热分解法和碳热还原法制备掺钨VO2/云母复合粉体的热致变色性能
赵 冬 黄婉霞*, 蔡靖涵 何 鹏 颜家振 吴 静 宋林伟
(1四川大学材料科学与工程学院,成都 610064)
(2四川大学制造科学与工程学院,成都 610064)
There have been at least 6 kind polymorphs of vanadium dioxide (VO2)reported up to now,such as VO2(A)[1],VO2(B)[2],VO2(C)[3],VO2(D)[4],VO2(M)[5]and VO2(R)[6].The reversible semiconductor-metal transition(SMT)between VO2(M)and VO2(R)[7]has attracted numerous attention due to enormous mutation of electrical resistivity, optical transmittance and reflectance at a critical transition temperature (Tt)of 68℃.These abrupt changes and the relatively low transition temperature bring VO2attractive materials for a wide variety of technological applications.One important potential is the use of VO2as intelligent window coatings-a window can change one or more of its properties in response to some external stimulus proposed by Granqvist[8].Because VO2(R)can block near infrared solar transmission (most of the heat from the sun comes in these wavelengths),while VO2(M)is almost infrared-transparent.More exciting,the visible light transmittance remains almost unchanged.Thus,it can replace curtain glass and low-emission(Low-E)glass and make the buildings adjust the indoor temperature automatically and intelligently.
For half a century,various approaches have been employed to make the characteristics of the SMT of VO2into practical application.The most direct method is using sol-gel,sputtering or deposition technology to adhere VO2on substrates, such as silicon[9],muscovite[10],quartz[11],to form thermochromic films.As the eigen-Ttof VO2is too high in practice,many dopants,such as W,Mo,F,have been tried to manipulate the Ttclose to room temperature,and W has been proved to be the most efficient dopant,which can decrease the Ttby 27℃ per 1at%doping[12].Nevertheless,we must face the fact that it is complex and expensive to prepare large area films using current techniques,thuslimiting the application ofVO2intelligent windows in practice.
Compared with thin films,VO2powder can not only prevent oxidation,but also reduce the phase change stress[13].In the meantime the cost is lower.In this regard,Valmalette[14]and Shi[12]have attempted to disperse VO2into polymer coatings,and obtained VO2/polymer composite films with good thermochromism.In general process of preparing VO2,it is necessary to use heat treatment to achieve the transformation of V5+to V4+,which is a much harsh reaction.This transition is usually achieved by vacuum thermal decomposition,atmosphere protected annealing, carbothermal reduction,etc.The heating temperature usually ranges from 460 to 650℃.In contrast,the vacuum thermal decomposition process is simple and clean,but requires a high degree of vacuum equipment,and thus not suitable for industrialization.Atmosphere protected annealing uses inert gas to achieve relative vacuum,which requires not too complex devices and is easy to implement.Carbothermal reduction has also been used to prepare VO2[15],and the only by-product is CO2,which can prevent the production from being contaminated by other impurities.So it has a great practical value.
In previous works[16-17],we have clad VO2on mica powder,i.e.VO2/Mica composite powder,and achieved fairly good performance.In this paper,as the continuation of the works,we select tungsten as the dopant to VO2/Mica composite powder and report a facile and effective technique to prepare W-doped VO2by carbothermal reduction.Then the W-doped powder is embedded into ultraviolet(UV)curing resin to form composite films so as to achieve relatively lower Ttand better thermochromic properties.
1 Experimental
Ammonium tungstate((NH4)5H5[H2(WO4)6]·H2O,>99.0%pure)was mixed with vanadium pentoxide(V2O5,>99.7%pure)and used as additives to introduce doping ions into the powder(the nW/nVwas chosen at 0at%,0.620at%,1.117at%and 1.622at%).The mixture was put into a ceramic crucible and heated to 800℃in ambient condition for 30 min,and then poured into deionized water at room temperature.After vigorous stirring for 2 h,a brownish sol was formed.The mica flakes(20 μm(625 mesh))were sequentially pretreated by ethylalcohol,acid solution (VHCl∶VH2O2∶VH2O=1∶2∶5)and alkaline solution (VNH3·H2O∶VH2O2∶VH2O=1 ∶2 ∶5),in order to remove organic contaminants and ions on the surface of mica.
The precursor V2O5-gel/Mica powder was prepared by spray-drying method:4 g pretreated mica powder was added into 20 mL V2O5sol with evenly stirring,and then the mixed sol was sprayed in a spray-dry vessel at 120℃.V2O5gel was coated on the surface of mica in this procedure and V2O5-gel/Mica powder was obtained.
The VO2/Mica composite powder was prepared by two different heat treatment processes:
(a)Thermal decomposition method:the precursor V2O5-gel/Mica powderwas spread on corundum crucible and heated in a tube furnace to 520℃for 90 min with a heating rate of 8 ℃·min-1, under a static atmosphere ofargon,and then cooled to room temperature along with the furnace cooling.After that,different W-doped VO2/Mica composite powder was obtained.
(b)Carbothermal reduction method:the precursor V2O5-gel/Mica powder was slowly ground with stoichiometrically corresponding carbon black in a ceramic crucible,and then put into a tube furnace.The furnace temperature was increased to 520℃and annealed at this temperature for 3.5 h,then cooled to ambient temperature in the argon flow to prevent vanadium dioxide from oxidation.
In order to evaluate the thermochromic properties of the VO2/Mica powder by FTIR,the transparent samples of composite films were prepared.5wt%VO2/Mica powder was inserted into the UV curing resin and stirred evenly.The mixture was spread on the flat glass sheet and followed by irradiation-curing in the air,then the composite films were removed from the glass sheet.The films were about 40 μm in thickness.
The surface morphology of the VO2/Mica powder was determined by SEM (HitachiS-4800).The crystalline structure ofthe doped powder was characterized at room temperature by X-ray diffraction with a Philips diffractometer and Cu Kα (λ=0.15418 nm)radiation.The thermochromic characteristics of the composite powder and composite films were investigated by DSC(Netzsch 204,Germany)and FTIR(Bruke Tensor27,Germany)equipped with a controllable heater cellule.
2 Results and discussion
2.1 X-ray diffraction
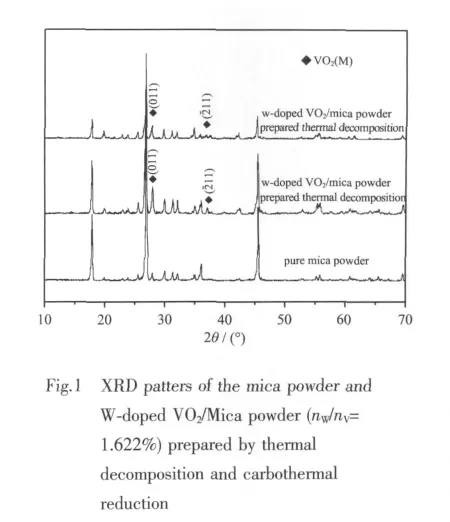
Fig.1 shows the XRD patterns of W-doped VO2/Mica powder with 1.622%W/V molar ratio.The mica′s three strong diffraction peaks are weakened after coating.In addition to the mica peaks,two peaks of VO2(011)(211)plane diffraction are found,which confirms that themain composition on micasubstrateis monoclinic VO2(PDF:43-1051).No peaks corresponding to tungsten are observed,because W dissolves in VO2grid to compose V1-xWxO2solid solution[18-19].This is the reason why tungsten has an effect on the Ttof the VO2(also discussed in section 3.3).If W is segregated from the VO2as a second phase,it would be no reduction of the Tt[20].
It also can be seen from the XRD that different heat treatment processes do not cause crystalline structure differences.The two processes both mainly achieve VO2(M).However,the powder prepared by thermal decomposition shows stronger VO2(M)peaks than carbothermal reduction, thus thermal decomposition samples have much better performance in the infrared range(shown in Fig.3).
2.2 SEM images
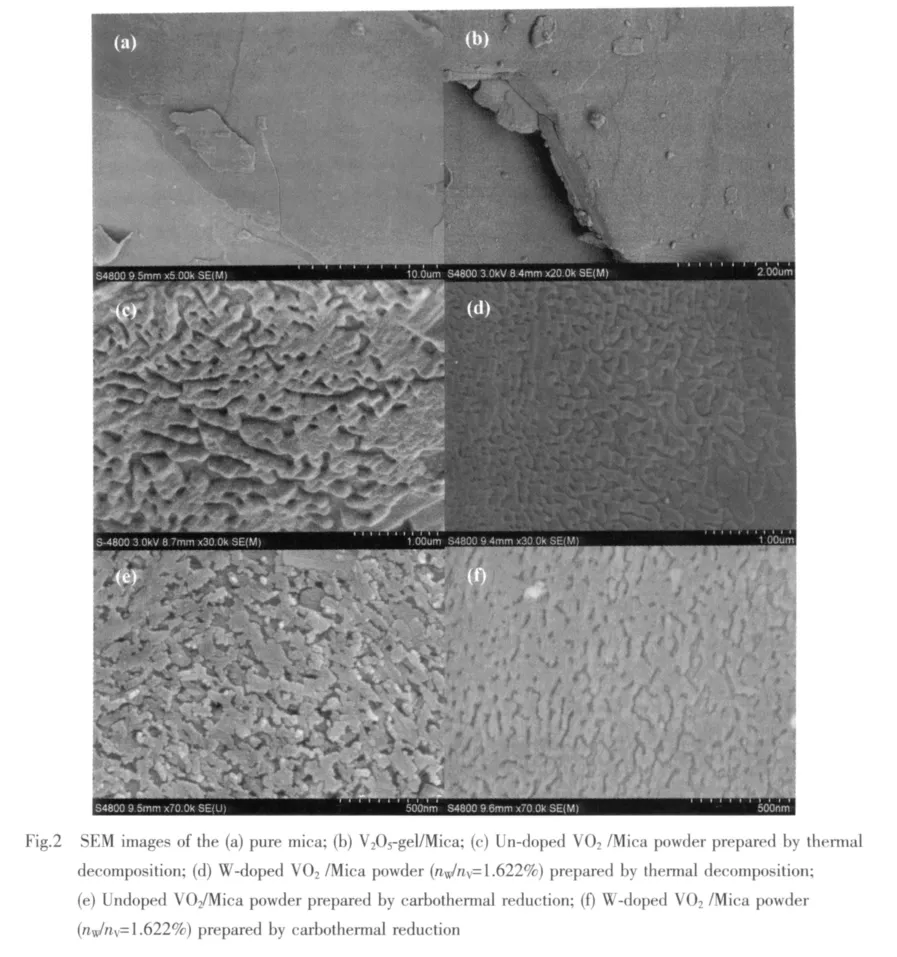
Fig.2(a)depicts a surface image of the mica flakes.Fig.2(b)shows the image of V2O5gel adhering to mica surface after a spray-drying process.From the image Fig.2(a)and Fig.2(b),the plane of mica with natural lamellar surface and cleavage layers can be easily seen.Fig.2(c)and Fig.2(d)show the surface micrographs of Un-doped and W-doped VO2/Mica composite powder with 1.622%W/V molar ratio prepared by thermal decomposition,while Fig.2(e)and Fig.2(f)prepared by carbothermal reduction.An of the surface consist of small worm-like grains and a lot of pores.The pores are formed because of the wetting properties difference between the mica substrate and the precursor sol[10],and contraction at spry-drying process and annealing.At the same time,the uncompact cladding and exist of pores make the mica substrate exposed,thus the mica′s diffraction peaks are still strong in the XRD patterns(show in Fig.1).
The natural sheet structure of mica makes it parallelly disperse in the medium,and worm-like VO2grains on its surface form similar to continuous film,which can show higher infrared radiation shielding efficiency compared to ultra-fine VO2powder.
Moreover,the doping leads to a noteworthy change in the surface micrographs.Compared with un-doped samples,doping samples have smaller particles and smoother surface,whether they are prepared by thermal decomposition or carbothermal reduction.
2.3 FTIR analyses
The transmittance spectra of W-doped VO2/Mica composite films with 1.622%W/V molar ratio,in the spectral range between 4 000 and 400 cm-1,is shown in Fig.3.The details of the absorption peaks have been discussed in reference 16.Clearly the transmittance of the composite films shows apparent changes.It is because that VO2undergoes transition from VO2(M)at 16℃ to VO2(R)at 70℃.The composite powder prepared by thermaldecomposition showslarger changes than that of carbothermal reduction,however.
The transmittance hysteresis loops of W-doped VO2/Mica composite films with different nW/nV,at the wavenumber of 2 200 cm-1,are shown in Fig.4.It can be seen from Fig.4 that,in the range of 10~100 ℃,the infrared transmittance of differentnW/nVcomposite films change greatly along with the temperature changes forboth thermal decomposition and carbothermal reduction.Undoped films exhibit the best switching property beyond 25%ΔTras the temperature increases from 20 to 100℃.ΔTris the maximum infrared transmittance changes of the films at 2 200 cm-1due to the semiconductor-metal transition.
With the nW/nVincreases gradually,the Tt(Tt=(Theating+Tcooling)/2)of the composite films decrease(shown in Table 1).The Theatingand Tcoolingare the temperatures of infrared transmittance dramatic change of VO2/Mica composite films when heating and cooling,respectively.The results show that,each addition of 1%nW/nVcan reduce the Ttby about 18℃,indicating a linear relationship between the doping dose and the reduction of Tt.When the molar ratio reaches 1.622%,the Ttreduces to about 40℃,which is closer to room temperature.The requirement of lower Ttcan be easily met by doping more tungsten.
However,it can not be ignored that,with an increase in the nW/nV,the ΔTrof the films minishes and the sharpness of the hysteresis loops declines.These phenomena are due to doping because doping leads to an increase in defects in VO2,and the defects cause a change in the performance ofphase transition[21].
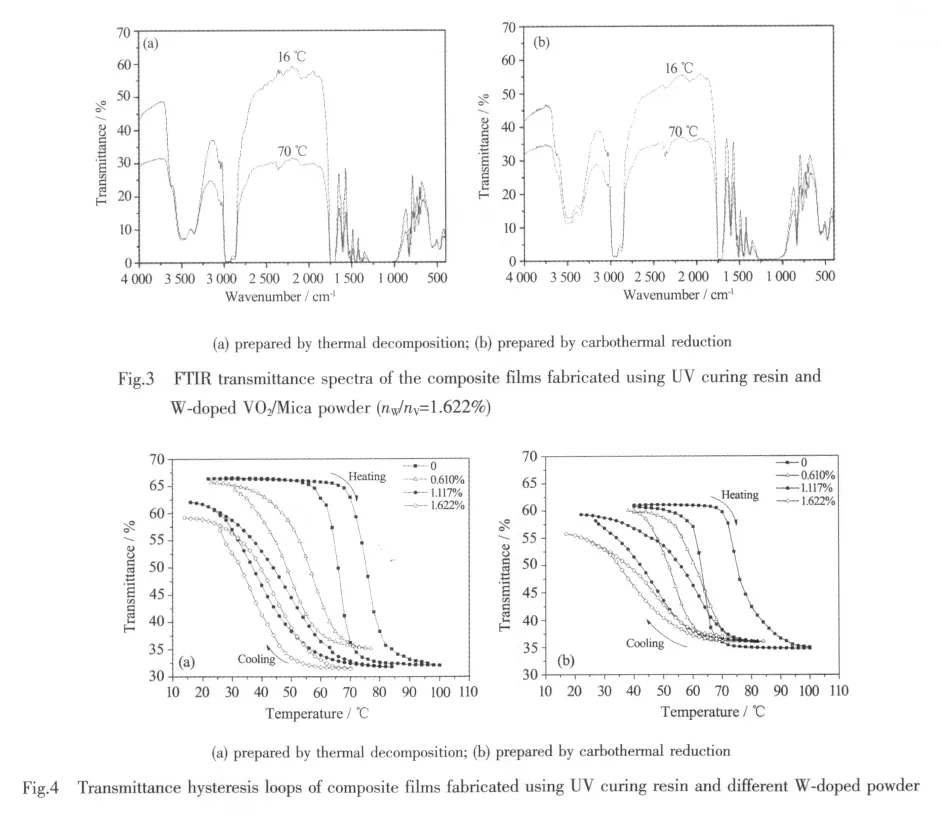
Compared with carbothermal reduction,samples prepared by thermal decomposition have larger ΔTrand smaller hysteresis width (Tw=Theating-Tcooling)(shown in Table 1),which are corresponding to the results of XRD and SEM.Maybe the litteryand irregular surface prepared by carbothermal reduction leads to more grain boundaries and stress.In addition,we find that samples prepared by carbothermal reduction have lower initial transmittance at low temperature.It may be due to the contamination of carbon black residual.
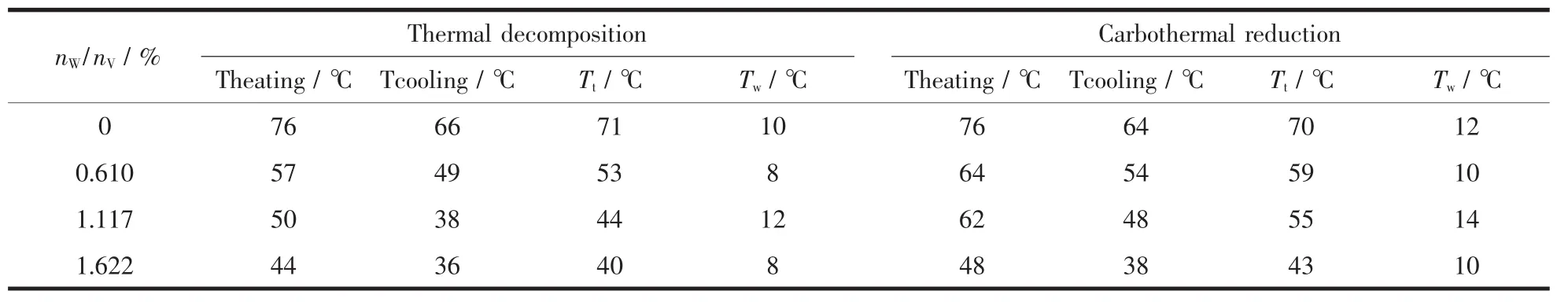
Table 1 Comparison of Ttand Twbetween two different heat treatment processes
2.4 DSC
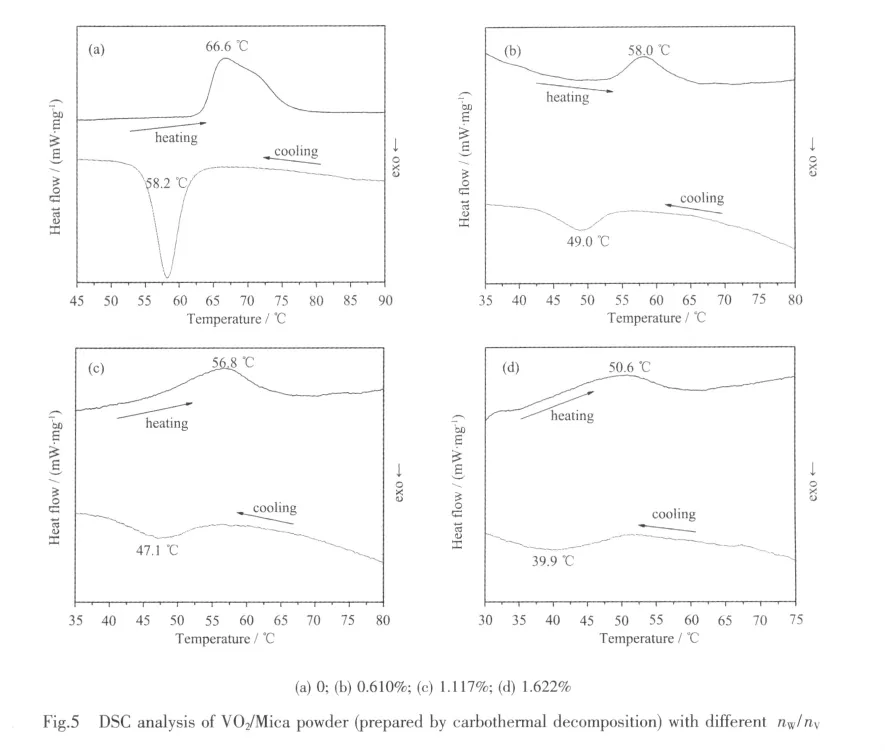
DSC test shows the same trend as FTIR analyses when doping W to VO2/Mica powder.Taking the powder prepared by carbothermal reduction as example,the typical DSC curves for W-doped VO2/Mica composite powder with different nW/nVare manifested in Fig.5.It can be seen that with increase in the nW/nV,the intensity of the endothermic and exothermic peaks weakens and the width of the peaks broadens,which is corresponding to the decreasing ofthe ΔTrand sharpness of the infrared transmittance hysteresis loops shown in Fig.4.The phase transition temperature(Tt)is related by Tt=(Ten+Tex)/2.Tenis the temperature of endothermic peak in the heating process and Texis the temperature of exothermic peak in the cooling process.The hysteresis width(Tw)is evaluated by Tw=Ten-Tex.
The values of Ttof W-doped VO2/Mica composite powder with different nW/nVprepared by carbothermal reduction obtained by FTIR and DSC are shown in Fig.6.Ttalso decreases about 18℃with per 1%nW/nVand can be tuned linear by W doping dose.According to the reported models[22-25],doping makes the band gap of the VO2reduced.On the one hand,due to the introduction of extra electrons into the vanadium d bands by the substitution of V4+with W6+.On the other hand,addition of tungsten might break V4+-V4+bonds and result in formation of two new bonds V3+-W6+and V3+-V4+.Therefore,with the increasing fraction of W6+in the lattice,the loss of homopolar V4+-V4+bonding destabilizes the semiconductor phase.This process can lower the semiconductor-to-metal transition temperature.
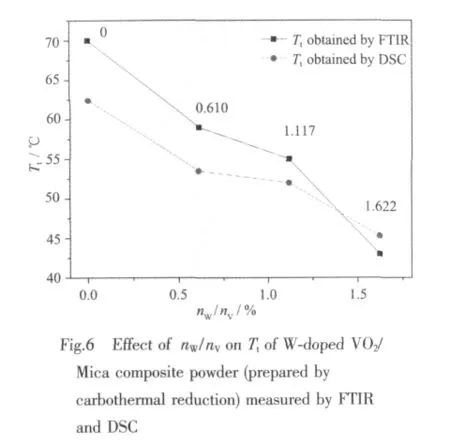
Ttmeasured by FTIR and DSC has difference about 4 to 7℃.The cause of the difference may be as follows:the samples for FTIR measurement are the thin films prepared by inserting VO2/Mica powder into the UV curing resin,and VO2/Mica powder is wrapped within the UV curing resin so that the surface and inside of the samples have the temperature gradient.However,the samples for DSC measurement are the pure W-doping VO2/Mica composite powder.
3 Conclusions
In this work,we have presented that the W-doped VO2/Mica powder is prepared by two different methods.Both methods obtained fairly pure VO2(M),and VO2crystals were clad like small worms on mica surface.Doping makes smaller particles and smoother surface than un-doped samples.The composite films prepared by thermal decomposition show better thermochromic performance than those prepared by carbothermal reduction.The phase transition temperature (Tt)decreases with an increase in the nW/nV.The Ttcould be reduced about 18℃by each addition of 1%W/V molar ratio,and Ttreaches to about 40℃when 1.622%W is doped.However,the infrared transmit-tance changes (ΔTr),the sharpness of the composite films become worse when nW/nVincreases.
[1]Yoshio O,Takeshi Y,Naoichi Y.J.Solid State Chem.,1990,86:116-124
[2]François T,Robert C,Jean B.J.Solid State Chem.,1976,17:431-438
[3]Douglas H,Jon Z,Christopher J W,et al.J.Solid State Chem.,1998,138:178-182
[4]QuBY,LiuL,XieY,etal.Phys.Lett.A,2011,375:3474-3477
[5]Andersson G.Acta Chem.Scand.,1954,8:1599-1606
[6]Andersson G.Acta Chem.Scand.,1956,10:623-628
[7]Morin F J.Phys.Rev.Lett.,1959,3:34-36
[8]Granqvist C G.Thin Solid Films,1990,193-194:730-740
[9]Shi Q W,Huang W X,Yan J Z.J.Sol-Gel Sci.Technol.,2011,59:591-597
[10]Yan J Z,Zhang Y,Huang W X,et al.Thin Solid Films,2008,516:8554-8558
[11]Dejene F B,Ocaya R O.Curr.App.Phys.,2010,10:508-512
[12]Shi J Q,Zhou S X,You B,et al.Sol.Energy Mater.Sol.Cells,2007,91:1856-1862
[13]YANG Xiu-Chun(杨修春),WANG Ying-Bo(王胤博),ZHOU Shi-Shan(周石山),et al.J.Build.Mat.(Jianzhu Cailiao Xuebao),2010,13:827-830
[14]Valmalettea J C,Gavarri J R.Sol.Energy Mater.Sol.Cells,1994,33:135-144
[15]Xu C Y,Pang M J,Yuan C G,et al.J.Mater.Sci.Eng.,2006,24:252-254
[16]He P,Huang W X,Yan J Z,et al.Mater.Res.Bull.,2011,46:966-969
[17]CAI Jing-Han(蔡靖涵),HUANG Wan-Xia(黄婉霞),HE Peng(何鹏),et al.Chinese J.Func.Mater.(Gongneng Cailiao),2011,42(S1):82-84
[18]YAN Jia-Zhen(颜家振),HUANG Wan-Xia(黄婉霞),LIU Yang-Si(刘阳思),et al.Rare Met.Mater.Eng.(Xiyou Jinshu Cailiao Yu Gongcheng),2009,37(9):1648-1651
[19]LUO Rong-Rong(罗蓉蓉),HUANG Wan-Xia(黄婉霞),HE Peng(何鹏),et al.Chinese J.Func.Mater.(Gongneng Cailiao),2010,41(S2):334-338
[20]Choa J H,Byuna Y J,Kima J H,et al.Ceram.Int.,2012,38:S589-S593
[21]Xu S Q,Ma H P,Dai S X,et al.J.Mat.Sci.,2004,39:489-493
[22]Pan M,Zhong H M,Wang S W,et al.J.Cryst.Growth,2004,265:121-126
[23]Romanyuk A,Steiner R,Marot L,et al.Sol.Energy Mater.Sol.Cells,2007,91:1831-1835
[24]Burkhardt W,Christmann T,Franke S,et al.Thin Solid Films,2002,402:226-231
[25]Jin P,Nakao S,Tanemura S.Thin Solid Films,1998,324:151-158