石墨烯-金纳米复合材料:水热合成及在生物传感器中的应用
冯晓苗 闫真真
(南京邮电大学信息材料与纳米技术研究院,有机电子与信息显示国家重点实验室培育基地,材料科学与工程学院,南京210046)
0 Introduction
Graphene, as an integral part of graphite, is a monolayer of sp2-bonded carbon atoms packed into a dense honeycomb crystal structure, attracting tremendous attention from both the experimental and theoretical scientific communities[1]. Graphene-based composite materials have shown to improve electronic and thermal conductivity[2]. The dispersion of metal nanoparticles on graphene sheets potentially provides a new way to develop catalytic, magnetic, and optoelectronic materials.
Myoglobin (Mb) is a single-chain protein of 153 amino acids containing a heme (iron-containing porphyrin) group in the center which is found in mammalian skeleton and muscle tissues with the functions to store and transport oxygen. Mb with an αhelical polypeptide chain folds into several segments that serve to stabilize the conformation of iron heme through hydrophobic interaction and hydrogen bonding. It is an ideal model molecule for the study of electron transfer reactions of heme proteins and also for biosensing and biocatalysis. However, the direct electron transfer between the redox center of the immobilized enzyme and an electrode is often shielded by the insulating outer protein shell[3]. Great efforts have been made to enhance its electron transfer by using mediators, promoters, or some special electrode materials[4]. The exploitation of Mbbased mediator-free H2O2biosensors is a promising area of research. Because of the good biocompatibility,graphene can act as a connector to bind the enzyme and enhance the possibility of direct electron transfer between protein and electrode surface. Au nanomaterials with good biocompatible can provide a mild microenvironment similar to that of redox proteins in native systems and give the protein molecules more freedom in orientation. They also have great potential to immobilize proteins via reducing efficiently the insulating property of the protein shell for direct electron transfer and facilitating the rate of electron transfer through the conducting tunnels of Au[5]. Therefore, exploitation of this hybrid material for construction of a Mb-based mediator free H2O2biosensor is a promising area of research.
Herein, we report the facile hydrothermal synthesis and application to the biosensor of graphene-Au nanocomposite. The product was characterized by TEM, XRD, and XPS. The obtained graphene nanosheets are almost transparent and Au nanoparticles with an average diameter less than 50 nm are dispersed on the graphene sheets substrate without aggregation. Based on the good biocompatibility and conductivity, the composite was applied to entrap Mb and construct a H2O2biosensor.Direct electron transfer between Mb and electrode could be easily achieved and the protein exhibits bioactivity in solutions with a wide range of pH value.
1 Experimental
1.1 Materials
Graphite, sulfuric acid, potassium permanganate,hydrogen peroxide (H2O2, 30% (W/V), solution),polyvinylpyrrolidone (PVP), trisodium citrate, HAuC14,Nafion, and Mb were purchased from Shanghai Chemical Reagent Co. All reagents were of analytical grade and used as received.
1.2 Synthesis of graphene-Au nanocomposite
Graphene oxide (GO) was prepared from graphite by the Hummers method[6-7]. GO (20 mg) was dispersed into 20 mL of deionized water containing 0.4 g of PVP by sonication. Then 1mL of HAuC14was added under stirring. Stirring was continued for 30 min. And then the mixture was transferred into a Teflon-lined stainless steel autoclave and treated hydrothermally at 150 ℃for 24 h. After the autoclave was cooled down to room temperature, the solution was centrifuged and the precipitate was washed with distilled water and ethanol for several times. The final product was dried in vacuum at 40 ℃for 24 h.
1.3 Fabrication of the biosensor
The glassy carbon electrode (GCE) was first polished with 1.0-, 0.3-, and 0.05-μm alumina powder successively, followed by rinsing thoroughly with double distilled water. The polished electrode was then sonicated in acetone and double distilled water and finally dried at room temperature. The graphene-Au nanocomposites obtained above were dispersed in water (2 mg·mL-1). 10 μL of this suspension and 10 μL of the Mb solution (5 mg·mL-1) were mixed, and then 5 μL of the mixture was deposited onto the surface of the pretreated GCE. It was left to dry at room temperature. 2 μL Nafion was then added for encapsulation. The electrode was left to dry and stored for at least 24 h at 4 ℃. The biosensor was stored under the same condition when not in use.
1.4 Characterization
The morphologies of the graphene-Au nanocomposites were investigated by transmission electron microscopy (TEM, JEOL JEM-200CX). TEM operated at 100 kV. X-ray diffraction patterns were taken on a Philip-XPert X-ray diffractometer with a Cu Kα X-ray source (tube voltage: 40 kV, current: 40 mA, scanning range: 5°~90°, scanning speed: 0.5°·s-1, Cu Kα1, λ=0.154 065 nm).X-ray photoelectron spectroscopic(XPS)analysis was performed on an ESCALAB MK ⅡXray photoelectron spectrometer. Electrochemical experiments were performed using a CHI660C workstation (Shanghai Chenhua, Shanghai). All experiments were carried out using a conventional three-electrode system, where the GCE modified with graphene-Au as the working electrode, a platinum wire as the auxiliary electrode and a saturated calomel electrode as the reference electrode. All solutions were deoxygenated by nitrogen (high purity: 99.999%)before and during the measurements.
2 Results and discussion
The shapes of the graphene-Au nanocomposites were analyzed by low and high resolution TEM observations, as shown in Fig.1. The graphene nanosheets are almost transparent and exhibit a very stable nature under the electron beam. From Fig.1, we also can see that Au nanoparticles with a diameter between 10 ~50 nm are dispersed on the graphene sheets substrate. Since GO and HAuC14could be reduced to graphene and Au by hydrothermal reduction,respectively[8-11]. When GO and HAuCl4were put into hydrothermal environment in the presence of PVP at the same time, graphene-Au nanocomposites could be obtained. Herein, PVP acts as an anchor agent.

Fig.1 Low and high resolution TEM images of graphene-Au nanocomposites

Fig.2 XRD pattern of graphene-Au nanocomposites
The XRD pattern confirms the presence of Au nanoparticles in the composites. As shown in Fig.2,the broad peak at 2θ value of 24.5° is ascribed to the graphene nanosheets[12]. Another five diffraction peaks above 30° correspond to Braggs reflections from (111),(200),(220),(311),and(222)of the face-centered cubic(fcc) Au crystal structure, and are in good agreement with the reported data[12], indicating that the Au is formed after being reduced by hydrothermal route.
The GO and graphene-Au were also characterized by XPS and the corresponding results are shown in Fig.3(a) and 3(b), respectively. The C1s XPS spectrum of graphene-Au nanocomposite shows the same oxygen functionalities with GO. However,the absorbance peaks of graphene-Au at 286.9 (C-O)and 287.2 (C=O and O-C=O) are sharply decreased,which indicates the reduction of GO. In addition,there is an additional component at 285.7 eV corresponding to the C-N bonds that might be caused by the presence of PVP in the resulting product[13].

Fig.3 C1s XPS spectra of GO (A) and graphene-Au (b) nanocomposites
In this work, the direct electron transfer between Mb and electrode can be achieved through the immobilization of Mb on a novel graphene-Au nanocomposite. The CVs of Mb/graphene-Au modified GCE without (a) and with 1 μmol·L-1(b) H2O2in nitrogen-saturated 0.1 mol·L-1PBS solution (pH=7.0)are given in Fig.4(A). A pair of quasi-reversible redox peaks could be observed, as shown in Fig.4a. With the addition of H2O2, an increase in reduction current for the Mb/graphene-Au modified GCE could be observed, indicating that Mb remains its bioelectrocatalytic activity and could catalyze the reduction of H2O2. The electrocatalytic process can be expressed as follows:
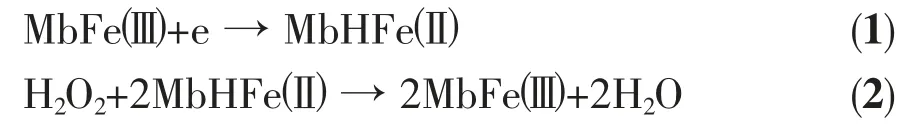

Fig.4 Cyclic voltammograms of the Mb/graphene-Pt (A), Mb (B), and graphene-Pt (C) modified GCE in the absence (a) and in the presence (b) of H2O2(1 mol·L-1) in N2 atmosphere at a scan rate of 100 mV·s-1
In the presence of H2O2, MbHFe(Ⅱ)is efficiently converted to its oxidized form, MbFe(Ⅲ). Consequently,more MbFe(Ⅲ)molecules are reduced at the electrode surface by the direct electron transfer. Fig.4(B) and 4(C) show the CVs of Mb and graphene-Au modified GCE before and after addition of 1 μmol·L-1H2O2in nitrogen-saturated 0.1 mol·L-1PBS solution (pH=7.0).From the figure, we could see that the catalytic ability of the pure Mb and graphene-Au is lower than that of Mb/graphene-Au modified GCE. Furthermore, Mb can be difficult to realize its direct electrochemistry without the help of graphene-Au. Therefore, a good catalytic effect on the reduction of H2O2of Mb/graphene-Au modified GCE is obtained by the coordinative interaction of Mb and graphene-Au.
The CVs of Mb/graphene-Au modified GCE show a strong dependence on the pH value of the solution(Fig.5). The formal potential shifts negatively with the increase in pH value, and it exhibits a linear relationship over a wide pH value range from 4.0 to 8.0 with a slope of 56.0 mV·pH-1. This value is very close to the expected value of 58 mV·pH-1, indicating a one-proton process coupled with single-electron transfer. It can be concluded that Mb exhibits excellent bioactivity in a pH value range from 3.0 to 8.0. And the results also indicate that graphene-Au nanocomposite can provide a suitable microenvironment for Mb.

Fig.5 Effect of pH value on the formal potential of the Mb/graphene-Au modified GCE
Fig.6 shows the differential pulse voltammetric(DPV) measurements using Mb/graphene-Au modified GCE in N2-saturated 0.1 mol·L-1PBS solution (pH value of 7.0) upon addition of H2O2with different concentrations. The peak current of Mb/graphene-Au modified GCE increases with the increasing concentration of H2O2. The linear calibration range for H2O2is 0.1 ~1.5 μmol·L-1(inset of Fig.6) with a detection limit of 0.05 μmol·L-1as defined by a signal-to-noise ratio of 3, which is lower than the reported data[14].
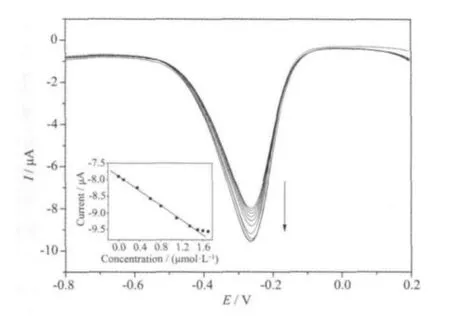
Fig.6 Differential pulse voltammograms obtained at the Mb/graphene-Au modified GCE in 0.1 mol·L-1 phosphate buffer solution (pH=7.0) containing 0,0.1, 0.35, 0.6, 0.8, 1.1, 1.35, 1.5, 1.6, and 1.7 mol·L-1 H2O2(from top to bottom); Inset:relationship between the peak current and the concentration of H2O2
The biosensor shows a good selectivity for H2O2.In a N2-saturated and stirring PBS solution (0.1 mol·L-1, pH=7.0) containing 100 μmol·L-1H2O2, the response of interest from 10 μmol·L-1uric acid (UA)and ascorbic acid (AA) is negligible. The peak current retains 98% of its initial response for Mb/graphene-Au modified GCE after 100 cycles at a H2O2concentration of 1 μmol·L-1(scan rate: 100 mV·s-1).Moreover, during storage in PBS solution with pH value of 7.0 (0.1 mol·L-1) at 4 ℃, the biosensor still remains about 8% of its initial sensitivity after 3 weeks. This could be due to protein loss from the electrode surface into the PBS solution. The relative standard deviation (RSD) of the peak current in five successive determinations at a H2O2concentration of 1 μmol·L-1is 0.5% for Mb/graphene-Au modified GCE.The corresponding RSD value determined at a H2O2concentration of 1 μmol·L-1is 4.4% for five Mb/graphene-Au modified GCEs fabricated independently.
3 Conclusions
In this work, graphene-Au nanocomposites were synthesized through facile hydrothermal route. TEM and XRD results show that Au nanopartices distribute on the single graphene sheet without obvious aggregations. As a model, a third-generation H2O2biosensor was developed based on the direct electrochemistry of Mb immobilized on graphene-Au nanocomposite. The graphene-Au exhibits excellent biocompatibility, high conductivity and good electrocatalytic performance toward the reduction of H2O2and could accelerate the electron transfer between the electroactive sites embedded in protein and the electrode. The proposed biosensor shows sensitive amperometric biosensing for H2O2with a linear range from 0.1 to 1.5 μmol·L-1and a detection limit of 0.05 μmol·L-1(S/N=3), high affinity, excellent selectivity, acceptable reproducibility and stability.
[1] Geim A, Novoselov K. Nat. Mater., 2007,6:183-191
[2] Yu A, Ramesh P, Itkis M, et al. J. Phys. Chem. C, 2007,111:7565-7569
[3] Yabuki S, Shinohara H, Aizawa M. J. Chem. Soc. Chem.Commun., 1989,14:945-946
[4] Nassar A, Rusling J. J. Chem. Soc., Chem. Commun., 1996,118:3043-3044
[5] Liu S, Ju H. Biosens. Bioelectron., 2003,19:177-183
[6] Hummers W, Offeman R. J. Am. Chem. Soc., 1958,80:1339-1339
[7] Cote L, Kim J, Huang F. J. Am. Chem. Soc., 2009,131:1043-1049
[8] Tang Z, Shen S, Zhuang J, et al. Angew. Chem. Int. Ed.,2010,49:4603-4607
[9] Xu Y, Sheng K, Li C, et al. ACS Nano, 2010,4:4324-4330
[10]Chen H, Wang Y, Dong S. Inorg. Chem., 2007,46:10587-10593
[11]Tang X, Jiang P, Ge G Y, et al. Langmuir, 2008,24:1763-1768
[12]Guo H, Wang X, Qian Q, et al. ACS Nano, 2009,3:2653-2659
[13]Shan C, Yang H, Song J, et al. Anal. Chem., 2009,81:2378-2382
[14]Zong S, Cao Y, Zhou Y, et al. Biosens. Bioelectron., 2007,22:1776-1782
——材料科学与工程

