2,4-二氯苯氧乙酸构筑的三核锌ギ配合物的合成、晶体结构及性能分析
杨颖群 陈志敏 匡云飞
(1衡阳师范学院化学与材料科学系,衡阳 421008)
(2衡阳市西城有机化工厂,衡阳 421008)
0 Introduction
The study of carboxylic complexes has become one of the hottest research subjects in coordination chemistry due to their versatile topological structures and potentialapplications in magnetism,optical device,catalysis and biological fields[1-6].Phenoxyacetic acid and its derivatives are a class of aromatic carboxylic acid with wide application.They can be used as conventional fungicides, plant growth regulators and agricultural herbicides[7].Moreover,they can be used as ligands to build complexes[8-14].In recent years,by using 2,4-dichlorophenoxy acetic acid(HL)as a ligand,we have synthesized some carboxylate complexes,and studied their properties[15-19].Our present work is just a continuous study of our research on the synthesis of carboxylate complexes by using HL.We report herein the synthesis method,crystal structure and magnetic,luminescence and electrochemical properties of a new trinuclear Znバcomplex[Zn3L6(2,2′-bipy)2](1)with bridged L-anions as ligands.
1 Experimental
1.1 Materials and instrumentation
All materials were of analytical grade and used without further purification.Crystal structure determination was carried out with a Bruker Smart CCD area diffractometer.Melting point measurement was executed on a Beijing-made XT4 binocular micromelting point apparatus.Magnetic measurements in the range of 2.8 ~300 K were performed on a MPMSSQUID magnetometer at a field of 2 kOe on a crystalline sample in the temperature settle mode.Luminescence spectrum was obtained at room temperature on a WGY-10 fluorescence spectrophotometer.Cyclic voltammogram was measured on a CHI660D electrochemical workstation from Shanghai Hua Chen.
1.2 Synthesis of the title complex
A mixture of zinc acetate dihydrate (0.27 mmol,0.060 g),HL(0.66 mmol,0.145 g)and 2,2′-bipyridine(0.12 mmol,0.018 g)was dissolved in in 6 mL mixed solvent of CH3OH/DMF/H2O(volume ratio:1∶1∶1).The reaction was kept under water-bath condition at about 343 K for 25 h.The resultant solution was filtered and the filtrate was kept untouched and evaporated slowly at room temperature.Colorless particles-shaped single crystals suitable forX-ray analysiswere obtained after six weeks.Yield:37% .M.p.:181.0~181.5 ℃.
1.3 Crystal structure determination
A crystal with the dimensions of 0.25 mm×0.24 mm ×0.23 mm was put on Bruker Smart CCD area diffractometer equipped with a graphite-monochromatic Mo Kα radiation(λ=0.071 073 nm).By using ω-φ scan mode at 296(2)K,we collected 9 395 reflections in the range of 1.52°≤θ≤25.01°,of which 6 405 were independent with Rint=0.011 1,and 5 437 were observed(I>2σ(I))and used for further refinement.The crystal structure was directly solved with SHELXS-97 program[20].Corrections for Lp factors and empirical adsorption adjustment were applied and all nonhydrogen atoms were refined with anisotropic thermalparameters.The final refinement including hydrogen atoms converged to R1=0.030 6,wR2=0.075 7,(Δ/σ)max=0.001,S=1.020.The crystallographic data and structure refinement of the title complex are shown in Table 1.

Table 1 Crystallographic data and structure refinement for the title complex
CCDC:874918.
2 Results and discussion
2.1 Crystal structure
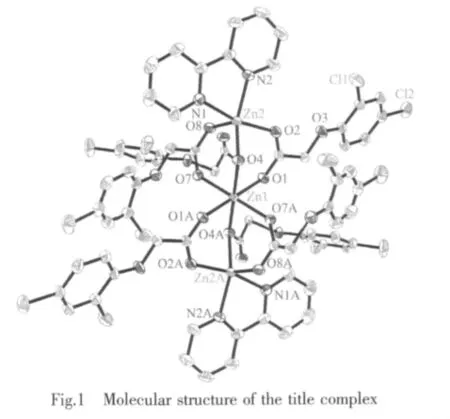
In Table 2 are some selected bond lengths and bond angles of 1.Fig.1 reveals its molecular structure.As shown in Fig.1,1 consists of three central Zn2+ions,six L-anions,and two 2,2′-bipyridine molecules.The six L-anions have two coordination modes:bidentatebridging coordination and monodentate-bridging coordination.Owing to the bridging coordination of L-anions,the whole molecule presents a trinuclear structure,where the adjacent Zn(1)-Zn(2)distance is 0.335 9 nm.The central Zn(1)ion is coordinated by six oxygen atoms from six L-anions to give a distorted octahedral coordination geometry in which O(1),O(7),O(1A)and O(7A)locate at the equator plane,O(4)and O(4A)occupy the axial positions.The sum of bond angles O(1)-Zn(1)-O(7),O(7)-Zn(1)-O(1A),O(1A)-Zn(1)-O(7A)and O(7A)-Zn(1)-O(1)at equator plane is 360°.Compared with Zn(1)ion,Zn(2)ion is located in a distorted trigonal bipyramid coordination environment,where O(4),N(2)and O(8)locate at the equator plane,but O(2)and N(1)occupy the axial positions.Bond angles O(4)-Zn(2)-N(2),O(8)-Zn(2)-N(2)and O(8)-Zn(2)-O(4)are142.85(7)°,106.80(8)° and 109.51(7)°,respectively,and the sum of the above angles is 359.16°.The O-Zn(1)-O angles of 1 vary considerably from 85.63(7)° to 180.00(6)°.The OZn(2)-O angles of 1 vary from 91.45(7)°to 109.51(7)°.The Zn-O distances are in the normal range of 0.201 12(16)~0.215 55(16)nm.In 1,the average length of Zn-N bonds is 0.212 4 nm,Shorter than that of Zn-N bonds (0.214 2 nm)in similar complex Zn3(phen)2(2,4-DAA)6[21].
2.2 Magnetic properties
The magnetic susceptibility data of 1 under variable temperatures (2.8 to 300 K)were colletedwith an applied magneticfield of2 kOe.The temperature dependence ofthe molar magnetic susceptibility of 1 is presented in Fig.2 in the form of χmvs T.The product of χmincreases slowly from-3.358 7 ×10-4cm3·mol-1at 300 K to-9.280 3×10-6cm3·mol-1at 28 K,and the value of χmis negative.Such magnetic behavior indicates 1 is a diamagnetism system in temperatures range of 28~300 K.

Table 2 Selected bond lengths(nm)and bond angles(°)of the title complex

2.3 Luminescent properties
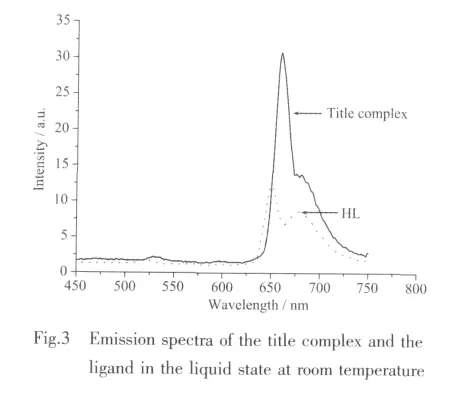
Under the same conditions,the fluorescence property of 1 and ligand HL were measured in the liquid state at room temperature in the range of 450~750 nm with the best excitation wavelength at 664 and 654 nm,respectively,and their emission spectrums are shown in Fig.3.1 exhibited one intense fluorescence emission band at around 660 nm.HL displayed fluorescence emission at about 650 nm.Compared with HL,1 has a similar emission band shape and position,which indicates that intraligand excitation is responsible for the emission of 1.In addition,probably due to the increased rigidity of the ligand coordinated with metal ions,the fluorescence intensity of 1 is stronger than that of HL[22-23].
2.4 Electrochemical properties
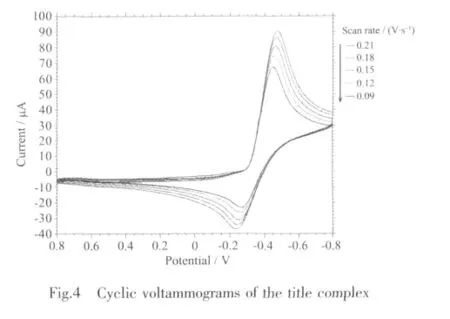
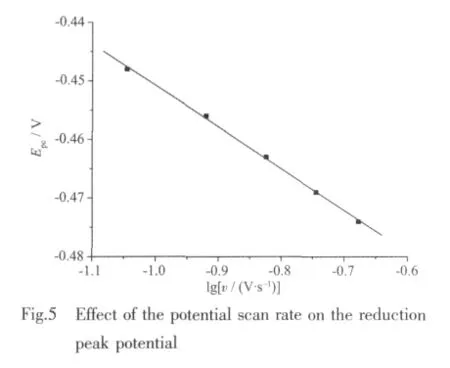
Fig.4 shows the cyclic voltammogram(CV)curve of1.In the CV measurement,we employed a conventional three-electrode system where a platinum electrode was chosen as the working electrode,a saturated calomel electrode (SCE)as the reference electrode,and glass/C as the counter electrode.1 was dissolved into the solvent of methanol,the resulted solution being of the concentration of 1 ×10-3mol·L-1.HAc-NaAc solution (pH=4.0)was used as buffer solution,and KCl is used as the supporting electrolyte.The scanning range is-0.8~0.8 V,and the scan rate is 0.09~0.21 V·s-1.As revealed by Fig.4,there exists a pair of redox peak in every CV curve,and ipais not equal to ipc,which demonstrates that the electron transfer in the electrode reaction is quasi reversible.In addition,the reduction peak potential(Epc)shifts to a more negative value with increasing scan rate(v),and it is proportional to lgv (Fig.5).The linear regression equation is Epc=-0.071lgv-0.521 8 with the correlation coefficient 0.998 6.Based on the slope of Epcwith lgv,the number of electrons involved in the reduction of 1 can be evaluated.The αn is calculated to be 0.83.Generally,the electron transfer coefficient α is about 0.5 in the totally quasi reversible electrode process.So,the value of n is about 2,indicating that two electron is involved in the reduction of 1,and the electrode reaction corresponds to Znギ/Zn(0).
[1]Creaven B S,Egan D A,Karcz D,et al.J.Inorg.Biochem.,2007,101(8):1108-1119
[2]Rafizadeh M,Amani V,Zahiri S.Acta Crystallogr.,Sect.E:Struct.Rep.Online,2007,63:m1938-m1939
[3]Braverman M A,Supkowski R M,Laduca R L.Solid State Chem.,2007,180(6):1852-1862
[4]Beko S L,Bats J W,Schmidt M U.Acta Crystallogr.,Sect.C:Cryst.Struct.Commun.,2009,65:m347-m351
[5]TIAN Jing(田婧),DONG Li-Na(董丽娜),ZHAO Kai(赵凯),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(1):113-118
[6]WANG Ji-Jiang(王记江),HOU Xing-Yang(侯向阳),CAO Pei-Xiang(曹 培 香 ),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(4):829-832
[7]YANG Ying-Qun(杨颖群),LI Chang-Hong(李昶红),LI Wei(李薇),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(10):1890-1894
[8]Du M,Cai H,Zhao X J.Acta Crystallogr.,Sect.E:Struct.Rep.Online,2004,60:m1139-m1141
[9]Gao S,Liu J W,Huo L H,et al.Acta Crystallogr.,Sect.E:Struct.Rep.Online,2004,60:m1308-m1310
[10]GAO Shan(高山),YUE Yu-Mei(岳 玉 梅),MA Dong-Sheng(马东 升),et al.Chinese J.Struct.Chem.(Jiegou Huaxue),2004,23:825-828
[11]Gao S,Huo L H,Ng S W.Acta Crystallogr.,Sect.E:Struct.Rep.Online,2005,61:m2270-m2272
[12]Zhang X F,Gao S,Huo L H,et al.Acta Crystallogr.,Sect.E:Struct.Rep.Online,2005,61:m2471-m2473
[13]HUO Li-Hua(霍丽 华)GAO Shan(高 山)LIU Ji-Wei(刘 继伟),et al.Chinese J.Struct.Chem.(Jiegou Huaxue),2005,24(3):334-338
[14]Wen Y H,Ng S W.Acta Crystallogr.,Sect.E:Struct.Rep.Online,2007,63:m2378-m2379
[15]YANG Ying-Qun(杨颖群),LI Chang-Hong(李昶红),LI Wei(李薇),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(9):1531-1534
[16]LI Wei(李薇),LI Chang-Hong(李昶红),YANG Ying-Qun(杨颖群),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(2):269-372
[17]YANG Ying-Qun(杨颖群),LI Chang-Hong(李昶红),LI Wei(李薇),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,25(6):1120~1123
[18]YANG Ying-Qun(杨颖群),LI Chang-Hong(李昶红),LI Wei(李薇),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,28:1385-1388
[19]Yang Y Q,Li C H,Li W et al.Z.Kristallogr.NCS,2010,225:255-256
[20]Sheldrick G M.Acta Crystallogr.,1990,A46:467-473
[21]YANG Ying-Qun(杨颖群),LI Chang-Hong(李昶红),LI Wei(李 薇 ),et al.Chem.J.Chinese Univ.(Gaodeng Xuexiao Huaxue Xuebao),2008,29:449-452
[22]XUE Ying-Wen(薛莹雯),XU Qing-Feng(徐庆锋),ZHANG Yong(张 勇),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2005,21(11):1735-1739
[23]LI Jing(李婧),ZHOU Jian-Hao(周建豪),LI Yi-Zhi(李一志),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2004,20(7):841-844