小鼠神经再生的检测(下)
潘宝晗,约翰 W.格里芬,2,4,迈克 A.波里,保罗 N.霍夫曼,3,默汗默德H.法拉
(1.约翰霍普金斯医学院 神经科,美国 马里兰州巴尔的摩21205;2.约翰霍普金斯医学院 神经科学系,美国 马里兰州巴尔的摩21205;3.约翰霍普金斯医学院 眼科,美国 马里兰州巴尔的摩21205;4.约翰霍普金斯医学院 病理科,美国 马里兰州巴尔的摩21205;)
4 Histological measures of nerve regeneration
In the mouse sciatic system faster growing sprouts outgrow the available length of nerve within 1week,so that data collection is restricted to that interval,must be done in daily increments,and requires assessment of short nerve segments of 1mm or less.For these reasons histological approaches that identify the regenerating fibers in longitudinal sections of the whole nerve or in whole mounts of the nerve are most satisfactory.Immunostaining for axonal markers can be useful in measuring the position of the fast growing axons at early times after injury in the mouse.With some antibodies penetration of the whole nerve is adequate,so that immunostaining of whole mounts of nerves can be done and no section-to-section variation is introduced.Optical sections of whole mounts can be made by confocal microscopy.Alternatively,the nerves can be examined in longitudinal sections.
The best markers are the products of genes that are rapidly and markedly upregulated by axotomy.The growth-associated protein GAP43is the most widely used of these.GAP43is a highly regulated cytoplasmic protein that is normally expressed at low levels and in only some peripheral axons,including the nociceptors innervating the epidermis.After axotomy,it is induced in the nerve cell body[29],rapidly transported down the axon,(Fig.7)and inserted into and retained within lipid rafts in the growth cone by dual palmitoylation(Nakamura et al.,1998).It is a PKC substrate.Its phosphorylated form interacts with a number of terminal components,and acts to cap actin filaments.For our present purposes the fact that it is largely absent from the axon before injury,that it becomes abundant rapidly after axotomy,that it is rapidly transported so that it accumulates within the growth cones even in the longest nerves of mice within short periods after synthesis,and that there are excellent antibodies to detect it immunohistochemi cally combine to make it a superb marker for studies of regeneration(Fig.7).
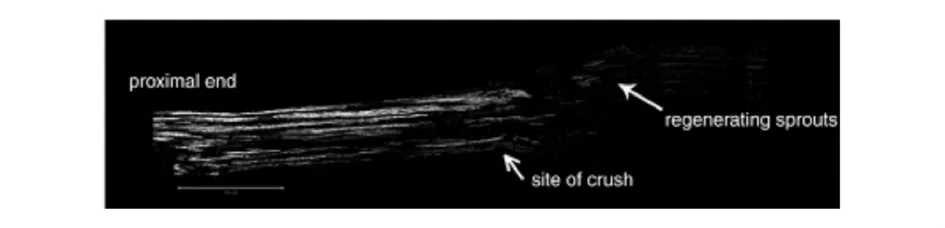
Fig.7 Immunostaining for GAP43 3 days after sciatic nerve crush.The axons in the proximal stump are intensely stained for the marker,which identifies sprouts that are beginning distal to the crush site.
Endogenous axonal proteins that are normally present in the axon,including neurofllament proteins and class IIIβ-tubulin,can be used to identify axonal sprouts.However,they have an inherent limitation in small animals because the axons contain these markers before injury.These markers identify axonal debris during the early stages of Wallerian degeneration,making the distinction from regenerating sprouts in the distal stump tenuous.This concern also applies to fluorescent proteins(XFP variants of the green fluorescent protein)con-trolled by neuronal promoters such as Thy1.2.Fig.5Aillustrates the appearance of the sciatic nerve 5 days after sciatic nerve transection in a line H YFP mouse.Because the Schwann cells of the denervated Bunger bands contain breakdown products of the axoplasm,they are fluorescent,and in this setting it is difficult to discriminate the regenerating axons from the residua of interrupted axons(Fig.5B).
4.1 Low molecular weight fluorescent markers introduced at sites of axotomy
Measurement of the lengths of regenerating sprouts in longitu-dinal sections of nerve can also be done following the injection of axonally transported compounds into the nerve proximal to the site of nerve injury.The strategy in these methods is to crush the proximal stump of a regenerating nerve above the site of the previous axotomy and then to inject the marker into the endoneurial space.Taken up into axons of the newly created distal stump,the marker passes the original site of axotomy and then to fills out the regenerated fibers(Fig.8).The biotin derivative biotinamide hydrochloride,or Neurobiotin(NB)has been shown to be a useful marker for intracellular tracing in slice preparations after electro-physiological recording of single living neurons[30].This compound has a very low molecular weight of 323Da and is transported in both retrograde and anterograde directions depending on the method of injection and the type of tissue.The rate of transport of this tracer is high[30].In addition,this compound has no signs of toxicity in any labeled neurons and significant effects on neuron membrane properties have not been identified.In mouse sciatic nerves the available length of nerve below a crush can be filled out within b 4hafter injection.This method is advantageous for identifying the transition from latency to outgrowth and for the early stages of outgrowth,because it does not depend on the induction of new neuronal proteins,as required for GAP43,and because for the most part it does not identify marker in axons undergoing Wallerian degeneration,just discussed.How ever,we have found that,when injected 48hafter the initial sciatic nerve crush,4hlater a percentage of fibers undergoing Wallerian degeneration in the distal stump is labeled.This is likely to represent uptake into axons that were still in continuity at the time of injection,but then entered the phase of explosive degradation of axoplasm and axonal segmentation within the next 4h.For this reason interpretation of data from early studies done the first few days after the nerve injury must be done cautiously.
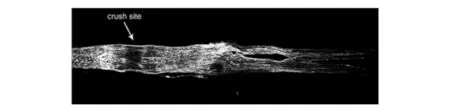
Fig.8 Longitudinal section of a neurobiotin-injected mouse sciatic nerve 5 days after nerve crush.The arrow identifies the crush site.Neurobiotin was injected at the site of a more proximal nerve crush 4 h before the nerve was removed.The sprouts in the distal stump are well displayed.
An alternative to these measures,all of which are based on longitudinal sections of the distal stump,are assessments of the numbers of fibers reaching a defined point distal to the crush at a specific time,as determined in transverse sections of plastic-embedded specimens.Because at the light level the number of myelinated fibers can be reliably counted,this measure appears foolproof,but there are several cautions in the interpretation of this data.Electron microscopy is required to assess premyelinated and unmyelinated sprouts.Importantly,myelinated parent axons can extend many sprouts that myelinate before reaching their targets.Thus counting only myelinated fibers may test the extent of axonal branching or the speed of myelination rather than the extent of reinnervation of targets.The accuracy of these axonal counts as a measure of regeneration also depends on having the specimens embedded in known proximal-distal orientation.I-magine that the front of regenerating sprouts on day 4after nerve crush has reached 6.5mm from the crush site.If the fixed mouse sciatic nerve is divided into seven 2-mm blocks,the site of interest will be the proximal face of the fourth block distal to the crush.If this nerve segment is cut on its distal face few sprouts will be seen.If in a second experimental condition with no biological difference in regeneration the block face was oriented with the proximal face forward and sprouts were seen the calculation might be made that the condition produced a 25%increase in the rate of regeneration.Such errors should wash out with large numbers of animals,but the data would remain unnecessarily noisy.An alternative to protect against this error is to cut every block to the center before taking sections for analysis.
4.2 Retrograde tracing
Various kinds of tracers have been developed as the fundamental methods to map connectivity in the nervous system.Aspects to be considered in selection of a tracer for measuring regeneration include:anterograde or retrograde tracing methods,uptake mechanisms,transport speed,toxicity and stability.To obtain optimal results,it is important to choose the tracer best suited for the system studied and for the specific question asked.
Retrograde tracing to label neurons regenerating into specific nerves or reaching specific targets is a well established approach that remains highly valuable.HRP has been used to analyze the consequences of peripheral nerve injury and to evaluate regeneration rates.Other tracers such as Fluoro-Gold and a series of fluorescent compounds,as well as biotinylated dextran amine [BDA],have been frequently used to retrogradely label motor neurons in the spinal cord.The nerves can be transected at a distal site and the cut end“dipped”in the retrograde marker,or with some of the markers they can be injected into the target(for example,a specific muscle or a foot pad).These techniques require practice and controls for diffusion and other technical concerns,but they can be used effectively and highly reproducible.Concerns include determining the degree of spread from the injection site and the fact that some of the retrograde markers produce longterm toxicity in the neurons that contain them.
5 Electrophysiological measures
In larger animals,it is possible to measure the progress of sprouts down the nerve by near-needle techniques.Such an approach can be adapted to the mouse in terminal in vitro or in in vivo studies.For in vitro analysis,the nerve is removed from above the site of the crush or repair to the most distal site available and hung on bipolar electrodes in an appropriate oxygenated salt solution.Both A and C waves can be detected and the amplitudes measured.The rate of recovery of A fiber and C fiber compound action potential(CAP)amplitudes,compared to u-ninjured control nerves,gives a useful measure of regeneration to the site of distal transection.In vivo electrophysiological measures can be used as time-to-target measures.
5.1 Radiolabeled axonal markers
Now rarely used,radioisotopic label of the fast anterograde component of axonal transport can give an efficient and vivid picture of the distribution of growth cones distal to a nerve injury.The technique depends upon the fact that anterogradely transported proteins accumulate in the cut ends of severed nerve fibers and,as the fibers regenerate,in the growth cones.Injection of labeled isotopes such as S35-methionine into the ventral horn or dorsal root ganglia labels fast axonal transport.In uninjured nerves the “peak”of pulse-labeled fast transport passed down the nerve to the nerve endings,and a relatively flat“shoulder”of radioactivity remains along the nerve.If the nerve is crushed a few days before labeling the radioactivity accumulates within the growth cones,so that when the distribution of radioactivity is plotted the relative position of the fastest growing fibers,and an estimate of the proportion growing at that rate can be determined[31].In young animals the front of radioactivity passes down the nerve as a sharply defined peak,reflecting the fact that many nerve fibers grow at rates approaching that of the fastest fibers.In older animals the proportion growing at slower rates increases,and more radioactivity remains near the crush site,reflecting fibers that have grown very little.The limitation to this approach is the degree of nonspe-cific radioactive contamination of nerves that results from the isotope injection.In the rat this is negligible,but in the mouse non-transported radioactivity can be a limiting confound.
5.2 Measurement of collateral sprouting and reinnervation
Collateral sprouting leading to reinnervation of denervated targets has arguably received insufficient research attention in relation to its potential therapeutic importance.In patients with proximal nerve injuries a realistic goal is to restore protective sensibility to a partially denervated foot or hand by collateral spouting of uninjured fibers.In the rodent collateral sprouting of nociceptors can result in recovery of protective sensibility in denervated skin[32].As with regeneration of interrupted fibers,rodents are much better at generating collateral sprouts than humans[33].However,the mechanisms underlying collateral sprouting may be more amenable to therapeutic manipulation in man than are those required for regeneration of interrupted fibers.The magnitude of scientifi advance required to improve collateral sprouting is likely to be less than that required for long distance regeneration of the interrupted fibers.
As noted,throughout this discussion collateral sprouting is used in the restricted definition to describe the process by which intact,uninjured nerve fibers extend axonal branches into the regions previously occupied by other nerve fibers that have undergone degeneration.What fiber types undergo collateral growth Unmyelinated fibers are well recognized for this capacity.Aβmechanosensory fibers can sprout.For Aβmyelinated fibers the evidence is mixed.Without question,sprouting to innervate nearby denervated muscles can occur from the nodes of uninjured myelinated motor fibers above the motor nerve terminals.Convincing physiological evidence also indicates that Aβmechan-osensitive afferents that innervate teeth can sprout in the jaw,and can even cross the midline to innervate teeth from the other hemijaw.However,this is decidedly the exception.The reason for the paucity of collateral growth from myelinated fibers may reflect the growth inhibitory properties of many of the consti-tuents of myelin-forming Schwann cells.In the myelin sheath,in the attachment sites of the paranode,and in the periaxonal membrane of the internode are at least two proteins with growth inhibitory potential,MAG and netrin-1.In addition,there is immunocytochemical data showing that OMgP is found in the Schwann cell fingers overlying the nodes[34].These molecules are likely to restrict collateral sprouting in fibers with intact nodes.
Extensive studies of collateral sprouting of uninjured C and Aβsensory fibers,as well as unmyelinated postganglionic sympathetic fibers,have demonstrated reinnervation of neighboring denervated skin.This has been satisfyingly detailed in the advantageous system of the cutaneous nerves of the thorax[35].The dorsal and lateral cutaneous nerves provide sensory innervation of the back.Section of one of these cutaneous nerves leaves an island without nociception or mechanoception.With collateral sprouting from neighboring segments sensation can restored.Conversely,section of several of these cutaneous nerves with sparing of only one results in an island of preserved sensibility that gradually expands as a result of because of collateral sprouting.The size of this island can be mapped by pinch or heat pain stimulation of the back skin to elicit the cutaneus trunci reflex.In this preparation,nociceptors undergo collateral sprouting that has been documented by electron microscopy.Strikingly,no Aβ fibers extended beyond the baseline boundaries of the island.
Comparable results have been achieved in the rodent foot.For example,following sciatic nerve ligation,the saphenous nerve sprouts into the sciatic distribution(Fig.6D).Again,Aβfibers are minor participants.However,in some other model systems Aβsprouting has been adduced by electrophysiological evidence.For example,collateral sprouting of trigeminal fibers across the midline of the jaw to innervate the dental pulp has been shown for Aβas well as nociceptor fibers.Some of these fibers can cross the midline of the jaw to reach denervated teeth.In the rat hindfoot,Kinman and Aldskogius[36]found that regeneration of the saphenous into the territory of the sciatic nerve was speeded by prior crush of the sciatic,suggesting that a“growth state”in the collaterally growing nerve cell speeded outgrowth.Bajrovic and Sketelj reached the opposite conclusion in studies in which the peroneal nerve was spared and the saphenous,sural,and tibial were cut.In this study either simultaneous or earlier crush of the peroneal nerve slowed regrowth into the denervated territories.The reasons for this striking discrepancy are unclear.
The Benowitz lab has shown that a focal injection of inosine,a metabolite of adenosine that activates N-kinase,is sufficient to inducecollateral sprouting from uninjured CNS fibers.The best defined drivers of collateral sprouting are growth factors.In models in which anti-NGF strategies have been used collateral sprouting is eliminated.In vitro studies using NGF-coated beads have formally shown that a gradient of a relevant growth factor along the shaft of an axon is sufficient to induce accumulation of actin and mitochondria[37]at the point nearest the bead,followed by extension of a sprout toward the bead.In vivo,a focal injection of cells expressing NGF under lentiviral control is suf?cient to induce collateral sprouting of medial septal fibers.
Denervated Schwann cells in the subepidermal plexus are a source of growth factors,and at least contribute to the measured increases in growth factors in denervated skin.It is likely that the basal epidermis is also a site of elevated growth factor production.Consistent with this is the observation of the responses of epidermal fibers to denervation of neighboring regions.A simple system is excision of a cylinder of skin(dermis and epidermis)by apunch of the type used in diagnostic biopsies[38].The site is rapidly covered by new epidermis.The dermal defect is filled by a connective tissue plug.The epidermal fibers on the margin of the incision begin within days to elongate at their endings(ultraterminal sprouting),and incline toward the new epidermis.They may in part be carried by the migration of keratinocytes into the defect,but the inclination persists for weeks.In addition some of these“tilted”fibers extend collaterals that grow into the new epidermis.These sprouts typically drop to the dermalepidermal junction and grow just on the epidermal side of the basal epider-mis.In man their growth is slow,but within months they innervate the entirety of a 3mm defect.These sprouts in turn send collaterals vertically into the epidermis,and this arrangement is maintained for months.
An “incision”model provides an instructive contrast.In this model,the skin punch is passed into the skin to produce a circular incision,but no skin is excised.The ultraterminal sprouting and growth along the dermalepidermal basal lamina occurs as in the excision model.The important difference in the preparation is that a dermal column topped by the original epidermis survives.Because the incision transected the subepidermal network of nerve fibers,both the epidermis and the dermis with its Schwann cell bands survive.Regenerating axons,at least in part from the transected ends of the fibers in the subepidermal network,grow into the dermis and grow up to the epidermis.As these regenerating fibers enter epidermal regions occupied by the collateral and ultraterminal sprouts they quickly out-compete them,so that the extended a-xons return to the borders of the incision[39].This observation suggests that epidermal axons can only maintain expanded terminal arbors if they have uncompeted access to growth factors from the fullfield.This in turn suggests that the relatively regular spacing of fibers in the normal epidermis may be determined by the available growth factor resources.
One of the most striking but relatively little explored enhancers of collateral sprouting appears to be electrical activity in the nerve.In the dorsal cutaneous nerve “islands”described above collateral reinnervation was accelerated by repeated mechanical stimulation of the skin within the innervated regions,as well as by electrical stimulation of the uninjured nerves.Electrical stimulation can also improve specificity of reinnervation by the sprouts of interrupted fibers.
6 Conclusions
Studies of peripheral nerve regeneration to this point have been dominated by measurements made in the sciatic system of the rat.Molecular genetic approaches will increasingly mandate the use of the mouse,and this transition will require in turn in-vestments of effort into developing improved measures of regeneration.These measures will complement anticipated advances in understanding the fundamental changes associated with the entry of the neuron into the axonal growth state,with the local mechanisms involved in axonal building during regeneration,the manipulation of growth factors and growth factor signaling,the roles of Schwann cells,and the clearance of myelin debris.The same functional,behavioral,electrophysiological,and histological tools are likely to be useful in the study of axonal degeneration and axonal protection in experimental neuropathies.
New model systems of regeneration in specific nerves will complement the established models using sciatic nerve regeneration.For many needs the use of tools that specifically assess muscle reinnervation,the reinnervation of the various sensory transducers,and reinnervation of autonomic targets are likely to become feasible.The correlation of reinnervation to function will depend in part on reestablishing the specificity of the connections.Measurements that are adapted for the mouse and that can assess the latency to growth,the rate of axonal growth,the specificity of reinnervation,and the extent of functional recovery will be particularly valuable.
Finally,it seems likely that increased attention will be paid to approaches that amplify collateral sprouting and reinnervation.Collateral sprouting can lead to functional improvement in strength of partially denervated muscle and to improvement in protective sensibility.Importantly,these functional improvements can occur without the need for long distance nerve fiber growth that regeneration of interrupted fibers may entail.Manipulation of growth factor signaling and possibly electrical stimulation of uninjured nerves have been shown in rodents to accelerate collateral growth,and are likely to be among the approaches that will be further tested for this purpose.
Acknowledgements
Funding for studies from the authors’laboratories was provided by the Adelson Program in Neural Repair and Rehabilitation of the Adelson Medical Research Foundation,the Robert Packard Center for ALS Research,the Muscular Dystrophy Association,and the NIH NINDS.Mr.Vamsi Kalari provided Figure 7.
[29]Hahm k,Sirdofsky M,Browen A,et al.Collateral sprouting of human epidermal nerve fibers following intracutaneous axotomy[J].J Peripher Nerv Syst,2006,11(2):142-147.
[30]Kita H,Armstrong W.A biotin-containing compound N-(2-aminoethyl)biotinamide for intracellular labeling and neuronal tracing studies:comparison with biocytin[J].J Neurosci Methods,1991,37(2):141-150.
[31]Hoffman P N,Lasek R J.Axonal transport of the cytoskeleton in regenerating neurons:constancy and change[J].Brain Res,1980,202(2):317-333.
[32]Jackson P C,Diamond J.Regenerating axons reclaim sensory targets from collateral nerve sprouts[J].Science,1981,214(4 523):926-928.
[33]Healy C,LeQuesne P M,Lynn B.Collateral sprouting of cutaneous nerves in man[J].Brain,1996,119(966):2 063-2 072.
[34]Kinman E,Aldskogius H.Collateral sprouting of sensory axons in the glabrous skin of the hindpaw after chronic sciatic nerve lesion in adult and neonatal rats:a morphological study[J].Brain Res,1986,(377):73-82.
[35]Huang J K,Phillips G R,Roth A D,et al.Glial membranes at the node of Ranvier prevent neurite outgrowth[J].Science,2005,310(5 755):1 813-1 817.
[36]Inserra M M,Bloch D A,Terris D J.Functional indices for sciatic,peroneal,and posterior tibial nerve lesions in the mouse [J].Microsurgery,1998,18(2):119-124.
[37]Jacquin M F,Hu J W,Sessle B J,et al.Intra-axonal Neurobiotin injection rapidly stains the long-range projections of identi?ed trigeminal primary afferents in vivo:comparisons with HRP and PHA-L[J].J Neurosci Methods,1992,45(1-2):71-86.
[38]Kong L,Wang X,Choe D W,et al.Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice[J].J Neurosci,2009,29(3):842-851.
[39]Laird F M,Farah M H,Ackerley S,et al.Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking[J].J Neurosci,2008,28(9):1 997-2 005.

