separation of tannic acid from aqueous solution by polymeric resins”, Sep. Sci. Technol., 43 (2), 389-402 (2008).
14 Wang, J., Deng, B.L., Chen, H., Wang, X.R., Zheng, J.Z., “Removal of aqueous Hg(II) by polyaniline: sorption characteristics and mechanisms”, Environ. Sci. Technol., 43 (14), 5223-5228 (2009).
15 Zhang, Y., Li, Q., Sun, L., Tang, R., Zhai, J.P., “High efficient removal of mercury from aqueous solution by polyaniline/humic acid nanocomposite”, J. Hazard. Mater., 175 (1-3), 404-409 (2010).
16 Ansari, R., “Application of polyaniline and its composites for adsorption/recovery of chromium (VI) from aqueous solutions”, Acta Chim. Slov., 53, 88-94 (2006).
17 Gupta, R.K., Dubey, S.S., “Removal of cesium Ions from aqueous solution by polyaniline: a radiotracer study”, J. Polym. Res., 12 (1), 31-35 (2005).
18 Ruotolo, L., Gubulin, J.C., “Chromium (VI) reduction using conducting polymer films”, React. Funct. Polym., 62 (2), 141-151 (2005).
19 Zhang, Y., Li, Q., Tang, R., Hu, Q.C., Sun, L., Zhai, J.P., “Electrocatalytic reduction of chromium by poly (aniline-co-o-aminophenol): An efficient and recyclable way to remove Cr (VI) in wastewater”, Appl. Catal. B: Environ., 92 (3-4), 351-356 (2009).
20 Mahanta, D., Madras, G., Radhakrishnan, S., Patil, S., “Adsorption of sulfonated dyes by polyaniline emeraldine salt and its kinetics”, J. Phys. Chem. B., 112 (33), 10153-10157 (2008).
21 Anbia, M., Ghaffari, A., “Adsorption of phenolic compounds from aqueous solutions using carbon nanoporous adsorbent coated with polymer”, Appl. Surf. Sci., 255 (23), 9487-9492 (2009).
22 Shimano, J.Y., MacDiarmid, A.G., “Polyaniline, a dynamic block copolymer: key to attaining its intrinsic conductivity”, Synth. Met., 123 (2), 251-262 (2001).
23 Wang, J.H., Zheng, S.R., Shao, Y., Liu, J.L., Xu, Z.Y., Zhu, D.Q.,“Amino-functionalized Fe3O4@SiO2core-shell magnetic nanomaterial as novel adsorbent for aqueous heavy metals removal”, J. Colloid Interf. Sci., 349 (1), 293-299 (2010).
24 Ücer, A., Uyanik, A., Cay, S., Ozkan, Y., “Immobilisation of tannic acid onto activated carbon to improve Fe (III) adsorption”, Sep. Purif. Technol., 44 (1), 11-17 (2005).
25 Hall, K., Eagleton, L., Acrivos, A., Vermeulen, T., “Pore-and soliddiffusion kinetics in fixed-bed adsorption under constant-pattern conditions”, Ind. Eng. Chem. Fund., 5 (2), 212-223 (1996).
26 Liu, F.L., Wang, J.H., Li, L.Y., Shao, Y., Xu, Z.Y., Zheng, S.R.,“Adsorption of direct Yellow 12 onto ordered mesoporous carbon and activated carbon”, J. Chem. Eng. Data, 54 (11), 3043-3050 (2009).
2012-02-09, accepted 2012-10-06.
* Supported by the National Major Research Plan for Water Pollution Control and Treatment of China (2008ZX07010-003-002), the National Natural Science Foundation of China (21107065) and the Scientific Research Program Funded by Shaanxi Provincial Education Department (HJK0769).
** To whom correspondence should be addressed. E-mail: wangjiahong@sust.edu.cn
 Chinese Journal of Chemical Engineering2013年6期
Chinese Journal of Chemical Engineering2013年6期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Hydrogen Reduction of Silver Ions on the Performance and Structure of New Solid Polymer Electrolyte PEI/Pebax2533/AgBF4Composite Membranes*
- Separation of Recombinant Geranylgeranyl Diphosphate Synthase of Deinococcus radiodurans from Expressed Strain Cell Homogenate by Immobilized Metal Affinity Chromatography on a Characterized Monolithic Cryogel Column*
- Immobilization of Papain in Biosilica Matrix and Its Catalytic Property*
- Comparison on Thermal Conductivity and Permeability of Granular and Consolidated Activated Carbon for Refrigeration*
- Supercritical Fluid Extraction of a Novel Template from Mesoporous Zirconia and the Effect on Porous Structure*
- Synthesis of 2-Methyl-4-methoxyaniline from o-Nitrotoluene Using Pt/C and Acidic Ionic Liquid as Catalyst System*
