Hydrothermal Syntheses,Crystal Structures and Properties of Keggin-type Polyoxometalate[ZnⅡO0.5(4,4'-bpy)1.5(H2O)2]2[HPMoⅥ12O40]·4H2O
Lü Xiuqing,LI Gaixian,CHEN Jianxin
(1.College of Chemistry and Chemical Engineering,Jinzhong University,Jinzhong 030600,China;2.College of Chemistry and Material Science,Fujian Normal University,Fuzhou 350002,China)
1 Introduction
The widespread contemporary interest in organic-inorganic hybrid compounds,which are expected to be endowed with enhanced or combined functionalization of both organic and inorganic substructures,has been focused on the richness of their structures and special properties in the fields of catalysis and materials science as well as in biology,magnetism,nonlinear optics and medicine[1-6].By incorporating organic and inorganic counterparts into a single structure,the functionality of organic-inorganic hybrid materials can be multiplied from both organic linker molecules and inorganic species[2-8].In this communication,we report the hydrothermal synthesis,crystal structure and catalytic properties of a novel organicinorganic hybrid polyoxovanadate:[ZnO0.5(4,4'-bpy)1.5(H2O)2]2[HPMoⅥ12O40]·4H2O.
2 Exiperimental
2.1 Materials and physical measurements
All AR grade chemicals and solvents were purchased commercially and used without further purification.
The IR spectra were measured on a Perkin-Elmer FT-IR 2000 spectrometer with KBr pellets in the 4000~400 cm-1region.Elemental analysis was carried out by an EA1112 CHNS elemental analyzer.UV/Vis absorption spectra were recorded on a Perkin-Elmer Lambda 900 spectrometer.
2.2 Synthesis of the complex
A mixture of MoO3(0.036 g),ZnO(0.020 g),P2O5(0.035 g),4,4'-bpy(0.078 g)and H2O(9ml)was sealed in a 15 ml Teflon-lined stainless steel autoclave with 50%filling.The resulting mixture was heated at a rate of ca.100℃·h-1to 160℃and held at this temperature for 2 days.Subsequently the autoclave was cooled to room temperature at a rate of ca.5℃·h-1.The resulting black platelet crystals were filtered and washed with water,then dried at ambient temperature.Yield:26.9%(based on Zn).Anal.Calcd(%)for:C,3.84;H,1.62;O,30.37;N,3.31;P,1.19;Mo,44.53;Zn,5.08.C30H41N6O49PZn2Mo12([ZnO0.5(4,4'-bpy)1.5(H2O)2]2[HPMoⅥ12O40]·4H2O)Found(%):C,13.95;H,1.32;O,30.35;N,3.25;P,1.20;Mo,44.58;Zn,5.06.
2.3 Crystallographic data collection and structure determination
A summary of the crystal data,data collection and refinement parameters for title compound is given in Table 1.Data were collected at 293 k on a Rigaku R-AXIS RAPID Weissenberg IP diffractometer equipped with a graphite-monochromatized Mo-Kα radiation(λ =0.71073 Å).Lorentz-Polarization corrections and empirical absorption correction were applied to the data.The structures was solved by direct methods with SHELXS-97[9].and refined on F2using full-matrix least-squares calculations with SHELXL-97[10].All non-hydrogen atoms were refined with anisotropic thermal parameters,and most hydrogen atoms were located in the calculated positions.All calculations were performed on a Pentium IV computer.Selected bond lengths and angles are provided in Table 2 and Table 3 for compound.

Tab.1 Crystallographic data for compound表1 化合物的晶体学数据
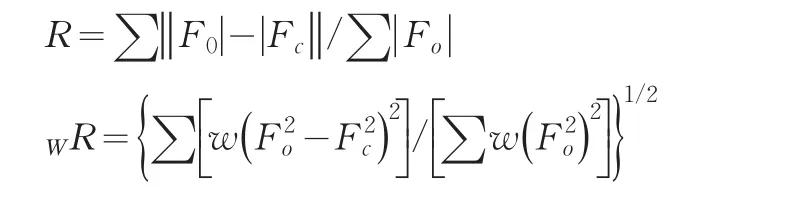
2.4 Catalytic reaction
0.05 g powder of title compound,5.0 ml(0.050 mol)benzaldehyde and 10.0 ml 30%H2O2(0.10 mol)were added to a 50 ml three-necked flask,which was placed on a magnetic stirring heater.Under stirringand the protection of N2,the reaction was carried out at 50℃for 1.0 h,then the reaction was stopped and the catalyst was separated off.The standard NaOH solution was used to titrate the reactant solution under the protection of N2.The catalytic activity was evaluated by the yield of benzoic acid.
Tab.2 Selected bond lengths(Å)表2 化合物主要键长
3 Results and discussion
3.1 Structure description
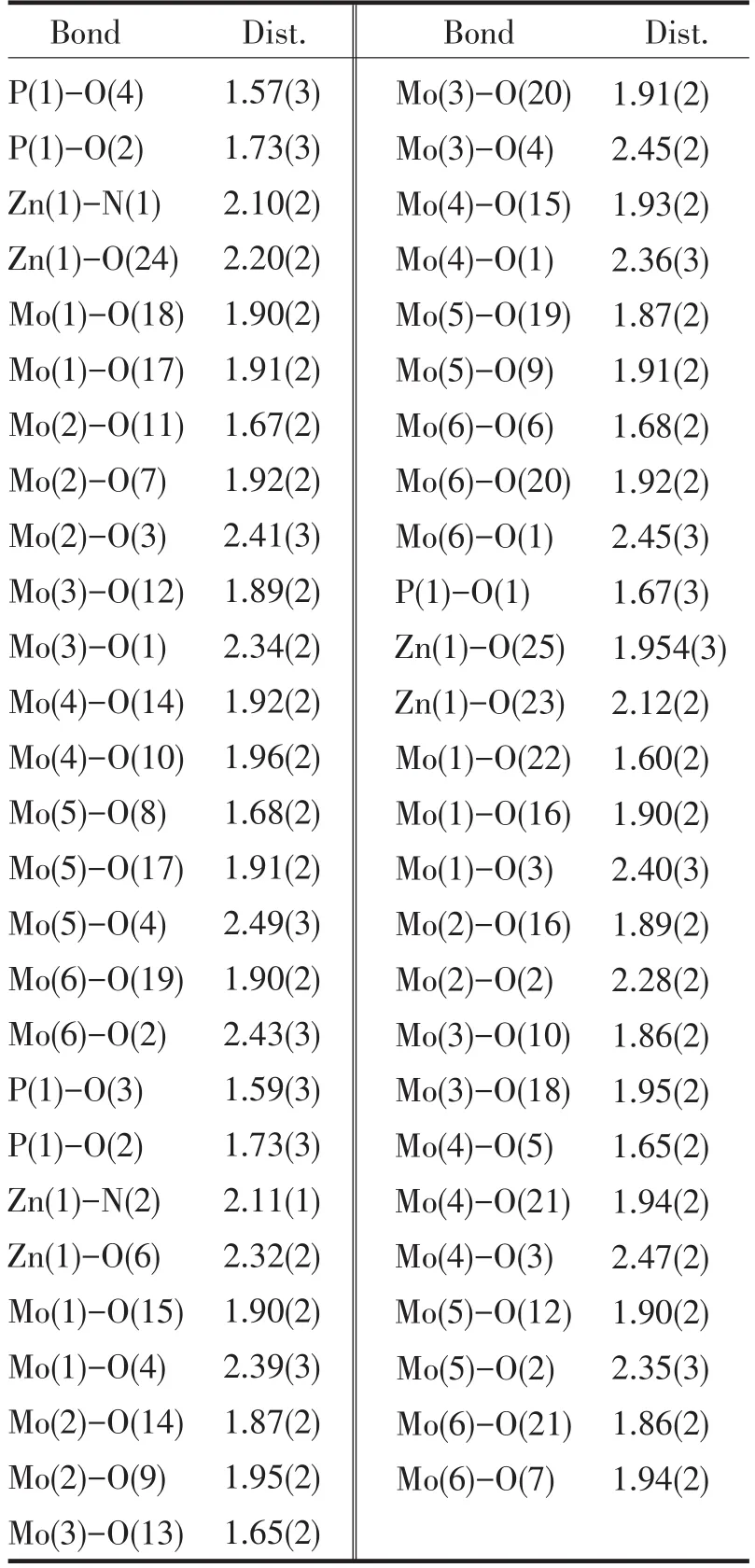
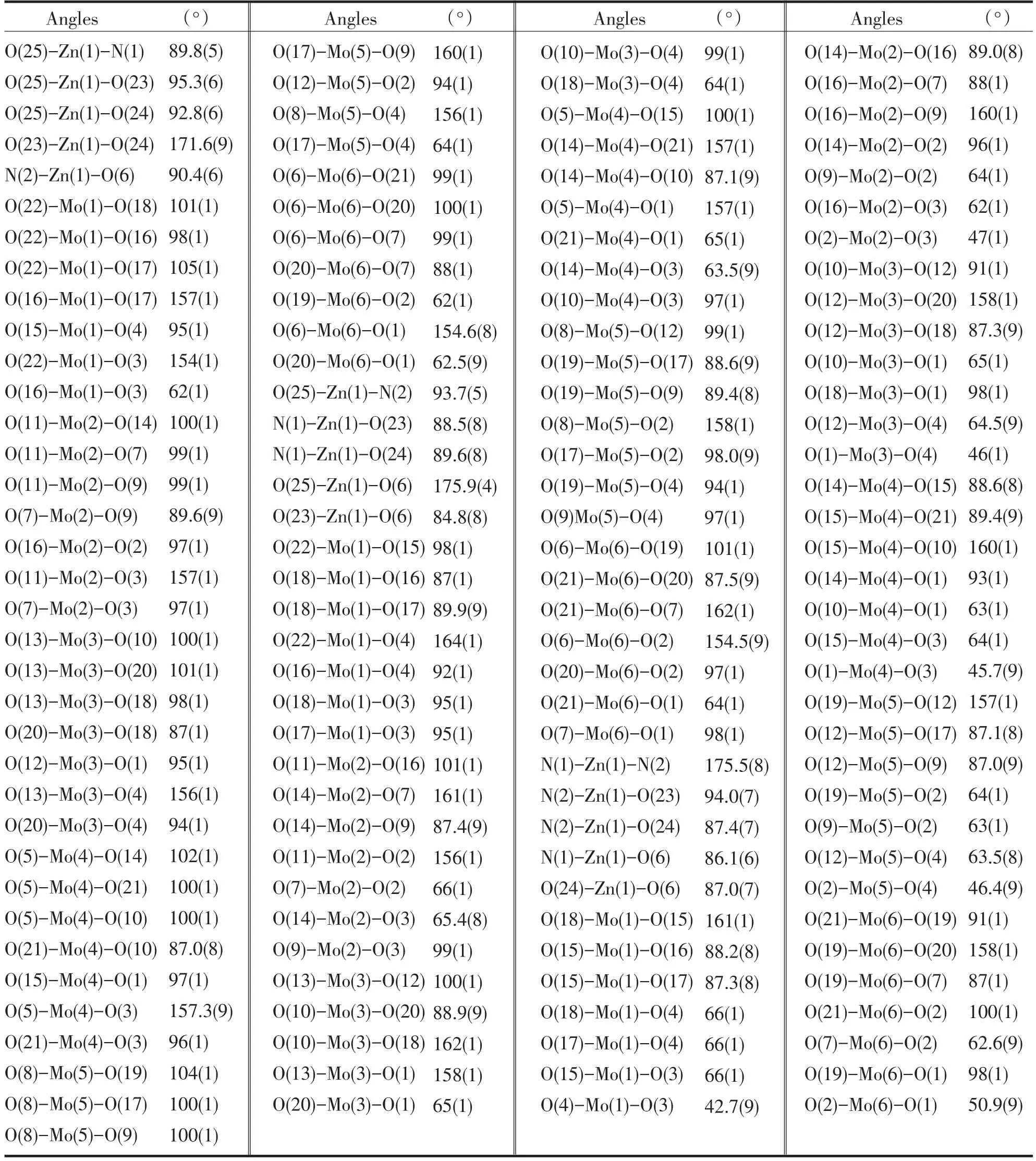
A single crystal analysis revealed that the complex consists of one Keggin polyanion[HPMoⅥ12O40]2-,two[ZnⅡO0.5(4,4'-bpy)1.5(H2O)2]+coordinated cations and four water molecules(Fig.1).The polyoxoanion is based on a central PO4tetrahedron surrounded by twelve MoO6octahedra arranged in four groups of three edge-shared octahedral Mo3O13.The Mo3O13groups were linked by sharing corners and linked to the central PO4tetrahedron.The P-O distances in the PO4tetrahedron are in the 1.573(3)-1.73(3)Å range.The Mo-O distances lie in the 1.60(2)~2.68(1)Å range for the terminal oxygen atoms(Mo-Ot),2.35(3)~2.49(2)Å for the edge-sharing oxygen(Mo-OC)and 1.86(2)~1.96(2)Å for double bridging oxygen(Mo-Ob),and the O-Mo-O angles fall in the 62(1)~160(1)°range.The P atom is located at the inversion center and surrounded by a cube of eight oxygen atoms with each oxygen site being half-occupied.The[HPMo12O40]2-unit bonded to two[ZnⅡO0.5(4,4'-bpy)1.5(H2O)2]+fragments through the terminal oxygen atoms.Each Zn atom is coordinated by two nitrogen atoms(N1 and N2)from two 4,4'-bpy ligands,one oxygen atom from[HPMoⅥ12O40]2-anion,two oxygen atom(O23 and O24)from two water molecules and a μ2-O(O25)to finish its distorted octahedral coordination environment[11].The Zn1-N distances lie in 2.10(2)~2.11(2)Å,The Zn1-O angles in the 1.954(3)~2.32(2)Å;The N-Zn1-O angles fall in the 86.1(6)~94.0(7)°.
The calculated results show that,in the structure of polyoxoanion,the Mo centers are in+6 oxidation state.Thus,the anion of the title compound is[HPMoⅥ12O40]2-.
3.2 IR Spectra and UV-visible spectra
The IR Spectrum(Fig.2)of compound exhibits prominent bands for the polyanion at 958,890 and 796 cm-1,attributed to ν(Mo-O)or ν(Mo-O-Mo).Bands in the 1604-1060 cm-1region are ascribed to characteristic vibration of 4,4'-bpy groups.The 3483 cm-1is due to characteristic vibration of water molecule.The results indicate that heteropolymolybdates in compound retain Keggin structure and has interactions with organic moieties in the solid state.
The oxygen-to-metal and/or phenyl ring π-π*charge transfer absorption maxima in the UV-visible spectrum occur at 248.0,319 and 379.0 nm(Fig.3).
Tab.3 Selected Bond Angles(°)表3 化合物主要键角
3.3 Catalytic activity determination
To investigate the oxidative catalytic activity of title compound,we have performed a probe reaction of the oxidation of benzaldehyde with H2O2using title compounds as catalysts.Under the earlier mentioned experimental conditions,the yield of benzoic acid is up to 89.1%in compound.In a comparative experiment where in no catalyst is added,the yield of benzoic acid is only 18.9%.This showed that compound has high catalytic activity for the oxidation of benzaldehyde with H2O2.
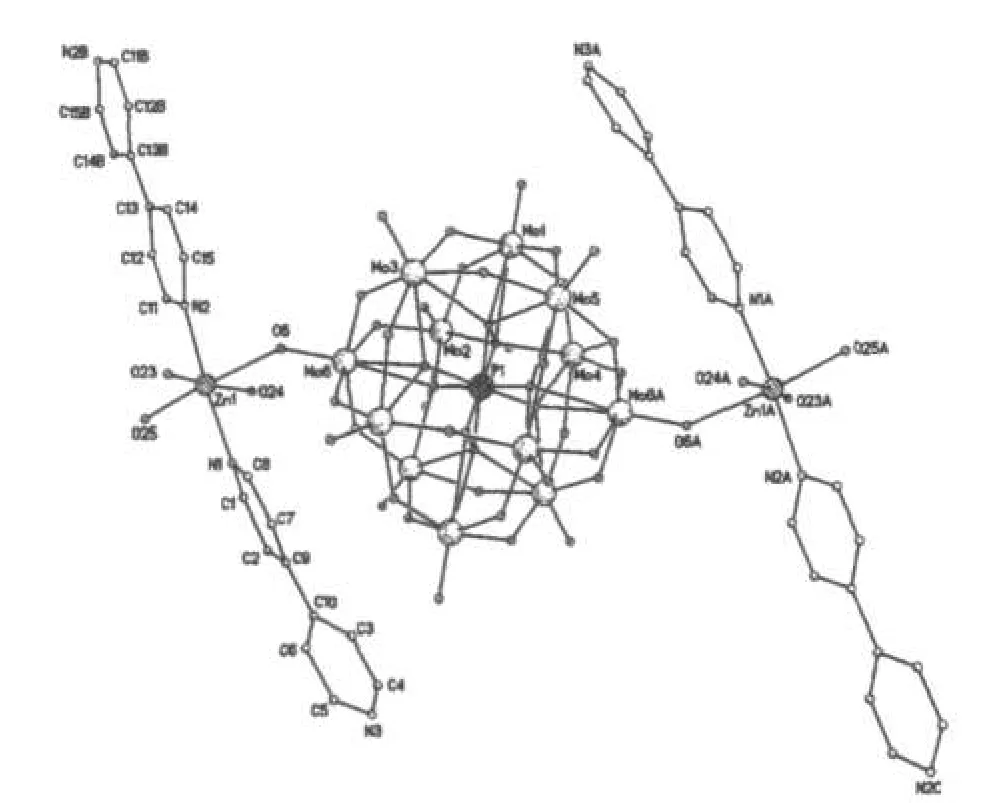
Fig.1 Molecular structure of the title complex图1 化合物的分子结构图
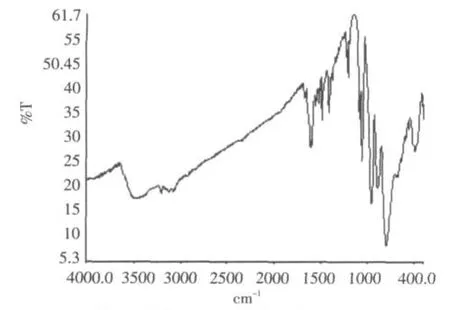
Fig.2 IR spectrum of the title compound图2 化合物的红外光谱图
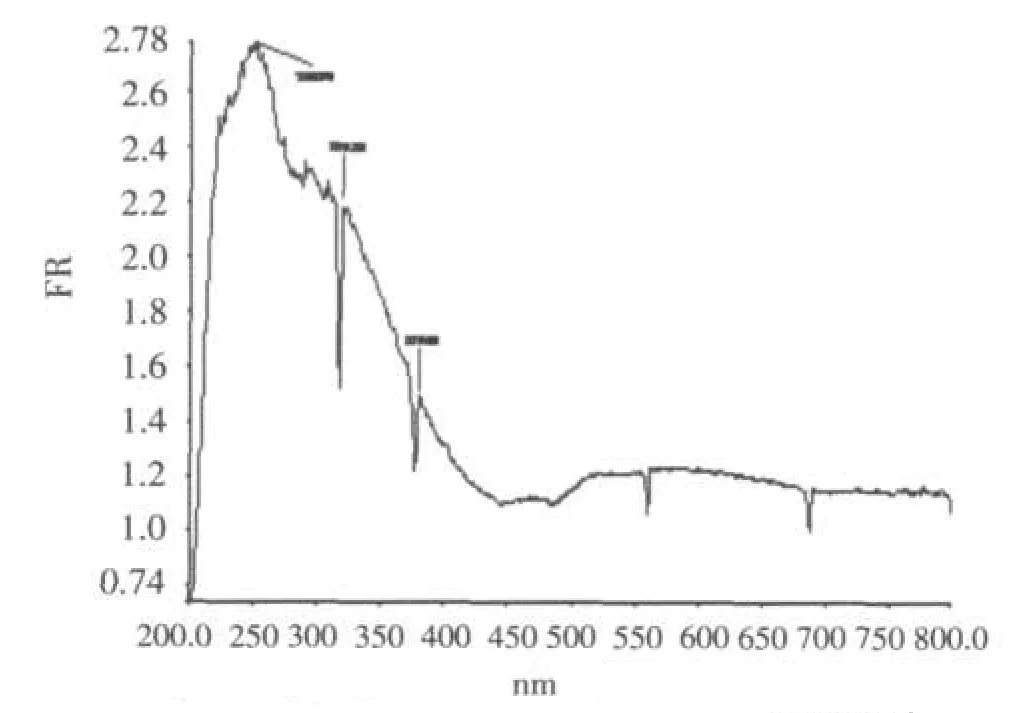
Fig.3 UV-Vis spectrum of the title compound图3 化合物的紫外光谱图
4 Conclusions
Organic-inorganic compound[ZnO0.5(4,4'-bpy)1.5(H2O)2]2[HPMoⅥ12O40]·4H2O.has been synthesized and the crystal structure of the compound was determined.Furthermore,the strong photoluminescence properties of compound may make it excellent candidates for potential photoactive materials.Meanwhile,the catalytic activities of the comound was determined by the oxidation of benzaldehyde to benzoic acid using H2O2as oxidant in a liquid-solid biphase system,which indicate that compound has high catalytic activity for the oxidation of benzaldehyde with H2O2.
[1]Hill C L.Polyoxome talates-multicomponent molecular vehicles to probe fundamental issues and practical problems[J].Chem Rev,1998,98(1):1-2.
[2]Wang E B,Hu C W.Introduction of heteropoly acid chemistry[M].Beijing:Chemical Industry Press,1984:16.
[3]Wang J P,Guo T J.Preparation,Characterization,Crystal Structure of a Charge-Transfer Complex Based on H4SiW12O40·nH2O and Diethanolamine[J].Chinese J Appl Chem,2002,19(9):852-856.
[4]Akutagawa T,Endo D,Imai H,et al.Formation of pphenylenediamine-crown ethe[PMo12O40]Salts[J].Inorg Chem,2006,45(21):8628-8637.
[5]Lu M,Wei Y G,Xu B B,et al.Hybrid molecular dumbbells:bridging polyoxometalate clusters with an organicconjugated rod[J].Angew Chem Int Ed,2002,41(9):1566-1568.
[6]Han Z G,Ma H Y,Peng J,et al.The first polyoxometalate polymer constructed by assembly of the heptamolybdic anion and copper coordination groups[J].Inorganic Chemistry Communications,2004,7:182-185.
[7]Joseph B,Glenn P A,et al.A new class of functionalized polyoxometalates:synthetic,structural,spectroscopic,and electrochemicalstudiesoforganoimido derivativesof[Mo6O19]2-[J].Am Chem Soc,2000,122:639-649.
[8]Xiao D R,Hou Y,Wang E B,et al.Hydrothermal synthesis and characterization of an unprecedented-type octamolybdate:[{Ni(phen)2}2(Mo8O26)][J].Inorganica Chimica Acta,2004,357:2525-2531.
[9]Sheldrick G M.SHELXS-97,Program for X-ray Crystal Structure Solution[D].University of Göttingen:Göttingen,Germany,1997.
[10]Sheldrick G M.SHELXL-97,Program for X-ray Crystal Structure Refinement[D]. University of Göttingen:Göttingen,Germany,1997.
[11]Lv X Q,Chen J X,Lan T Y,et al.Hydrothermal synthesis and crystal structureof a bivanadyl capped keggin polyoxometalate[Zn(2,2'-bpy)2(H2O)]2[HPMo12O40(VO)2][J].Chinese J Struct Chem,2005,24(2):139-144.

