Preparation and Catalytic Performance of Potassium Titanate Used as Soot Oxidation Catalyst
Gao Yuan; Meng Xiuhong; Pan Lihong; Song Lijuan; Duan Linhai
(1.Key Laboratory of Petrochemical Catalytic Science and Technology, Liaoning Shihua University, Fushun, Liaoning 113001; 2. Shandong Tianhong New Energy Chemical Co. Ltd.)
Preparation and Catalytic Performance of Potassium Titanate Used as Soot Oxidation Catalyst
Gao Yuan1,2; Meng Xiuhong1; Pan Lihong1; Song Lijuan1; Duan Linhai1
(1.Key Laboratory of Petrochemical Catalytic Science and Technology, Liaoning Shihua University, Fushun, Liaoning 113001; 2. Shandong Tianhong New Energy Chemical Co. Ltd.)
To prepare potassium titanate catalyst, a novel citrate acid complex-combustion method using CH3COOK and Ti(OC4H9)4as raw materials was developed. The crystalline phase and surface morphology of K2Ti2O5were investigated by X-ray diffraction (XRD), field emission scanning electron microscope (FE-SEM) and transmission electron microscope (TEM). The impact of some factors, such as the type of contact between K2Ti2O5and soot, the content of water vapor and SO2in exhaust, and the repeated use on catalytic activity of K2Ti2O5were studied by temperature programmed reaction (TPR). A comparison between the new method and the reported ones on catalytic activity of potassium titanate was investigated. The results showed that K2Ti2O5had high catalytic activity and good stability.
citrate acid complex-combustion method; diesel exhaust; potassium titanate; soot oxidation
1 Introduction
Over the past decade, diesel engine is becoming increasingly widely used since its invention for its high fuel economy, long lifetime and low annual maintenance costs. However, the emission of various pollutants in the exhaust gases leads to severe atmospheric pollution and climate change[1-2]. In order to control the emission of particulate matter, the most feasible way is a combination of traps and oxidation catalysts[3-4](using diesel particulate filter combined with diesel oxidation catalysts). The catalyst equipped in the traps should lower the soot ignition temperature for noncatalytic combustion (about 600 ℃) down to the temperature range characteristic of diesel exhaust gases (180—350 ℃) to enable self-recovery of traps. Till now, platinum-based catalysts[5-6]seem to represent the best catalytic activity, which allows soot oxidation at a quite low temperature, viz. the ignition temperature at 247 ℃[7]. However, the high catalyst cost and its sensitivity to SO2have restricted its application.
The perovskite-type oxides[8-10]and CeO2based oxides[11-13]have been proved to possess certain soot oxidation activities, but the oxidation temperature is still not low enough for practical application. Molten salt catalysts, especially potassium salts due to their high fluidity, have promising catalytic performance for soot combustion under realistic conditions[14]through enhancing their efficiency on contact with the soot. Up to now, extensive investigations on potassium-promoted catalysts for soot combustion have been performed[15-17].
In this study, potassium titanate catalyst K2Ti2O5was successfully prepared via the citrate acid complex-combustion method using CH3COOK and Ti(OC4H9)4as starting materials. The crystalline phase and surface morphology of K2Ti2O5were investigated by X-ray diffraction (XRD), field emission scanning electron microscope (FE-SEM) and transmission electron microscope (TEM). Its catalytic activity on soot oxidation was evaluated in a temperatureprogrammed oxidation (TPO) equipment (consisting of a packed-bed made of quartz tube) in terms ofTig(beginning of the carbon black oxidation) andTm(peak-top temperature) together. The influence of some factors, such as the type of contact between K2Ti2O5and soot, the content of water vapor and SO2in exhaust, and the repeated use of K2Ti2O5on its catalytic activity was also investigated. The results showed that K2Ti2O5prepared by the citrate acid complex-combustion method exhibited high activity andbetter stability than that prepared by the sol-gel method or the solid-phase method.
2 Experimental
To obtain the potassium precursor, potassium nitrate and citric acid (CA) at a molar ratio of 2:1 were added into deionized water under vigorous stirring. To obtain the titanium precursor, a 50% citric acid solution was pre-adjusted to a pH value of 7.0 with ammonia water, to which Ti(OC4H9)4was added under constant stirring. Flake precipitate occurred first and then was dissolved gradually. The solution was divided into upper and lower layers on standing, with the upper layer composed of butanol and lower layer-citric titanium solution.
Titanium precursor solution was added into potassium citrate to reach a K/Ti molar ratio of 2:1, to which ammonia water was added to make the solution slightly alkaline. The mixture was dried at 100 ℃ overnight followed by calcination in air.
2.1 Catalyst characterization
X-ray diffraction patterns were obtained with an X-ray analyzer (type M18XHF, made by Mac Science Co., Japan). Cu Kα radiation (λ=0.154 15 nm) was used with an X-ray gun operated at 40 kV and 100 mA. Diffraction patterns were obtained within the range of 2θ=10°—80° with a step size of 0.02º and a scanning velocity of 4°/min. The morphology of the catalysts was observed by FESEM (type S-4200, made by Hitachi, Japan) and TEM (type JEM-2000EX, made by Hitachi, Japan).
2.2 PM oxidation activity tests
All activity tests of K2Ti2O5on catalytic oxidation of soot were evaluated in a fixed-bed quartz reactor (10 mm in I. D.) using a TPO system in the temperature range of 373 K to 723 K (at a heating rate of 2 K/min). The activity of the catalyst is represented byTigandTmtogether. The soot used was Printex-U supplied by Degussa and the simulated diesel exhaust consisting of 10% O2in Ne gas passed through the reactor at a flow rate of 100 ml/min. The catalyst-soot mixture at a weight ratio of 9:1 was ground in an agate mortar for 30 minutes to simulate a mode of ‘tight contact’, while upon mixing the mixture with a spatula a mode of gently ‘loose contact’ was simulated. The outlet gas composition was automatically injected into and analyzed by an online gas chromatograph (modified type of Beifen GC-3420) equipped with a thermal conductivity detector (TCD) and a column Porapak Q for separating CO2and O2.
3 Results and Discussion
3.1 The effect of aging
Figure 1 gives the XRD patterns of aged catalyst as well as the non-aged sample, the doping of K2Ti2O5·xH2O can be reflected judging from the XRD patterns of non-aged K2Ti2O5via comparison with a single crystal phase after aging. It can be seen from Figure 1 that with respect to K2Ti2O5prepared by citrate acid complex-combustion method, the aging process could gradually eliminate water in the reactant and make the crystal phase regular and single. It can be seen from Figure 2 that water in K2Ti2O5·xH2O could not be completely removed even under a sintering temperature of 700 ℃, which could al-ter the structure of K2Ti2O5to affect its catalytic activity. Therefore, the aging process is critical for the catalyst preparation method.
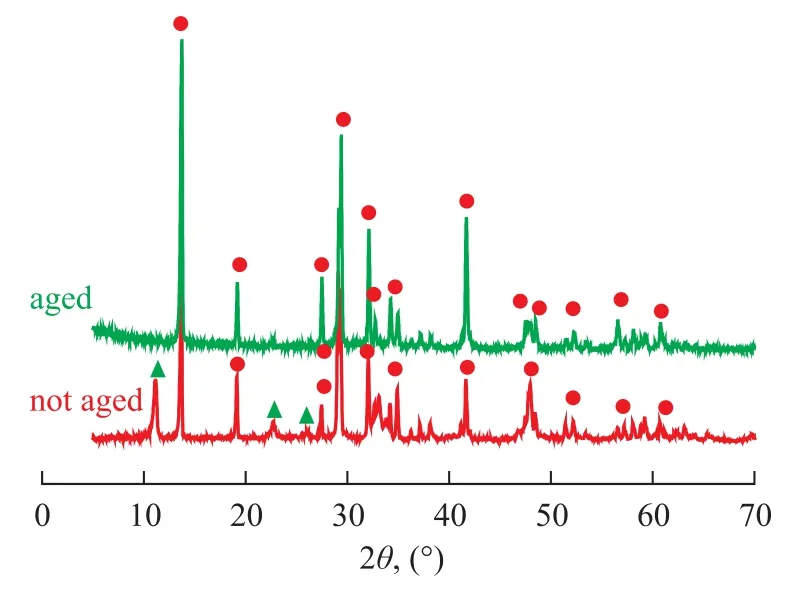
Figure 1 X-ray patterns of K2Ti2O5prepared by aging for 24 hours (a) and K2Ti2O5sample without aging (b)
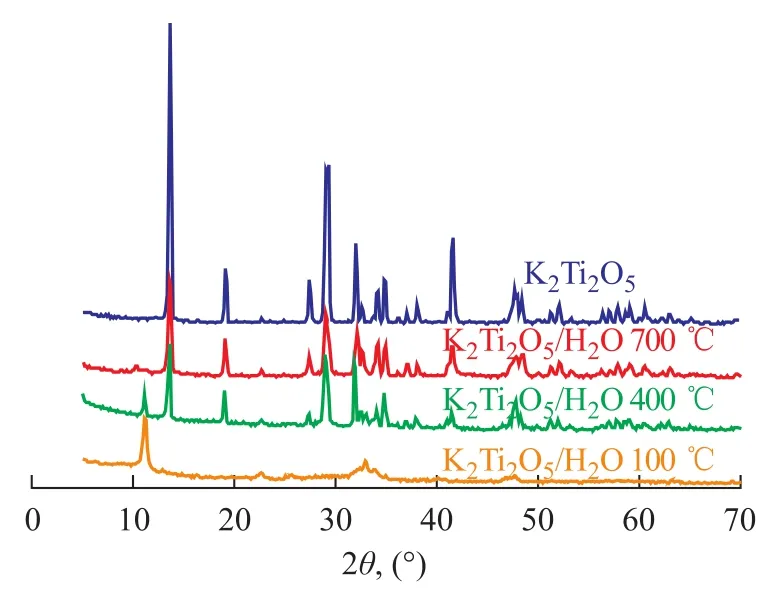
Figure 2 XRD patterns of water-dissolved K2Ti2O5at 100—700
3.2 Structural analysis
Figure 3 presents the SEM and TEM images of K2Ti2O5, from which we can see both the inner and outer structure of K2Ti2O5are regular and dense, with the grain size mostly concentrated in the range of 20—40 μm.
Figure 4 shows the idealized crystal of K2Ti2O5with a layered structure. The potassium ions are located in the middle of the layered structure to form a sandwich-like structure, which can promote the fluidity of potassium ions and the activity of K2Ti2O5in certain reactions.
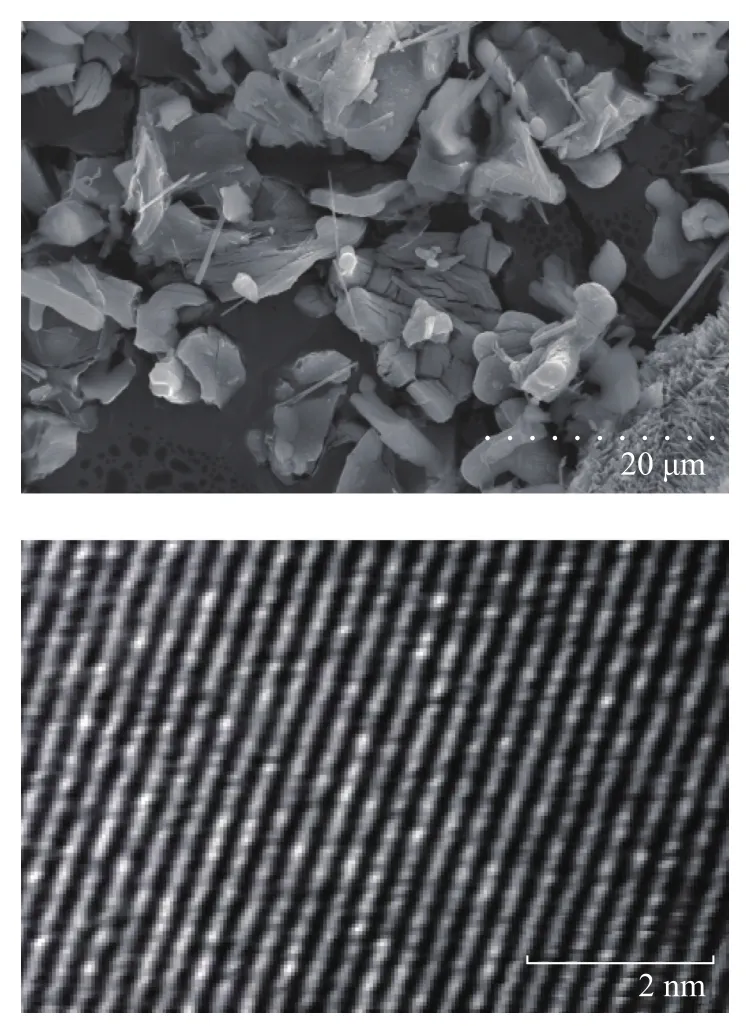
Figure 3 SEM and TEM images of K2Ti2O5
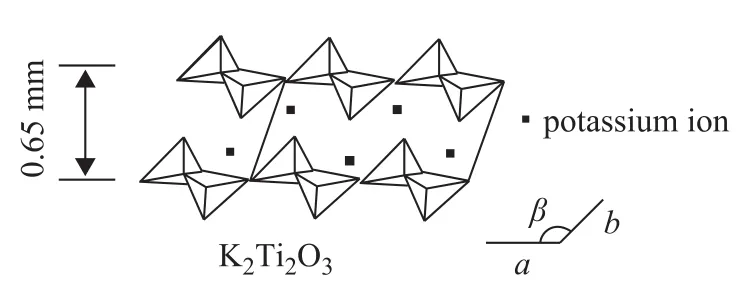
Figure 4 Idealized crystal of K2Ti2O5with a layered structure
3.3 Effect of calcination temperature
Figure 5 shows the XRD patterns of K2Ti2O5calcined under different temperatures. When the sintering temperature is 550 ℃, the characteristic peaks are only observed at 48°, indicating to the absence of K2Ti2O5. When the sample calcination occurred at 600 ℃, all the peaks matched well with the characteristic peaks belonging to K2Ti2O5and the XRD spectra showed small peaks and uneven baseline, suggesting that the mixture did not react fully. When the temperature reached up to 650 ℃, all characteristic narrow peaks with even baseline were identified. The analytical results have revealed that 650 ℃ is the best sintering temperature, since at a lower temperature all the precursors could not react fully to form the expected structure of K2Ti2O5.
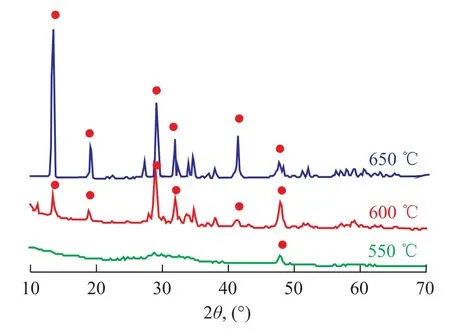
Figure 5 XRD patterns of samples prepared by sintering K-Ti raw materials mixture at 650600, and 550respectively
In previous work performed by our team[20-21], K2Ti2O5with high crystallinity was successfully prepared by the solid-phase method and the sol-gel method. It is found out that the calcination temperature of the samples prepared by these two methods should be 810 ℃ and 850 ℃, respectively. However, the adoption of citric acid complex-combustion method can reduce the calcination temperature down to 650 ℃, resulting in shortening of preparation cycle and energy saving.
3.4 Effect of contact mode
It has already been found out that the contact type between soot and catalyst is one of the major factors that can determine the soot oxidation rate. Loose contact condition is more close to real condition while intimate contact is helpful to reflect the intrinsic characteristics of the catalyst. The soot oxidation activity of the catalyst prepared by two methods (citric acid complex-combustion method and solid-phase method) and both of two contact modes were chosen for comparison, and the TPR profilesare presented in Figure 6.

Figure 6 TPR results for screening test of catalyst samples
It can be easily found out that generally soot combustion occurs at lower temperature under tight contact mode than the case under loose contact mode since its peak temperature moved to lower temperature for soot oxidation, which might be attributed to the better dispersion on the catalyst surface of particulate material (PM) and higher utilization efficiency of specific surface area in the former mode. Additionally, in the tight contact mode K2Ti2O5samples prepared by both of the two methods have nearly the same catalytic activity. But in the loose contact mode, K2Ti2O5samples prepared by the citric acid complex-combustion method demonstrate a better catalytic activity. This case might be related with the fact that catalyst samples prepared by the citric acid complexcombustion method have higher specific area which can enhance the interaction between soot and catalyst. K2Ti2O5samples prepared by the new method are preferable because of their high activity under loose contact with the soot, which is closer to the real condition in the PM filter.
3.5 Effect of active metal component
Figure 7 shows the TPR curves of five kinds of catalysts containing different active metal components, the catalytic activity of catalyst samples decreases in the following order: K2Ti2O5>Na2Ti3O7=Pt/TiO2>Cr2Ti2O7>TiO2. TiO2has low activity, which suggests that the activity of these catalysts is related with active metal components rather than TiO2. K2Ti2O5has a best catalytic activity, which may be related with the sandwich-like structure that can promote the fluidity of potassium ions.

Figure 7 TPR results for screening the catalytic activity of K2Ti2O5, Na2Ti3O7, TiO2, 1%Pt/TiO2, and Cr2Ti2O7
3.6 Effect of SO2and water vapor on exhaust composition
Figure 8 gives the TPR profiles of soot oxidation on K2Ti2O5in the presence of water vapor and sulfur dioxide. It can be seen from Figure 8 that the catalytic effect of K2Ti2O5does not change when the content of SO2is below 10 mg/L. When the SO2content reaches up to 30—60 mg/L, the catalytic activity of K2Ti2O5significantly declines. Since the SO2content in diesel exhaust is 8—16 mg/L under normal conditions, its concentration would not impede the soot oxidation, suggesting that K2Ti2O5is suitable for functioning as a particulate trap. When 10% (by volume) of water vapor was introduced into the exhaust, the poisoning effect of SO2weakens, which may be caused by the presence of water vapor that can enhance the contact between soot and catalyst to further increase the surface utilization rate and thereby reduce the poisoning effect of SO2. For traditional noble metal loaded catalyst, such as Pt/Al2O3, the presence of SO2will lead to a depletion of support materials. K2Ti2O5can be a good candidate for noble metal loaded catalysts thanks to its stability in a SO2-containing atmosphere.
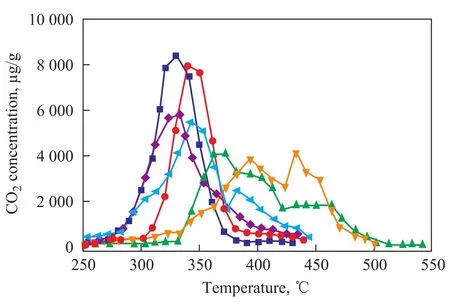
Figure 8 Effect of SO2and water vapour on activity of K2Ti2O5
3.7 Effect of repeated use on activity of K2Ti2O5
The used K2Ti2O5catalyst and another 0.01 g of soot were carefully ground in an agate mortar and placed in a quartz tube reactor again. After the same reaction cycle with the same catalyst bling repeatedly used for three times, its catalytic effect did not change much judging from its fixed perovskite structure, as shown in Figure 9. This result indicates that K2Ti2O5can satisfy the demand for this catalyst in terms of its catalytic stability.
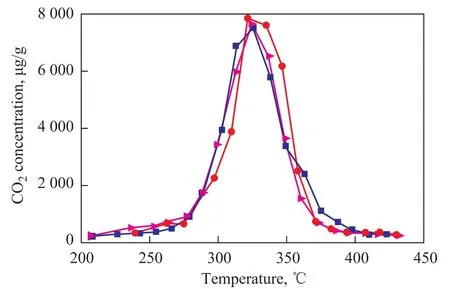
Figure 9 Effect of repeated use on the catalytic activity of K2Ti2O5
4 Conclusions
A new way of citric acid complex-combustion method for preparation of potassium titanate catalyst was developed, by which the catalyst could be obtained with a high crystallinity and large specific surface area. The K2Ti2O5catalyst samples tested under the intimate contact mode has a higher activity than the reported commercialized Ptbased catalysts, the transition-metal-based catalysts and perovskite-structured catalysts, and the K2Ti2O5catalyst assumes a highest activity among active metal loaded TiO2catalysts. Compared with the reported method (solid-phase), the optimum calcination temperature adopted by the citric acid complex-combustion method is lower (650 ℃) and the samples prepared have a better activity in the loose contact mode; K2Ti2O5catalyst also has good stability for soot oxidation in the presence of water vapor and SO2, and this catalyst can be used repeatedly to retain its activity. In summary, the citrate acid complex-combustion method possesses a practical value featuring simple operation, short preparation cycle and readiness for commercialization.
Acknowledgement:The authors are grateful for the financial supports provided for this research by the Education Department of Liaoning Province of China (No.2009T061) and the Ministry of Education of China (No.[2010]1561).
[1] Reichert D, Bockhorn H, Kureti S. Study of the reaction of NOxand soot on Fe2O3catalyst in excess of O2[J]. Applied Catalysis B: Environmental, 2008, 80(3/4): 248-259
此汽车各轮制动力和已达标,因其是前轮左轮制动力偏小,跑偏趋势是向右,后轮是右轮制动力偏小,跑偏趋势是向左,这样,前后轮跑偏趋势互相抵消了。本人认为,如上述超标车,因其制动力小的轮的制动力数据已超过轴重的30%,而制动力差为非同测车轮,应按合格车对待。
[2] Caroca J, Villata G, Fino D, et al. Comparison of different diesel particulate filters[J]. Topics in Catalysis, 2009, 52(13-20): 2076-2082
[3] Raux S, Frober A, Jeudy E. Low temperature activity of euro 4 diesel oxidation catalysts: Comprehensive material analyses and experimental evaluation of a representative panel[J]. Topics in Catalysis, 2009, 52(13-20): 1903-1908
[4] Keita T, Takahiro H, Tsuyoshi T, et al. Mechanical activation of self-propagating high-temperature-synthesized LaFeO3to be used as catalyst for diesel soot oxidation[J]. Catalysis Letters, 2009, 130(3/4): 362-366
[5] Krishna K, Makkee M. Pt-Ce-soot generated from fuelborne catalysts: soot oxidation mechanism[J]. Topics in Catalysis, 2007, 42-43(1-4): 29-236
[6] Oi-Uchishawa J, Wang S D, Nanba T, et al. Improvement of Pt catalyst for soot oxidation using mixed oxide as a support[J]. Appl Catalysis B, 2003, 44(3): 207-215
[7] Oi-Uchisawa J, Akira Obuchi, Ryuji Enomoto, et al. Oxidation of carbon black over various Pt/MOx/SiC catalysts[J].Applied Catalysis B: Environmental, 2001, 32(4): 257-268
[8] Peng Xiaosheng, Lin He, Shangguan Wenfeng, et al. A highly efficient and porous catalyst for simultaneous removal of NOxand diesel soot[J]. Catalysis Communications, 2007, 8(2):157-161
[9] Zhu Ling, Wang Xuezhong, Liang Cunzhen. Catalytic combustion of diesel soot over K2NiF4-type oxides La2-xKxCuO4[J]. Journal of Rare Earths, 2008, 26(2): 254-257
[11] Atribak I, Bueno-Lopez A, Garcta-Garcfa A. Thermally stable ceria-zirconia catalysts for soot oxidation by O2[J]. Catalysis Communications, 2008, 9(2): 250-255
[12] Krishna K, Bueno-Lo´pez A, Makkee M, et al. Potential rare earth modified CeO2catalysts for soot oxidation I. Characterisation and catalytic activity with O2[J]. Applied Catalysis B: Environmental, 2007, 75(3/4): 189-200
[13] May I, Corinne P, Alain B, et al. Oxidation of carbon by CeO2: Effect of the contact between carbon and catalyst particles[J]. Fuel, 2008, 87(6): 740-750
[14] Neeft J P A, Makkee M, Moulijn J A. Catalysts for the oxidation of soot from diesel exhaust gases. I. An exploratory study[J] . Applied Catalysis B: Environmental, 1996, 8(1): 57-78
[15] Zhang Z L, Mou Z G, Yu P F, et al. Diesel soot combustion on potassium promoted hydrotalcite-based mixed oxide catalysts[J]. Catalysis Communications, 2007, 8(11): 1621-1624
[16] An H, Kilroy C, McGinn P J. Combinatorial synthesis and characterization of alkali metal doped oxides for diesel soot combustion[J]. Catalysis Today, 2004, 98(3): 423-429
[17] Zhang Y H, Zou X T. The catalytic activities and thermal stabilities of Li/Na/K carbonates for diesel soot oxidation[J]. Catalysis Communications, 2007, 8(5): 760-764
[18] Lee Dae-Won, Song Seung-Jin, Lee Kwan-Young. Reduction of lean NO2with diesel PM over metal-exchanged ZSM-5, perovskite and γ-alumina catalysts[J]. Korean J Chem Eng, 2010, 27(2): 452-458
[19] Milt V G, Banu′s E D, Ulla M A, et al. PM combustion and NOx adsorption on Co, Ba, K/ZrO2[J]. Catalysis Today, 2008, 133-135: 435-440
[20] Yin G S, Meng X H, Liu L L, et al. Application of K2Ti2O5in removing soot from diesel engine exhaust[J]. Petrochemical Technology & Application, 2011, 29(6): 498-501 (in Chinese)
[21] Meng X H, Gao Y, Liu L L, et al. Catalytic oxidation of soot in exhaust of diesel engine using K2Ti2O5[J]. Journal of Petrochemical Universities, 2011, 24(6): 63-67 (in Chinese)
Recieved date: 2012-12-27; Accepted date: 2013-02-05.
Professor Duan Linhai, Telephone: +86-24-56860757; E-mail: lh.duan@126.com.
- 中国炼油与石油化工的其它文章
- Influence of Carbon Content on S Zorb Sorbent Activity
- Effects of Fatty Acids on Low-Sulfur Diesel Lubricity: Experimental Investigation, DFT Calculation and MD Simulation
- Research on Catalytic Properties of Palladium Catalyst Prepared by Biological Reduction Method
- Propylene Polymerization Catalysts with Sulfonyl Amines as Internal Electron Donors
- Isolation and Characterization of a Thermophilic Oil-Degrading Bacterial Consortium
- A Probe into Process for Maximization of Low-carbon Olefins via Co-processing of Methanol and Heavy Oil

