杨梅酮的抗氧化活性
谢湖均 牟望舒 林芙蓉 徐结慧 雷群芳 方文军
(1浙江工商大学应用化学系,杭州310035;2浙江大学化学系,杭州310027)
1 Introduction
Flavonoids are found in fruits,vegetables,bark,nuts,tea,grains,flowers,and wine.1-3The general structures of flavonoids share a common three-ring structure in which two of them are aromatic rings A and B and one of them is heterocyclic C,which are shown in Fig.1.4-7Myricetin(3,5,7,3',4',5'-hexa-hydroxyflavon),belonging to flavonoid compounds,is a natural antioxidant that is present in waxberry.8-10The molecular structure of myricetin is also presented in Fig.1.Previous studies showed that myricetin has a variety of biological activities.11,12Myricetin has shown to possess anti-inflammatory,antiallergic,antimutagenic,cancer chemo-preventive and antioxidant activities.13-15Hence,in the past few years,many scientists have devoted to the research of the biological activity,especially the antioxidant activity of this compound,since free radical-induced peroxidation of membrane lipids has been considered to be associated with a wide variety of chronic health problems,such as cancer,atherosclerosis,and aging.16,17Myricetin has been reported to be a good antioxidant and can reduce levels of plasma low-density lipoproteins.18,19
The antioxidant activity of myricetin is related to its hydroxyl(OH)groups which can scavenge free radicals produced in vivo.20,21Previous experimental and theoretical studies showed that myricetin is a stronger antioxidant than quercetin,which has been attributed to the presence of the 5'-OH group that allows a further stabilization of the semiquinone myricetin radical via hydrogen-bond interaction.22,23Moreover,the pyrogallol moiety present in myricetin is a better superoxide scavenger than the catechol moiety present in quercetin.Wang et al.24evaluated the cytoprotective effect of myricetin on oxidative stress damaged cells by assessment of the scavenging effect of reactive oxygen species(ROS)and the activities of antioxidant enzymes.The results show that the myricetin has the scavenging effect of 1,1-diphenyl-2-picrylhydrazyl(DPPH)radicals on intracellular ROS.Moser25revealed that the antioxidant activity of myricetin was determined in soybean oil methyl esters(SME),and myricetin had greater antioxidant activity than atocopherol,but was inferior to tert-butylhydroquinone.
Justino and Vieira26performed semi-empirical PM6 calculations to obtain the heat of formation,frontier molecular orbitals,atomic charge,as well as bond dissociation enthalpy,in order to discuss the antioxidant activity of myricetin.However,generally speaking,the PM6 method can not give the fine geometry and unpaired electron distribution of a molecule.In contrast,density functional theory(DFT)has proved to be reliable in the study of geometrical and energetic properties of proton transfer and other ion-molecule reactions.27-29Sadasivam and Kumaresan30have reported the antioxidant activity of myricetin via DFT calculations.The results showed that a hydrogen abstraction in the para position is more favored than the other positions,showing that the myricetin is a potent antioxidant due to its semiquinone structure of dehydrogenated radicals,in which the unpaired electrons are mainly distributed on the para position of the O-atom and B ring.
In the present calculations,the antioxidant activity and rate constant for H-atom abstraction reaction of myricetin have been performed by DFT calculations.Our work can provide the reasons of high antioxidant activity of myricetin and determine which structural and electronic features are behind the high antioxidant activity of myricetin and telucidate the possible mechanisms underlying antioxidant activity.
2 Computational details
Geometry optimizations and frequency calculations have been carried out using the M06-2X functional31,32in combination with the 6-31++G(d,p)basis set.Unrestricted calculations were used for open shell systems,and local minima and transition states(TS)were identified by the number of imaginary frequencies(NIMAG=0 or 1,respectively).For each transition state,intrinsic reaction coordinate(IRC)calculations were also performed to guarantee its correct connection to the designated local minima.The TS Gibbs free energy allowed us to evaluate the Gibbs free energies of activation(ΔG#).To examine solvent effect on hydrogen atom abstraction reaction,the conductorlike polarizable continuum model(CPCM)33,34with a dielectric constant of water(ε=78.35)has been employed on the basis of the optimized structures in the gas phase.All the electronic calculations were performed with the Gaussian 09 package of programs.35
Atom in molecule(AIM)theory36,37was performed at the M06-2X/6-31++G(d,p)level of theory to investigate the hydrogen bond property of studied structures.The AIM analysis was carried out by means ofAIM2000 program.38
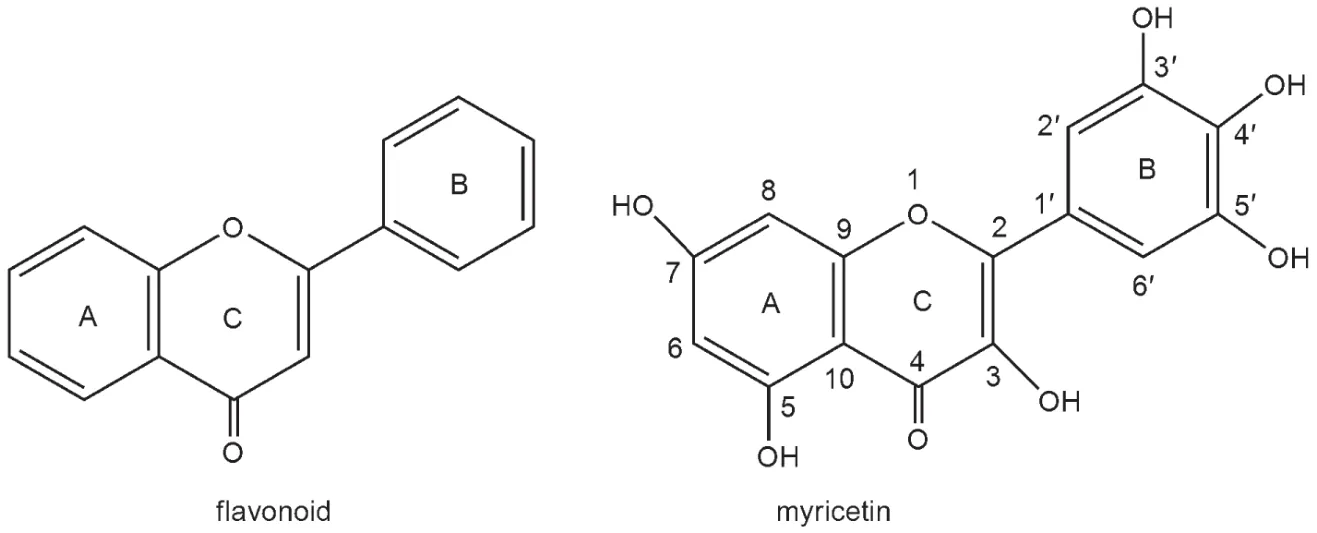
Fig.1 Molecular structure of flavonoid and atom numbering of myricetin(R)
Bond dissociation enthalpy(BDE,ΔH)of each OH group in flavonoids(ArOH)was calculated for different compounds according to the following equation:

where H was the enthalpy that took into account temperaturedependent corrections(zero point energy(ZPE),translational,rotational,and vibrational energies at 298 K).H(ArOH,298 K)corresponded to the enthalpy of the molecule and H(ArO·,298 K)was the enthalpy of the phenoxy radical where the H atom was removed.The BDE of each OH group of a compound was quoted BDE(i-OH).
The rate constants appeared more relevant than ΔG#to compare the different reactions since hydrogen atom transfer(HAT)reactions may involve tunneling effect.Rate coefficients were calculated at 298 K within the conventional transition state theory(TST)39,40framework.

where kBand h are the Boltzmann and Planck constants,the κ(T)transmission coefficient(quantum tunneling along the reaction coordinate)was evaluated by Wigner method.41This method is the most widely used and the simplest approximation to account for tunneling through the reaction barrier.Wigner method assumes a parabolic potential for nuclear motion near the transition state and κW(T)is given by
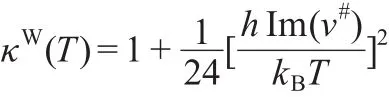
where ν#is the transition states(TS)imaginary frequency.
3 Results and discussion
3.1 Geometry analysis of myricetin
The nomenclature of H-deleted radicals is based on the molecular structure of myricetin(Fig.1).For example,the hydrogen atom is deleted from 3 site,then corresponding compounds are denoted with 3-OH radical.The same notation is used for the other radical forms,corresponding intermediates(Int),TS,and products(P).
Structure-activity relationship researches42-44revealed that flavonoids have effective radical-scaveng capacities,which is originated from unique geometry conformation,that is,the ortho-trihydroxyl structure in the B-ring,the 2,3-double bond allows π electron delocalization from the B-ring,as well as the 3-and 5-OH with 4-oxo function in the A-and C-rings.As shown in Fig.1,myricetin belongs to flavonoid and possesses the similar structure characteristic,indicating strong antioxidant activity.
Fig.2 depicts the evolution of the energy with respect to the C2'-C1'-C2-O1torsion angle around C1'-C2bond of myricetin between the rings B and C,and their preferred relative positions are presented.It has been found that the zero degree torsion angle is the most stable conformer of myricetin in gas phase.The curve also shows that the maximum value of potential energy lies at 90°,and the rotational barrier for interconversion is predicted to be 26.5 kJ·mol-1,which is consistent with previous results obtained from other flavonoids.45-48All hydroxyl groups are oriented in such way to form the stable structure.

Fig.2 Relative energy profiles of myricetin obtained from rotation of the dihedral angle C2′−C1′−C2−O1around C1′−C2bond
Many researchers have reported conformational calculations of flavonoids.Van Acker et al.49have obtained a planar geometry for quercetin via ab initio method,with the STO-3G basis set.Sadasivam and Kumaresan30have showed that the myricetin has a highly planar structure,the result is important because planarity influences π electron delocalization,one of the important parameters for an antioxidant.Fig.3 represents a three-dimensional(3D)visualization HOMO of myricetin,and it shows the characteristic of π state.For the myricetin,the HOMO mostly remains on the B-ring and little in the C2-C3double bond,indicating that the hydroxides in B-ring have strong antioxidant activity,which is in good agreement with previous calculated results.30,46The present calculations also suggested the presence of H-bond interactions between 3-OH and the carbonyl groups,3'-OH and 4'-OH groups,as well as 4'-OH and 5'-OH groups.Based on the calculations,the predicted H-bond distance of O3- H3…O4is about 0.1914 nm,which may play an important role in the myricetin stabilization and antioxidation activity.50,51

Fig.3 Frontier molecular orbitals of myricetin(isovalue=0.05)
3.2 Bond dissociation enthalpy
As Fig.1 shows,among the six hydroxyl groups of myricetin,the 3'-OH,4'-OH,and 5'-OH groups are in the B-ring(pyrogallol moiety),which are responsible for the strong antioxidant activity of myricetin.In addition,the 3-OH is in the C-ing,while the 5-OH and 7-OH groups are in theA-ring.
On the basis of previous studies,46,52-54flavonoids can react with radicals by hydrogen atom transfer(HAT)due to the low bond dissociation enthalpy of the O-H bond.Also,hydrogen atom transfer from an antioxidant to the oxygen-containing radical is a thermodynamically favorable event.Such as the BDE of phenol is 370 kJ·mol-1,which is generally chosen as the zero compound.55,56According to present calculations,all the BDEs of myricetin are lower than that of phenol as shown in Table 1,indicating that the hydrogen atom transfer is more favorable than the electron transfer.The BDE for breaking the O-H bond is characteristic of this antioxidant pathway.The weaker the OH bond(low BDE),the faster the reaction with free radicals.
Theoretical calculations of the bond dissociation enthalpy by means of semi-empirical,Hartree-Fock,as well as DFT methods,have been useful for elucidating the high capacity of the OH groups of phenolic antioxidants.Nevertheless,quantumchemical studies on flavonoids are far from being complete.The previous theoretical investigations focused only on the B-ring(particularly the catechol moiety)and the most reliable methodology still has to be established.Although the B3LYP method is used in many investigations on antioxidant activity,57-60and in this study,the M06-2X method developed by Zhao and Truhlar31,32is applied.The main reason lies in the fact that sometimes there are some problems in the prediction of bond dissociation enthalpy,as well as energies and geometries of transition states by means of the B3LYP method.61-63As shown in Table 1,it can be seen the sequence of the BDE of OH groups calculated from M06-2X/6-31++G(d,p)level of theory:4'-OH<5'-OH<5-OH<3-OH<3'-OH<7-OH,meaning that the 4'-OH group has the strongest antioxidation activity with the BDE value of 303.7 kJ·mol-1,which is consistent with the calculations obtained by Sadasivama and kumaresan.30
The relative low values of bond dissociation enthalpy(ΔH)are found for the 4'-OH and 5'-OH myricetin radicals,which clearly demonstrates that the B-ring is the most important site for hydrogen atom transfer.The role of the B-ring found here,especially that of the 4'-OH group,is in accordance with all structure-activity relationship described in the literature.30,46,64,65The role of ortho-substitution on the B-ring has also been intensively discussed,the 4'-OH seems to be the most thermo-dynamically favorable site,and similar results are obtained for quercetin and taxifolin.66,67The low ΔH value of 4'-OH group could be attributed to weak hydrogen-bond interactions,that is,the keto(C=O)group and remaining 3'-OH and 5'-OH groups(O3'-H'…O4'and O5'-H'…O4'hydrogen bonds).After the O-H bond is broken in the parent compound,the radical is able to rearrange to the more stable conformation,corresponding to the formation of a new hydrogen-bond in contrast to myricetin molecule.Thus,the hydrogen-bond has a stabilizing effect after radical formation on the B-ring and rearrangement,by twisting the remaining OH bond,is possible in the present calculations.
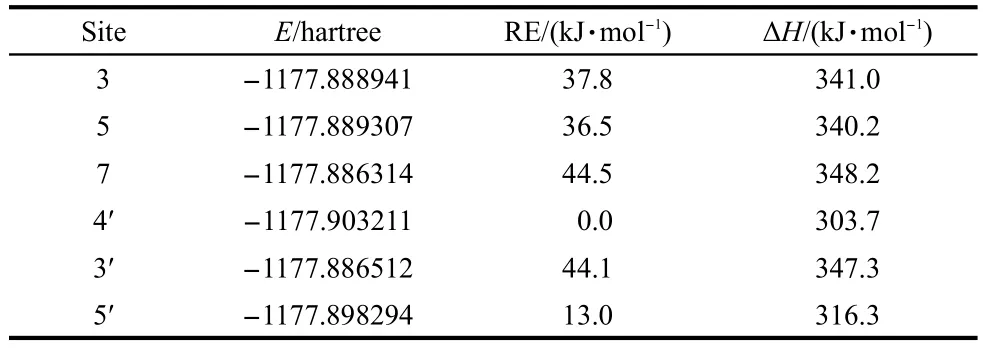
Table 1 Total energies(E)and relative energies(RE)of dehydrogenated myricetin radicals and O-H bond dissociation enthalpies(ΔH)derived from M06-2X/6-31++G(d,p)calculations
The 3-OH,5-OH,and 7-OH radicals were also considered in the present study.The ΔH values(Table 1)show that the role of the A-ring and C-ring is clearly less important than that of the B-ring.In contrast to B-ring,a difference of about 42 kJ·mol-1is observed for the bond dissociation enthalpy of the A-ring and C-ring.Again,this is in agreement with all previous studies.30,46The calculated BDE values confirm that even the 3-OH group in the C-ring of myricetin,in some cases,can be also responsible for antioxidant activity.46,68After H removal of the 3-OH group,the stabilizing effect,due to an hydrogenbond with the 4-keto group,disappears in myricetin.
Fig.4 presents the singly occupied molecular orbitals(SOMO)of dehydrogenated myricetin radicals.As Fig.4 shows,the SOMO of radicals can be correlated with the free radical-scavenging activity due to their delocalization of the unpaired electron and conjugation effects.Lien et al.69used this parameter in a quantitative structure-activity relationship(QSAR)study on phenolic compounds.The SOMO is important because it corresponds to the energy level of the unpaired electron.We have therefore analyzed the spatial distribution of SOMO.This parameter gives important information about possible stabilization by delocalization of the flavonoxy radical.It is noticed from Fig.4 that the SOMO is delocalized over the B-ring for the 4'-OH radicals of myricetin.Possibility of π electron delocalization in the radical,after hydrogen atom transfer,largely influences the ΔH values.This analysis supports the results obtained for the HOMO distribution and ΔH values,which are in agreement with previous structure-activity relationship.30
3.3 Antioxidation mechanism of myricetin

Fig.4 SOMO of H-deleted myricetin radicals
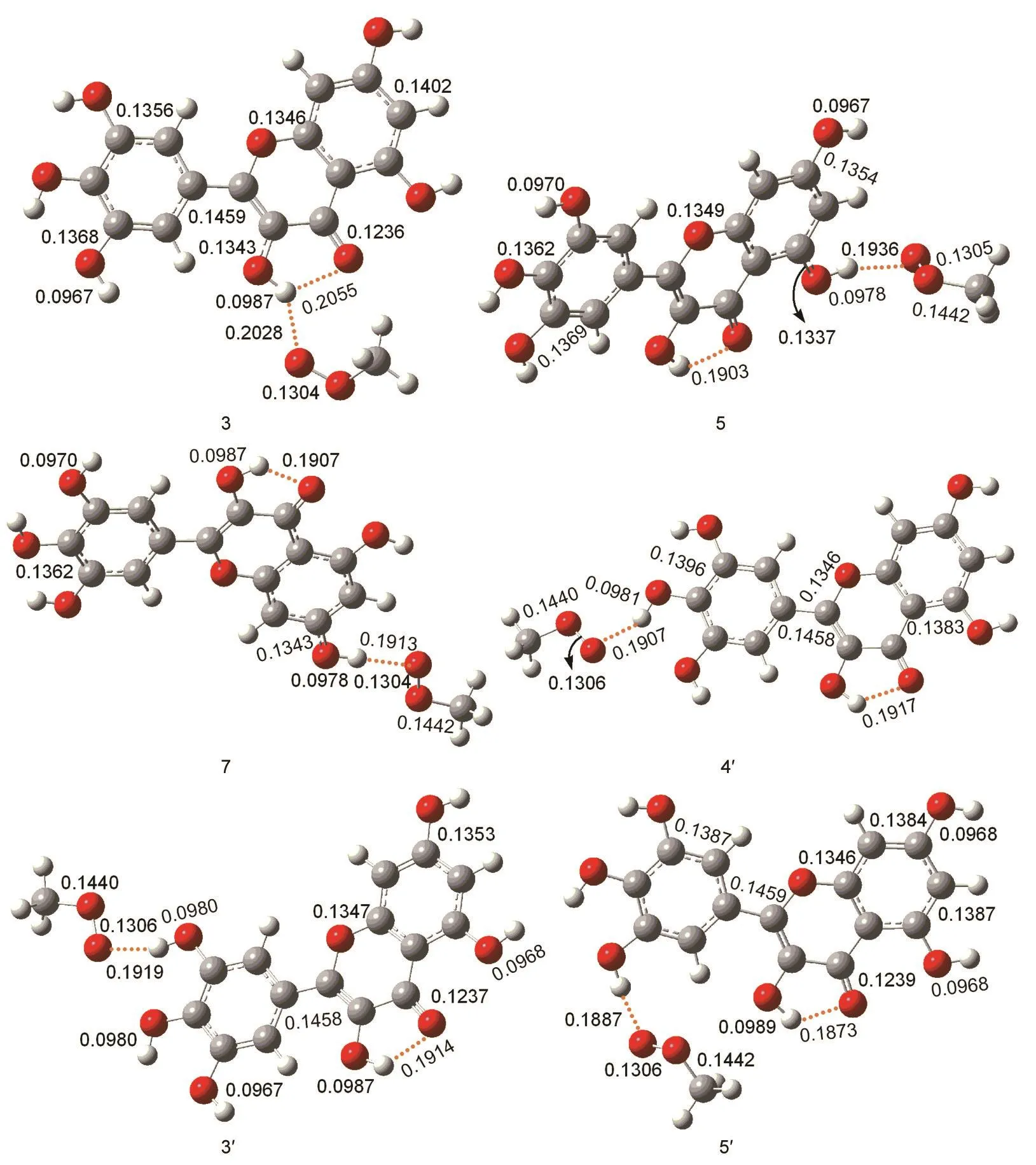
Fig.5 Optimized geometries of Int1derived from M06-2X/6-31++G(d,p)calculations bond distance in nm
The low O-H bond dissociation enthalpy of myricetin means that the hydrogen atom transfer reaction is easy to occur.In order to elucidate the reactivity of OH groups of myricetin,we calculated the free energy profiles for the reaction between the CH3OO·radical with myricetin(R).A simple model for the lipid peroxide radical CH3OO·is chosen because nu-merous natural peroxides are of the similar structure(ROO·).Thus,the obtained results could demonstrate their general behaviors.The calculations reveal that detachment of H atom by CH3OO·radical can be performed with all six hydroxyl groups of myricetin.Each reaction proceeds via one transition state(TS)and two intermediates(Int1and Int2).The relative energies of the different compounds in the hydrogen abstraction reaction in the gas phase and aqueous solution are given in Table 2,and the optimized geometries of the stationary points concerning Int1,TS,Int2,and P in the antioxidation reaction are available in Figs.5-8.
The optimized geometries of all stationary points encountered during the H-atom transfer reaction between the 4'-OH group of myricetin and CH3OO·radical are reported in Figs.5-8.The 4'-OH group is considered to be the most reactive,since,from its radicalization,a very stable radical species is obtained,stabilized by delocalization and conjugation phenomena,as well as by an internal H-bond,established between the radicalized O·and the adjacent OH groups,which agrees with the smallest value for bond dissociation enthalpy of 4'-OH group.Myricetin is a planar species,and the OH groups are arranged in such a way as to maximize the number of hydrogenbonds.The reaction between myricetin(R)and CH3OO·radical starts with the formation of a reactant complex(Int1),in which the radical is hydrogen bonded to the 4'-OH group of the ortho-diphenolic moiety of myricetin.As Fig.5 displays,the predicted 4'-OH…OOCH3hydrogen-bond distance in Int1is 0.1907 nm.The H-atom transfer reaction passes through a transition state(TS)in which the hydrogen is bonded to the ox-ygen atoms of both the 4'-OH and the CH3OO·radical.By inspecting Table 2,one can observe that the smallest activation barriers of 34.5 and 37.3 kJ·mol-1in the gas phase and aqueous solution are required for the reaction in the position of 4'-OH.The activation barrier required to transfer the hydrogen atom from the phenolic OH to the methyl peroxide radical is calculated as the difference in the total energy between the transition state(TS)and corresponding reactant(Int1).As shown in Fig.6,the critical distances of 4'-O…H and H…OOCH3in TS are 0.1122 and 0.1289 nm,respectively.After the hydrogen atom transfers,a complex of the intermediates(Int2)is found.The H-OOCH3bond is completely established,and the methyl peroxide is hydrogen bonded to the 4'-O radical of myricetin as shown in Fig.7.In the latter,an internal hydrogen bond is formed and involves the radicalized oxygen of the 4'-OH group and the adjacent 3'-OH in the B-ring.Finally,the CH3OOH and H-deleted myricetin radical(P)are generated as shown in Fig.8.On the basis of present calculations,the patterns of the hydrogen bonds present in myricetin are retained in going from reactant to Int1,TS,Int2,and P,which is validated by AIM analysis as shown in Fig.9.Generally speaking,hydrogen bond is characterized by positive value of Laplacian∇2ρ(r)and low electronic density ρ(r)value(<0.1)at bond critical point(BCP).Inspection of Fig.9 reveals obviously that the ρ(r)and ∇2ρ(r)of all compounds for the 4'-H atom abstraction reaction fit the characteristics of hydrogen bond.
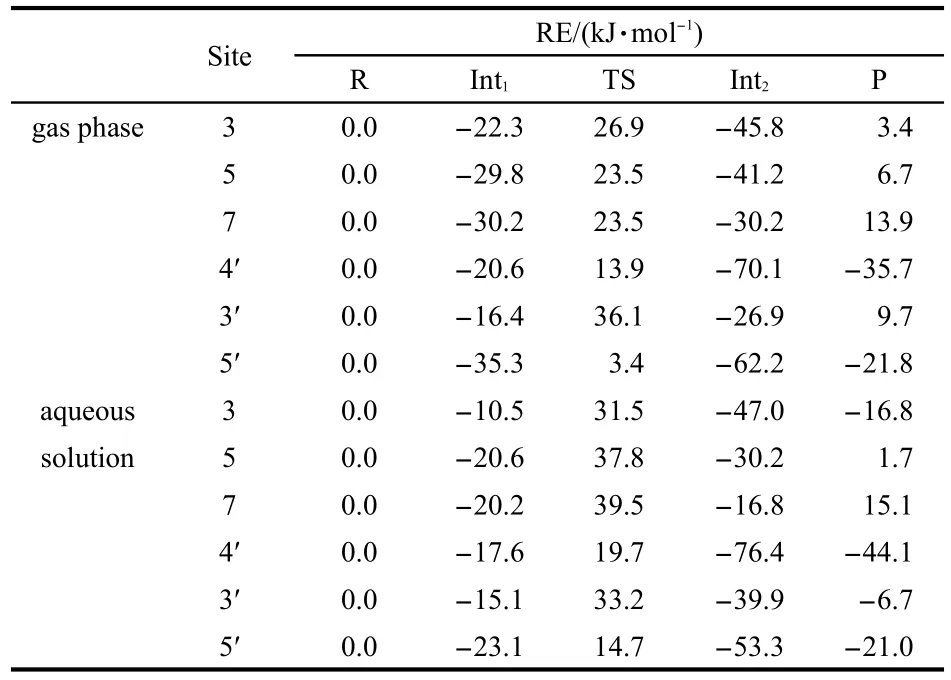
Table 2 Relative energy of the different compounds in the hydrogen abstraction reaction of myricetin

Fig.6 Optimized geometries of TS for hydrogen atom transfer derived from M06-2X/6-31++G(d,p)calculations
The H-atom transferreaction occurring between the CH3OO·radical and the 5'-OH group of myricetin is qualitatively similar to the 4'-OH group.The reactant complex(Int1)is obtained with hydrogen-bond 5'-OH…OOCH3distance of 0.1887 nm displayed in Fig.5.The transition state,necessary to proceed towards the product complex,is characterized by a 5'-O…H and H…OOCH3distances of 0.1105 and 0.1313 nm,respectively(Fig.6).As Table 2 shows,the predicted activation barriers for the hydrogen atom transfer are about 38.7 and 37.8 kJ·mol-1in the gas phase and aqueous solution,respectively.On the basis of the calculations,it leads to a conclusion that the reaction can take place in both 4'and 5'positions.As Table 2 depicts,the reverse reactions in these positions are unlikely,since both reactions for the formation of the final products are significantly exergonic in the gas phase to prevent the reverse process.
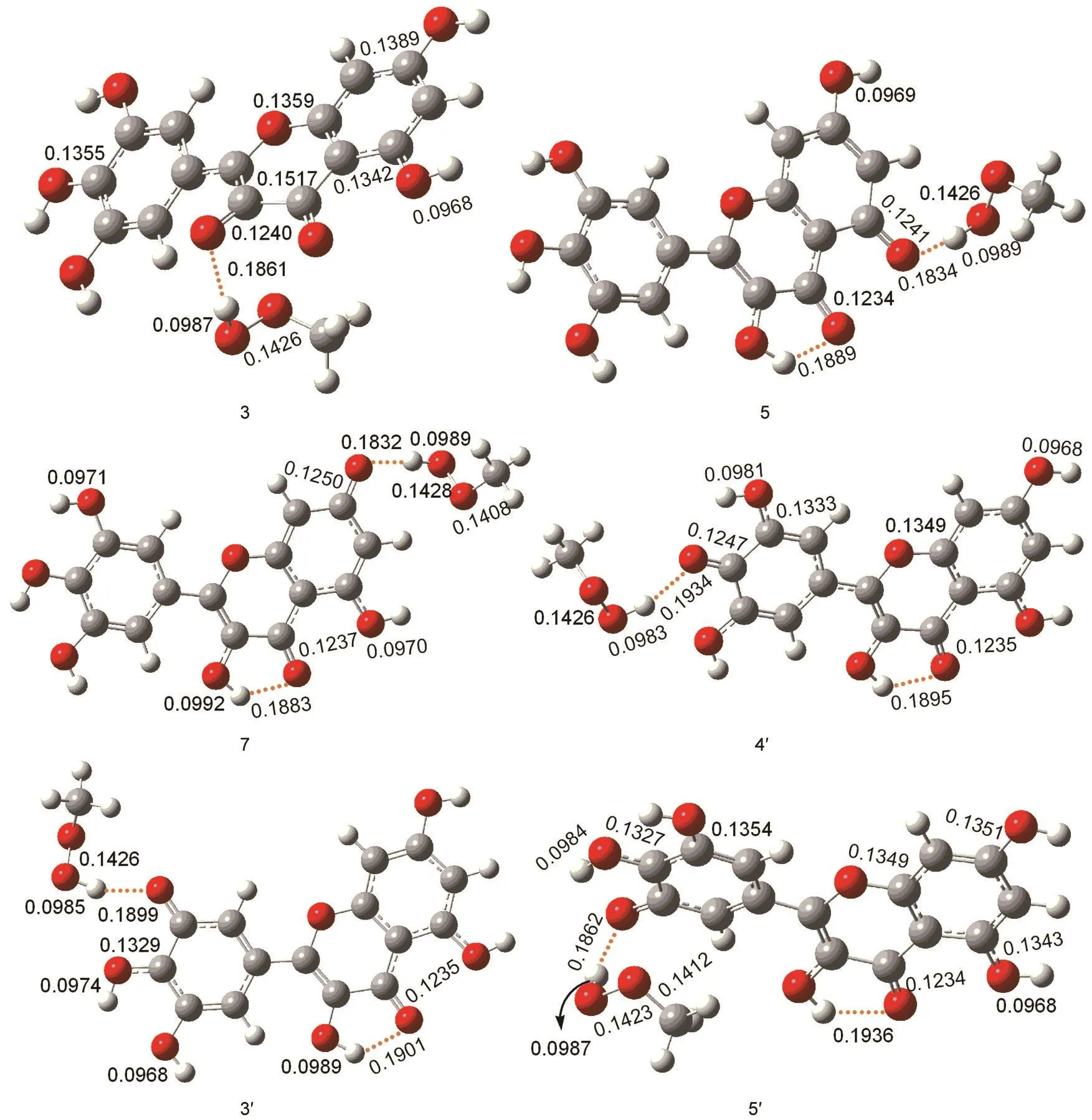
Fig.7 Optimized geometries of Int2derived from M06-2X/6-31++G(d,p)calculations
It is noted that the pyrogallol functionality in the B-ring of myricetin has a reasonable effect on the barrier heights.The greater reactivity of the 4'-OH and 5'-OH groups towards the CH3OO·radical is mainly due to the stability generated by the intramolecular H-bond between radicalized oxygen of the reactive OH group and the adjacent OH in the B-ring,which is retained in going from free myricetin to reactant complex to transition state.This H-bond helps in stabilizing the electronic deficiency generated on the radicalized oxygen during the H abstraction reaction.The important role of intramolecular hydrogen bonds in stability of the radicals has been validated by previous studies.7,42,46,67For the other OH groups,this stability cannot be achieved.
In the case of the 3'-OH group,the hydrogen-bond distance between the oxygen from the CH3OO·radical and the hydrogen from the 3'-OH group in Int1is about 0.1919 nm(Fig.5).As can be noted from Fig.6,the 3'-O…H and H…OOCH3distances at the saddle points for the hydrogen atom transfer are calculated to be 0.1174 and 0.1219 nm,respectively.The corresponding reaction barriers are equal to 52.5 and 48.3 kJ·mol-1in the gas phase and aqueous solution,respectively.
The 3-OH,5-OH,and 7-OH groups in C-ring of myricetin are recognized as the other possible sites for the reaction with the free radicals,from both theoretical and experimental points of view,due to the fact that in the radical arising from the oxidation process,the odd electron can be delocalized over both B-and C-rings.On the basis of the calculations,the H-atom transfer reactions of the 3-OH,5-OH,and 7-OH groups in C-ring of myricetin are less favorable than that of the 4'-OH group of myricetin.Also,the H-atom transfer reactions between the 3-OH,5-OH,and 7-OH groups with the CH3OO·show the similar potential energy surfaces.Thus,we will only talk about the reaction between the 3-OH group with the CH3OO·.For 3-OH group,a stable complex between reactants is obtained,through a weak interaction involving the hydrogen of the 3-OH and the oxygen of the CH3OO·.As Fig.5 shows,the distance of hydrogen-bond in Int1amounts to 0.2028 nm.The hydrogen of the 3-OH is also involved in the hydrogenbond with the 4-carbonyl oxygen(0.2055 nm).For the hydrogen that is going to be transferred,the reaction proceeds towards the transition state in which this hydrogen is contemporaneously bonded to the 3-OH and to the CH3OO·as shown in Fig.6(0.1082 and 0.1354 nm),and at the same time,the internal 4-keto…3-OH hydrogen-bond is destroyed.The activation barriers for the hydrogen atom transfer are predicted to be 49.2 and 42.0 kJ·mol-1in the gas phase and aqueous solution,respectively.
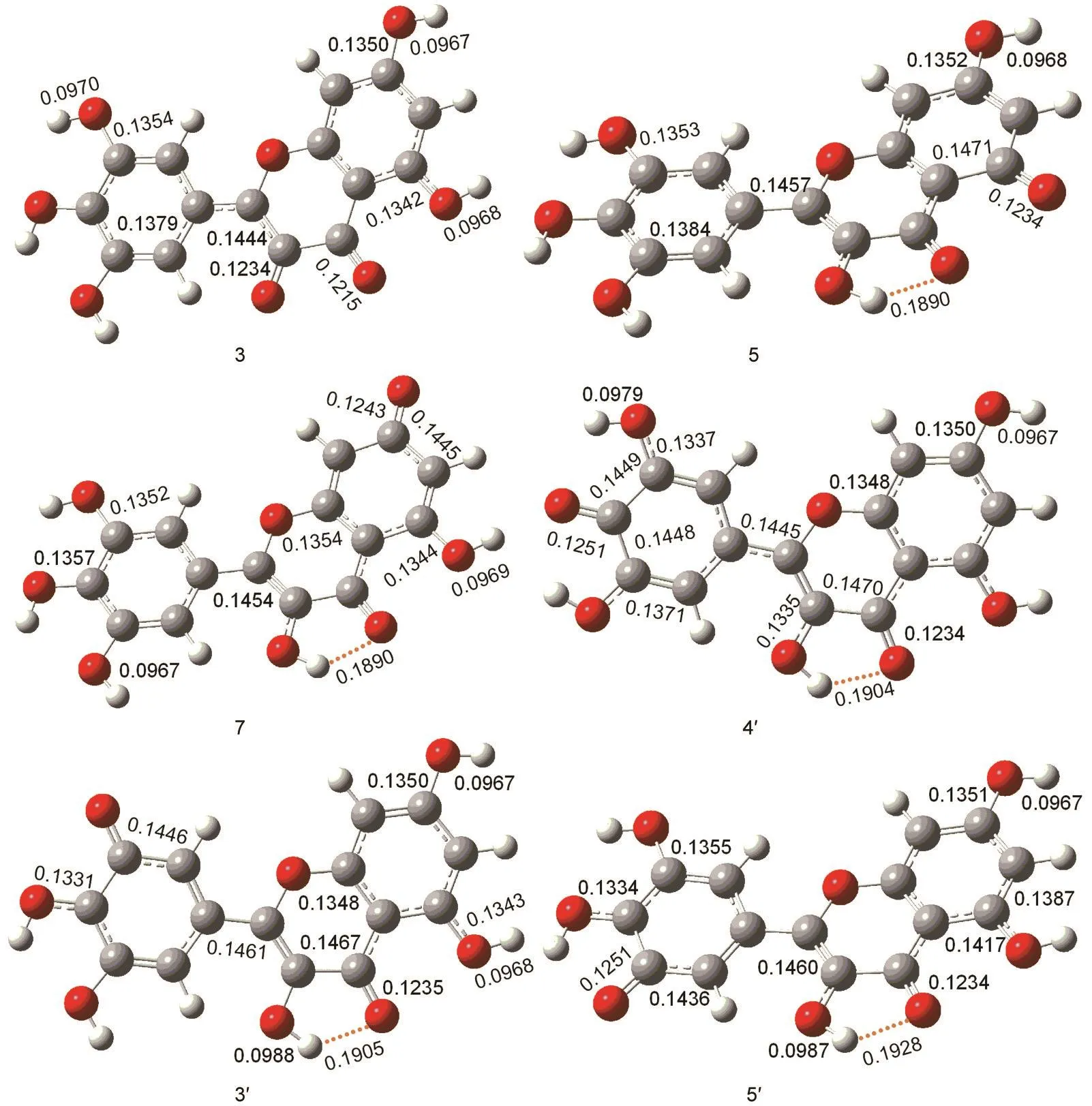
Fig.8 Optimized geometries of product(P)derived from M06-2X/6-31++G(d,p)calculations
On the basis of present calculations,the following activation energy sequence for the OH groups is found in the gas phase:4'-OH<5'-OH<3-OH<3'-OH<5-OH<7-OH,which agrees with the result from aqueous solution.The 4'-OH site seems to be the most reactive one,also indicated by previous works.30,46
A detailed analysis of the transition states shows that the spin density(Fig.10)is located on CH3OO·and H-deleted ring.In comparison to the reactants,the spin density in TS associated with H-deleted ring moiety is increased,while that associated with CH3OO·fragment is decreased.
3.4 Rate constants of H-atom abstraction reaction
The rate constants for the H atom abstraction from the different OH groups of myricetin by the CH3OO·radical are calculated via M06-2X/6-31++G(d,p)method,together with the tunnelling transmission coefficients as a function of the temperature,which are collected in Table 3.In the present calculations,only the M06-2X functional is performed since this functional provides more reliable kinetics and thermodynamics.
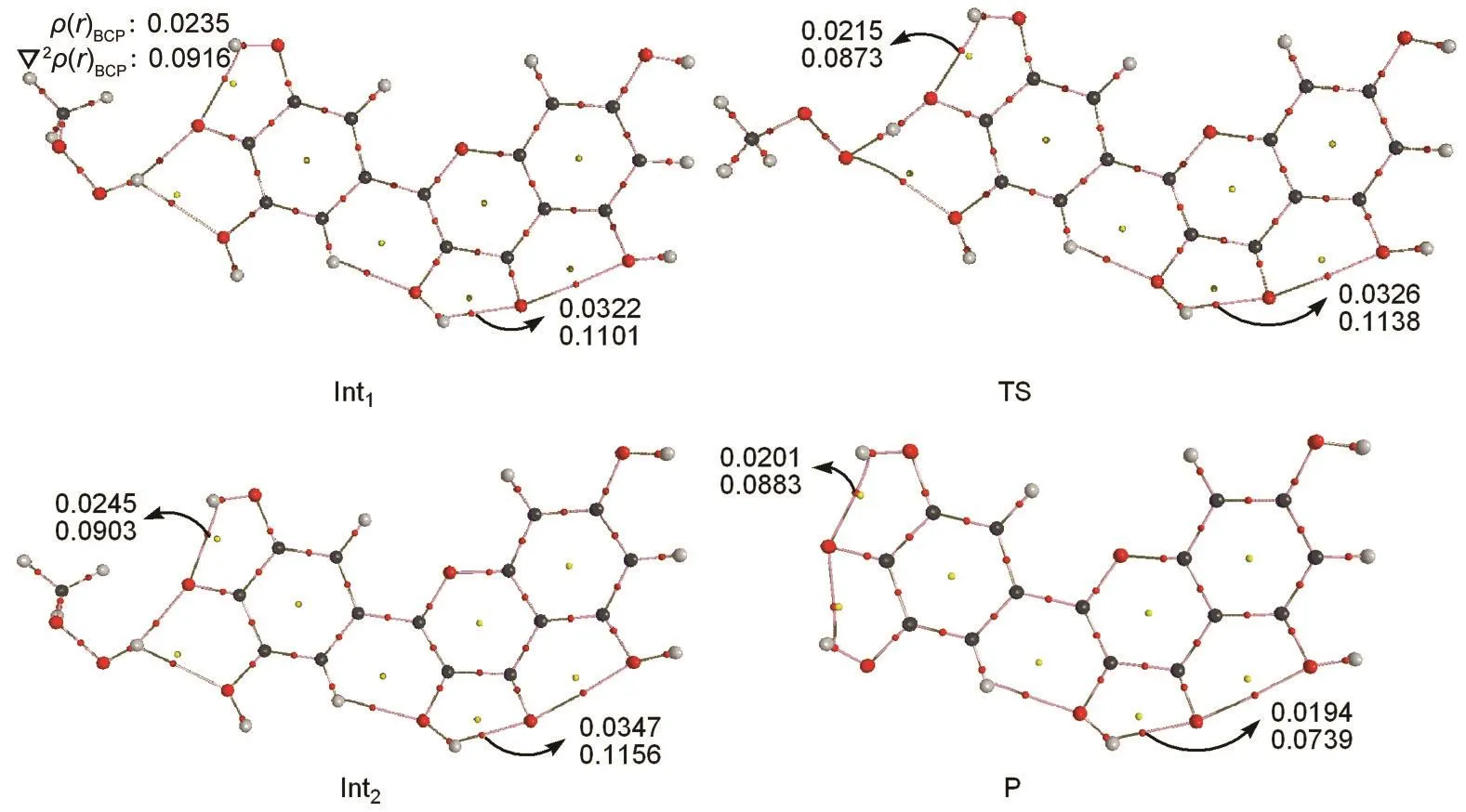
Fig.9 Electronic density ρ(r)BCPand Laplacian ∇2ρ(r)BCPat bond critical point(BCP)of Int1,TS,Int2,and Pfor the 4′-H atom abstraction reaction

Fig.10 Spin densities of TS for the hydrogen atom transfer reaction between myricetin and CH3OO·radical

Table 3 Rate constants for the H-abstraction reaction between CH3OO·free radical and myricetin presented along with the tunneling transmission coefficients as a function of temperature
The conventional transition state rate constant value,kTST,mainly depends on the high classical potential energy barrier(besides entropic contributions).As Table 3 shows,the rate constant for the 4'-H atom abstraction reaction is predicted to be 1.5×108L·mol-1·s-1at 298 K.After quantum tunneling correction,the rate constant of k(T)is equal to 6.0×108L·mol-1·s-1.In addition,with the increase of temperature,the rate constant is increased significantly.The predicted rate constants kTSTand k(T)at 1000 K are 8.7×1011and 1.1×1012L·mol-1·s-1,respectively.The rate constant at 1000 K is 3 orders of magnitude larger than that at 298 K.The present calculations indicate that the rate constant of 4'-H atom abstraction reaction shows the largest value,which is consistent with the previous researches30,46,64that a high rate of hydrogen atom transfer is expected to be related to a low BDE of phenolic O-H bond.The overall reactivity of myricetin toward CH3COO·radicals was found to be fast,supporting the excellent radical scavenging activity of myricetin,in agreement with the available experimental evidence.25Also,the κ(T)transmission coefficient is gradually decreased with the increase of temperature,indicating that the entropic effects become more significant.
As indicated in the previous section,the formation of the reactant complex and the dissociation of the product complex do not affect kinetics since these two processes occur with no classical energy barriers,and the overall reaction is governed only by the H-atom abstraction bottleneck.According to the calculations,the tunnelling effects are slightly important in this reaction.Our findings are in qualitative agreement with those obtained in a study of the reaction between the methyl peroxide radical and epicatechin,70also showing the pyrogallol functionality in the B-ring.
4 Conclusions
On the basis of the most stable conformations of myricetin,its electronic structure has been calculated by means of M06-2X/6-31++G(d,p)treatments.The BDEs of all OH groups of myricetin were calculated,and the antioxidation activity and rate constant for hydrogen atom transfer were discussed.All these results confirm the predominant H-transfer capacity of the 4'-OH group,compared to the other OH groups in the myricetin.The relative high antioxidation activity of 4'-OH group in myricetin is attributed to very stable H-deleted radical species,delocalization and conjugation phenomena,as well as by an internal H-bond,established between the radicalized O·and the adjacent OH groups.The theoretical investigations on the antioxidation mechanism of myricetin could provide the insights into the design and synthesis of novel antioxidants with enhanced activity.
(1) Kuhnau,J.World Rev.Nutr.Diet.1976,24,117.
(2)Miean,K.H.;Mohamed,S.J.Agric.Food Chem.2001,49,3106.doi:10.1021/jf000892m
(3)Li,M.J.;Zhang,L.M.;Liu,W.X.;Lu,W.C.Chin.J.Chem.Phys.2011,24,173.
(4) Rüfer,C.E.;Kulling,S.E.J.Agric.Food Chem.2006,54,2926.doi:10.1021/jf053112o
(5)Amat,A.;Clementi,C.;DeAngelis,F.;Sgamellotti,A.;Fantaccia,S.J.Phys.Chem.A 2009,113,15118.doi:10.1021/jp9052538
(6) Horvath,C.R.;Martos,P.A.;Saxena,P.K.J.Chromatogr.A 2005,1062,199.doi:10.1016/j.chroma.2004.11.030
(7) Nenadis,N.;Sigalas,M.P.J.Phys.Chem.A 2008,112,12196.doi:10.1021/jp8058905
(8) Rashid,U.;Anwar,F.;Moser,B.R.;Knothe,G.Bioresour.Technol.2008,99,8175.doi:10.1016/j.biortech.2008.03.066
(9) Gunesekaran,R.;Ubeda,A.;Alcaraz,M.J.;Jayaprakasam,R.;Nair,A.G.R.Pharmazie 1993,48,230.
(10)Mehrdad,M.;Zebardast,M.;Abedi,G.;Koupaei,M.N.;Rasouli,H.;Talebi,M.J.Aoac.Int.2009,92,1035.
(11) Burda,S.;Oleszek,W.J.Agric.Food.Chem.2001,49,2774.doi:10.1021/jf001413m
(12) Mira,L.;Fernandez,M.T.;Santos,M.;Rocha,R.;Florencio,M.H.;Jennings,K.R.Free Radic.Res.2002,36,1199.doi:10.1080/1071576021000016463
(13)Ko,C.H.;Shen,S.C.;Lee,T.J.;Chen,Y.C.Mol.Cancer Ther.2005,4,281.
(14) Morales,P.;Haza,A.I.J.Appl.Toxicol.2012,32,986.doi:10.1002/jat.v32.12
(15) Rasulev,B.F.;Abdullaev,N.D.;Syrov,V.N.Leszczynski,J.QSAR Comb.Sci.2005,24,1056.
(16)DeToma,A.S.;Choi,J.S.;Braymer,J.J.;Lim,M.H.ChemBioChem 2011,12,1198.doi:10.1002/cbic.v12.8
(17) Delgado,M.E.;Haza,A.I.;Garcia,A.;Morales,P.Toxicolin.In Vitro 2009,23,1292.doi:10.1016/j.tiv.2009.07.022
(18)Oyama,Y.;Fuchs,P.A.;Katayama,N.;Noda,K.Brain Res.1994,635,125.doi:10.1016/0006-8993(94)91431-1
(19)Gordon,M.H.;Roedig-Penmanm,A.Chem.Phys.Lipids 1998,97,79.doi:10.1016/S0009-3084(98)00098-X
(20) Lalas,S.;Tsaknis,J.J.Am.Oil.Chem.Soc.2002,79,677.doi:10.1007/s11746-002-0542-2
(21) Shahidi,F.;Wanasundara,U.Dev.Food Sci.1995,37A,469.
(22) Robak,J.;Gryglewski,R.J.Biochem.Pharmacol.1988,37,837.doi:10.1016/0006-2952(88)90169-4
(23) Angelone,T.;Pasqua,T.;Di Majo,D.;Quintieri,A.M.;Filice,E.;Amodio,N.;Tota,B.;Giammanco,M.;Cerra,M.C.Nutr.Metab.Cardiovas.2011,21,362.doi:10.1016/j.numecd.2009.10.011
(24)Wang,Z.H.;Kang,K.A.;Zhang,R.;Piao,M.J.;Jo,S.H.;Kim,J.S.;Kang,S.S.;Lee,J.S.;Park,D.H.;Hyun,J.W.Environ.Toxicol.Phar.2010,29,12.doi:10.1016/j.etap.2009.08.007
(25) Moser,B.R.Eur.J.Lipid Sci.Technol.2008,110,1167.doi:10.1002/ejlt.v110:12
(26) Justino,G.C.;Vieira,A.J.S.C.J.Mol.Model.2010,16,863.doi:10.1007/s00894-009-0583-1
(27)Mendoza-Wilson,A.M.;Sotelo-Mundo,R.R.;Balandran-Quintana,R.R.;Glossman-Mitnik,D.;Santiz-Gomez,M.A.;Garcia-Orozco,K.D.J.Mol.Struct.2010,981,187.doi:10.1016/j.molstruc.2010.08.005
(28) Leon-Carmona,J.R.;Galano,A.J.Phys.Chem.B 2011,115,4538.doi:10.1021/jp201383y
(29) Anouar,E.;Calliste,C.A.;Kosinova,P.;Di Meo,F.;Duroux,J.L.;Champavier,Y.;Marakchi,K.;Trouillas,P.J.Phys.Chem.A 2009,113,13881.doi:10.1021/jp906285b
(30) Sadasivam,K.;Kumaresan,R.Spectrochim.Acta A 2011,79,282.doi:10.1016/j.saa.2011.02.042
(31) Zhao,Y.;Truhlar,D.G.Theor.Chem.Acc.2008,120,215.doi:10.1007/s00214-007-0310-x
(32) Zhao,Y.;Truhlar,D.G.Accounts Chem.Res.2008,41,157.doi:10.1021/ar700111a
(33) Barone,V.;Cossi,M.J.Phys.Chem.A 1998,102,1995.doi:10.1021/jp9716997
(34)Cossi,M.;Rega,N.;Scalmani,G.;Barone,V.J.Comput.Chem.2003,24,669.doi:10.1002/jcc.10189
(35) Frisch,M.J.;Trucks,G..W.;Schlegel,H.B.;et al.Gaussian 09,RevisionA.01;Gaussian Inc.:Wallingford,CT,2009.
(36) Bader,R.F.W.Chem.Res.1991,91,893.
(37) Bader,R.F.W.J.Phys.Chem.A 1998,102,7314.doi:10.1021/jp981794v
(38) Biegler-Konig,F.AIM2000;University ofApplied Sciences:Bielefeld,Germany.
(39) Eyring,H.J.Chem.Phys.1935,3,107.doi:10.1063/1.1749604
(40) Evans,M.G.;Polanyi,M.Trans.Faraday Soc.1935,31,875.doi:10.1039/tf9353100875
(41) Wigner,E.J.Chem.Phys.1937,5,720.
(42) Russo,N.;Toscano,M.;Uccella,N.J.Agric.Food Chem.2000,48,3232.doi:10.1021/jf990469h
(43)Bors,W.;Heller,W.;Saran,M.Methods in Enzymology;Academic Press:San Diego,1990;Vol.186,p 343.
(44) Leopoldini,M.;Rondinelli,F.;Russo,N.;Toscano,M.J.Agric.Food Chem.2010,58,8862.doi:10.1021/jf101693k
(45) Estvez,L.;Mosquera,R.A.J.Phys.Chem.A 2007,111,11100.doi:10.1021/jp074941a
(46) Xie,H.J.;Lei,Q.F.;Fang,W.J.Acta Chim.Sin.2010,68,1467.
(47) Markovic,Z.S.;Dimitric,J.M.;Markovic,D.;Dolicanin,C.B.Theor.Chem.Acc.2010,127,69.doi:10.1007/s00214-009-0706-x
(48)Sadasivam,K.;Kumaresan,R.Comput.Theor.Chem.2011,963,227.doi:10.1016/j.comptc.2010.10.025
(49) VanAcker,S.A.B.E.;DeGroot,M.J.;Van den Berg,D.J.;Tromp,M.N.J.L.;Den Kelder,G.D.O.;Van der Vijgh,W.J.F.;Bast,A.Chem.Res.Toxicol.1996,9,1305.doi:10.1021/tx9600964
(50) Rice-Evans,C.A.;Miller,N.J.;Paganga,G.Free Radic.Biol.Med.1996,20,933.doi:10.1016/0891-5849(95)02227-9
(51)VanAcker,S.A.B.E.;Van Den Berg,D.J.;Tromp,M.N.J.L.;Griffioen,D.H.;Van Bennekom,W.P.;Van Der Vijgh,W.J.F.;Bast,A.Free Radic.Biol.Med.1996,20,331.doi:10.1016/0891-5849(95)02047-0
(52) Guzman,R.;Santiago,C.;Sanchez,M.J.Mol.Struct.2009,935,110.doi:10.1016/j.molstruc.2009.06.048
(53) Chiodo,S.G.;Leopoldini,M.;Russo,N.;Toscano,M.Phys.Chem.Chem.Phys.2010,12,7662.doi:10.1039/b924521a
(54)Li,M.J.;Li,Y.J.;Peng,C.R.;Lu,W.C.Acta Phys.-Chim.Sin.2010,26,466.[李敏杰,李亚军,彭淳容,陆文聪.物理化学学报,2010,26,466.]doi:10.3866/PKU.WHXB20100230
(55) Wright,J.S.;Johnson,E.R.;Di Labio,G.A.J.Am.Chem.Soc.2001,123,1173.doi:10.1021/ja002455u
(56) Trouillas,P.;Fagnere,C.;Lazzaroni,R.;Calliste,C.;Marfak,A.;Duroux,J.L.Food Chem.2004,88,571.doi:10.1016/j.foodchem.2004.02.009
(57) Aparicio,S.Int.J.Mol.Sci.2010,11,2017.doi:10.3390/ijms11052017
(58) Zhang,J.H.;Du,F.P.;Peng,B.;Lu,R.H.;Gao,H.X.;Zhou,Z.Q.J.Mol.Struct.-Theochem 2010,955,1.doi:10.1016/j.theochem.2010.04.036
(59) Kosinova,P.;Di Meo,F.;Anouar,E.H.;Duroux,J.L.;Trouillas,P.Int.J.Quantum Chem.2011,11,1131.
(60)Zhang,H.Y.;Wang,L.F.;Sun,Y.M.Bioorg.Med.Chem.Lett.2003,13,909.doi:10.1016/S0960-894X(03)00013-1
(61)Zhang,I.Y.;Wu,J.M.;Luo,Y.;Xu,X.J.Comput.Chem.2011,32,1824.doi:10.1002/jcc.v32.9
(62)Wu,J.M.;Zhang,I.Y.;Xu,X.ChemPhysChem 2010,11,2561.doi:10.1002/cphc.201000273
(63) Alecu,I.M.;Truhlar,D.G.J.Phys.Chem.A 2011,115,2811.doi:10.1021/jp110024e
(64) Dhaouadi,Z.;Nsangou,M.;Garrab,N.;Anouar,E.H.;Marakchi,K.;Lahmar,S.J.Mol.Struct.-Theochem 2009,904,35.doi:10.1016/j.theochem.2009.02.034
(65)Mikulski,D.;Gorniak,R.;Molski,M.Eur.J.Med.Chem.2010,45,1015.doi:10.1016/j.ejmech.2009.11.044
(66) Trouillas,P.;Marsal,P.;Siri,D.;Lazzaroni,R.;Duroux,J.L.Food Chem.2006,97,679.doi:10.1016/j.foodchem.2005.05.042
(67) Leopoldini,M.;Pitarch,I.P.;Russo,N.;Toscano,M.J.Phys.Chem.A 2004,108,92.doi:10.1021/jp035901j
(68) Bowater,L.;Fairhurst,S.A.;Just,V.J.;Bornemann,S.FEBS Lett.2004,557,45.doi:10.1016/S0014-5793(03)01439-X
(69) Lien,E.J.;Ren,S.;Bui,H.H.;Wang,R.Free Radic.Biol.Med.1999,26,285.doi:10.1016/S0891-5849(98)00190-7
(70) Tejero,I.;Gonzalez-Garcia,N.;Gonzalez-Lafont,A.;Lluch,J.M.J.Am.Chem.Soc.2007,129,5846.doi:10.1021/ja063766t

