Antioxidant potential of Rumex vesicarius L.: in vitro approach
Tajdar Husain Khan, Majid Ahmad Ganaie, Nasir Ali Siddiqui, Aftab Alam, Mohd Nazam Ansari
1Department of Pharmacology, College of Pharmacy, Salman Bin Abdulaziz University, Kingdom of Saudi Arabia
2Department of Pharmacognosy, College of Pharmacy, King Saud University, Kingdom of Saudi Arabia
3Department of Pharmacognosy, College of Pharmacy, Salman Bin Abdulaziz University, Kingdom of Saudi Arabia
Antioxidant potential of Rumex vesicarius L.: in vitro approach
Tajdar Husain Khan1*, Majid Ahmad Ganaie1, Nasir Ali Siddiqui2, Aftab Alam3, Mohd Nazam Ansari1
1Department of Pharmacology, College of Pharmacy, Salman Bin Abdulaziz University, Kingdom of Saudi Arabia
2Department of Pharmacognosy, College of Pharmacy, King Saud University, Kingdom of Saudi Arabia
3Department of Pharmacognosy, College of Pharmacy, Salman Bin Abdulaziz University, Kingdom of Saudi Arabia
PEER REVIEW
Peer reviewer
Dr. Sheikh Fayaz Ahmad, Ph.D., Assistant Professor of Immunology Department of Microbiology and Immunology, College of Medicine, Seventh of April University in Zawia-Libya. Tel: +218924069582, E-mail: fayaz2kin@yahoo.co.in
Co-reviewers: Atanu Bhattacharjee, Karnataka, India. Salim Khan, Riyadh, Kingdom of Saudi Arabia. Prawez Alam, Al Kharj, Kingdom of Saudia Arabia.
Comments
This manuscript is well written and contains advanced methodological qualitative analysis. The materials and methods are written precisely and well referenced. Manuscript contains good data about the use of natural products against the free radical mediated damage.
Details on Page 543
Objective:To assess in-vitro antioxidant activity of different fraction and perform high performance thin layer chromatography fingerprint analysis of most active fraction of Rumex vesicarius L. (R. vesicarius).
Rumex vesicarius L., DNA sugar damage, DPPH, antioxidant, HPTLC
1. Introduction
Plants and plant-based products are gaining importance against variety of diseases due to their non-toxic and no/ less side effects. Hence, we have been continuously working on the identification and characterization of medicinal plants[1-5]. Phytochemicals are experimentally proved and used as effective protection against oxidative stress and chemical carcinogenesis in various organs[1-5]. It has been demonstrated that some edible plants as a whole, or their identified ingredients, have substantial protective effects on various diseases[6].Rumex vesicarius(R. vesicarius) is an annual herb, which belongs to the family of Polygonaceae[7]. It spreads throughout desert and semi-desert areas of North Africa, Asia and Australia[8]. In Saudi Arabia,R. vesicariusis widely used as food, as a medicinal herb and as an antidote to scorpion stings[9]. Despite its importance, only few studies have been conducted onR. vesicarius.
Standardization of plant materials is needed in the present time. Numerous pharmacopoeias having monographs ofthe plant materials only describe the physico-chemical parameters. The WHO has stressed the need to ensure the quality of medicinal plant products by using modern controlled techniques and applying suitable standards[10,11]. High performance thin layer chromatography (HPTLC) fingerprinting technique offers better resolution and estimation of active constituents, which can be done with reasonable accuracy in a short time[12]. It can serve as a tool for identification, authentication and quality control of herbal drug[13].
The medicinal importance ofR. vesicariusis a reflection to its chemical composition since the plant contains many bioactive substances such as flavonoids (vitexin, isovitexin, orientin and isorientin). The plant is also rich in anthraquinones, particularly emodin and chrysophanol in roots. The plant also contains carotenoids, vitamins (especially vitamin C), proteins, lipids and organic acids[14-16]. The previously mentioned bioactive phytochemicals (such as polyphenols, flavonoids, carotenoids, tocopherols and ascorbic acid) have a role as antioxidant and detoxifying agents. The intake of dietary antioxidant phytochemicals like carotenoids, phenolic compounds and flavonoids may lead to the protection against non-communicable diseases in human beings such as cancer, cardiovascular diseases and cataract[17,18].
There was a need to evaluate fingerprint profile of most active antioxidant fraction of such an edible plant rich in bioactive substances specially flavonoids, total phenolics and total proanthocyanidin. Therefore, this study was designed to assessin-vitroantioxidant activity of different fractions and perform HPTLC fingerprint analysis of most active fraction ofR. vesicarius.These investigations were carried out to chemically determine the bioactive agents that may be responsible for biological activities (antioxidant activities).
2. Material and methods
2.1. Chemicals
All the chemicals and solvents used were of analytical grade and commercially available.
2.2. Plant material
Healthy whole plants ofR. vesicariuswere collected from the Al-Kharj area (Riyadh province, Kingdom of Saudi Arabia). The plants were cleaned, dried under shade, ground to a coarse powder and stored in an airtight container at 25 °C for further use. Plant specimens were botanically identified and authenticated by expert taxonomist. A voucher specimen has been deposited at the herbarium of the Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
2.3. Extraction
Air-dried and fine powder ofR. vesicariuswas subjected to extraction with methanol three times per day. The container with its contents was sealed and kept for a period of four days accompanying occasional shaking and stirring. The extract was filtered by Buchner funnel and the filtrate was concentrated with rotary evaporator at bath temperature not exceeding 40 °C to have gummy concentrate of greenish black extract (yield approximately 16.55%). The methanolic extract was further fractionated with different solvent system (increasing polarity). Four extracts (methanol, acetone, ethyl acetate and butanol) were separated by using the column.
2.4. Assessment of the potential to inhibit lipid peroxide
To assess the potential to inhibit lipid peroxide, different extracts of plant were selected. Group I served as a control group in which the reaction mixture consisted of 0.58 mL phosphate buffer (0.1 mol/L, pH 7.4), 0.2 mL ascorbic acid (100 mmol/L) and 0.02 mL ferric chloride (100 mmol/L). In other groups in addition to the control reaction mixture, different extracts of plant were also added. This reaction mixture was then incubated at 37 °C in a shaking water bath for 1 h. The reaction was stopped by the addition of 1 mL of trichloro acetic acid (10%). Following addition of 1.0 mL thio barbituric acid (TBA) (0.67%), all the tubes were placed in a boiling water bath for a period of 20 min. The tubes were then centrifuged at 5000 r/min for 10 min for 10 min. The amount of malondialdehyde formed in each of the sample was assessed by measuring the optical density of the supernatant at 535 nm. The results were expressed as the nmol malondialdehyde formed/h/g tissue at 37 °C by using a molar extinction coefficient of 1.56×105 /M/cm[19].
2.5. Inhibition ofDNA-sugar damage in calf thymusDNA
Hydroxyl radical scavenging activity was measured by studying the competition between deoxyribose and the test compounds. Group I served as a control group. In control the reaction mixture consisted of 0.5 mL calf thymus DNA (1 mg/mL of 0.15 mol/L NaCl), 0.5 mL phosphate buffer (0.1 mol/L, pH 7.4) and 0.05 mL of FeCl3(100 µmol/L in final concentration). In other groups in addition to the above mixture, different extracts of plant were also added. The reaction mixture was incubated for 1 h at 37 °C in a water bath shaker. After the incubation, 1 mL TBA (0.67%) was added to the reaction mixture and then it was kept in boiling water bath for 25 min. The TBA reacting species hencegenerated forms of an adduct showing a characteristic absorption at 535 nm which was monitored by using spectrophotometer[20].
2.6. Scavenging of hydrogen peroxide
The superoxide radical scavenging activity of the plant was performed as described by Liu,et al[21].The superoxide is generated in 3 mL of tris HCl (16 mmol/L, pH 8.0) containing 1 mL of nitro blue tetrazolium (50 µmol/L) solution, 1 mL nicotinamide adenine dinucleotide-reduced (78 µmol/L) solution and sample solution of different extracts in a concentration of 1-10 µg/mL. The reaction was started by adding 1 mL of phenazine methosulphate solution (10 µmol/L) to the mixture. The reaction mixture was incubated at 25 °C for 5 min, and the absorbance at 560 nm was measured against the blank samples. L-ascorbic acid was used as a control and butylated hydroxyanisole was used as a positive control.
2.7. DPPH·
radical scavenging assay
Free-radical scavenging capacities of the extracts were evaluated using the 2,2-diphenylpicrylhydrazyl radical (DPPH·)[22]. Briefly, the reaction was carried out in 1 mL of methanol containing freshly made DPPH·(100 µmol/L) in methanol and the different extracts of plant (400 µg/mL). The contents were vigorously mixed and incubated at room temperature (RT) in the dark for 20 min, and the absorbance was measured at 517 nm. In each experiment, the tested sample alone in methanol was used as blank and DPPH·alone in methanol as control.
2.8. Assessment of total polyphenol content
The total polyphenol content of the extracts was determined by using the Folin-Ciocalteu reagent[23]. Folin-Ciocalteu reagent (0.5 mL) and distilled water (5 mL) were added to the sample (0.1 mL). It was incubated for 3 min at RT and was subsequently mixed with 25% w/v solution of sodium carbonate (1.4 mL) and distilled water (3 mL). Following 1 h incubation at RT in the dark, the absorbance was measured at 765 nm. Blank contained Folin-Ciocalteu reagent and distilled water without the extract. The optical density of the sample (0.1 mL) in 25% w/v solution of sodium carbonate (1.4 mL) and distilled water (8 mL) at 765 nm was also measured. The total polyphenol content was determined by a standard curve of absorbance values in correlation with standard concentrations (0, 50, 150, 250, 500 µg/mL) of gallic acid. The total polyphenol content is presented as mg of gallic acid per g of extract.
2.9. Determination of total flavonoids
Measurement of total flavonoid content in the investigated extracts was determined spectrophotometrically according to Ordoñez,et al.[24] using a method based on the formation of flavonoid-aluminium complex with the absorption maxima at 510 nm. The reaction mixture contained 0.5 mL of extract in dimethylsulfoxide or standard solutions of quercetin, diluted with 2 mL distilled water and 0.15 mL of 5% NaNO2. After 5 min, 0.3 mL of 10% AlCl3was added. After 6 min, 1 mL of 1 mol/L NaOH was added and the total volume was made up to 5 mL with water. The solution was mixed well and the absorbance was measured against a prepared reagent blank at 510 nm. The flavonoids content was expressed as mg of quercetin equivalents per g of dried extract, by using a standard graph (y=0.0025x,R2=0.9974).
2.10. Determination of total proanthocyanidins
Determination of proanthocyanidin was done by using the procedure described by Sun,et al[25]. A volume of 0.5 mL of 1 mg/mL of extract solution was mixed with 3 mL of 4% vanillin-methanol solution and 1.5 mL hydrochloric acid; the mixture was allowed to stand for 15 min for color development. The absorbance was then measured at 500 nm. Total proanthocyanidin content was expressed as catechin equivalents (mg/g) using the equation based on the calibration curve.
2.11. HPTLC profile of extract
HPTLC finger print profile studies of methanol extract was carried out separately, following standard methodology of Harborne[26], and Wagner,et al[27].
2.11.1. Sample preparation
The methanol extract was dissolved separately with HPLC grade methanol, which was used for sample application on pre-coated silica gel G-F254 aluminum sheets.
2.11.2. Developing solvent system
A number of solvents were tried but satisfactory resolution was obtained in the developing solvent ethyl acetate: glacial acetic acid (GAA): methanol: water (6:2:1:0.2) for methanol extract.
2.11.3. Sample application
The samples (5 µL, 10 µL, 15 µL and 20 µL) were spotted in the form of bands of width 6 mm with a 100 µL sample using a Hamilton syringe on silica gel which was pre-coated aluminum plate GF-254 plates (20 cm×10 cm) with the help of Linomat 5 applicator attached to Camag HPTLC system,which was programmed through WIN CATS software.
2.11.4. Development of chromatogram
The mobile phase consisted of ethyl acetate: GAA: methanol: water (6:2:1:0.2) for methanol extract and 15 mL of mobile phase was used per chromatogram run. The linear ascending development was carried out in a 20 cm×10 cm twin through glass chamber saturated with the mobile phase.
2.11.5. Detection of spots
The developed chromatogram was dried by hot air to evaporate solvents from the plate. The developed plate was sprayed with anisaldehyde sulphuric acid as spray reagent and dried at 100 °C in hot air oven for 3 min and scanned at 270 nm in ultraviolet (UV) scanner. The plate was kept in photo-documentation chamber (Camag REPROSTAR 3) and the images were captured under UV light at 254 nm and 366 nm. The radio frequency values and fingerprint data was recorded by WIN CATS software.
2.11.6. Peak development of different extracts
Separate concentration (5 µL-20 µL) of methanolic extract was performed separately, and separate track was maintained for each concentration with separate peak development was done.
2.12. Statistical analysis
All values were expressed as mean±SE. One-way analysis of variance was applied to test for significance of biochemical data of the different groups. Significance is set atP<0.05.
3. Results
3.1. Inhibition of lipid peroxidation
We can suggest from our study thatR. vesicariuscan combat against free radical mediated peroxidation of lipids which may eventually damage the integrity of membrane. The potential ofR. vesicariusto inhibit iron ascorbate induced by lipid peroxidation may be due to the presence of their active chemical constituents. These chemical constituents may block the injurious chain reaction, which lead to lipid peroxidation. Free radicals generated by the iron/ascorbate system were inhibited by the addition of different extracts ofR. vesicarius(20.0%-33.8%). Methanolic extract of the plant shows maximum efficacy as shown in Table 1.
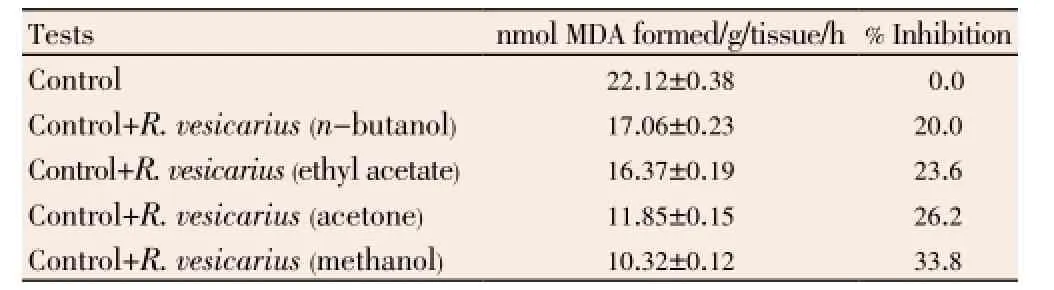
Table 1 Inhibition of lipid peroxidation.
3.2. Inhibition ofDNA-sugar damage in calf thymusDNA
There was inhibition in DNA sugar damage (37.0%-66.3%) with maximum efficacy being shown by methanolic extract ofR. vesicarius(66.3%) as shown in Table 2.
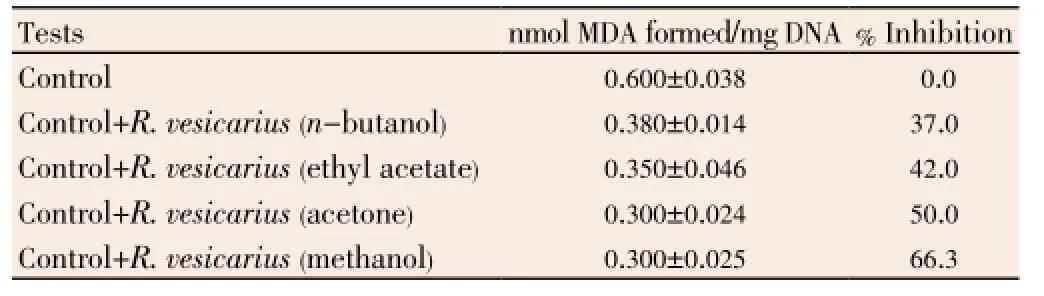
Table 2 Inhibition of DNA -sugar damage in calf thymus DNA.
3.3. Scavenging of hydrogen peroxide
The decomposition of H2O2by extracts ofR. vesicariusmay results from its antioxidant and free radical scavenging activity. Acetone extract ofR. vesicariusshows maximum efficacy.
3.4.DPPHradical scavenging activity
Table 3 illustrated the DPPH radical scavenging capacity of all the tested extracts. The results showed that methanolic extract ofR. vesicariuspossess highest scavenging capacity among all the tested extracts. In contrast,n-butanol extract showed the lowest scavenging capacity.

Table 3 Inhibition of total phenol, total flavonoids, proanthocyanidin and DPPH.

Table 4 Number of compounds detected in methanolic fraction R. vesicarius, with RF values and peak area.
3.5. Total phenolic, total flavonoids and total proanthocyanidin content
Table 3 demonstrates the results for total phenolic, total flavonoids and total proanthocyanidin content. The methanolic extract ofR. vesicariusshowed the highest total phenolic [(121.80±0.03) mg gallic acid/g)], total flavonoids [(43.00±0.03) mg quercetin/g)] and total proanthocyanidin [(74.90±0.02) mg catechin/g)] content.

Figure 1. Photo documentation of methanolic extract of R. vesicarius at 254 nm.
3.6.HPTLCfinger print profiles of methanol extract of R. vesicarius
The HPTLC finger print profiles of methanolic extract ofR. vesicariusshowed the presence of eight major compounds (in 20 µL) when developed in mobile phase of ethyl acetate: GAA: methanol: water (6:2:1:0.2) and scanned at 270 nm in UV scanner. Out of these eight compounds, compound number 4 was found in maximum concentration whereas compound number 6 was in minimum concentrations (in 20 µL). The photo documentation of methanol extract observed 254 nm and 366 nm is given (Figures 1 and 2). The radio frequency (RF) value of methanolic extract was given in Table 4.

Figure 2. Photo documentation of methanolic extract of R. vesicarius at 366 nm.
4. Discussion
Antioxidants are crucial in the prevention of human diseases. Herbal compounds with antioxidants activity may function as free radical scavengers, reducing agents, and quenchers of single-oxygen formation or reactive oxygen species, thereby protecting the body from degenerative diseases such as cancer. The reactive oxygen species are damaging byproducts generated during normal cellular metabolism or from toxic insult. They lead to a state of oxidative stress that contributes to the pathogenesis of a number of human diseases by damaging lipids, proteins and DNA[28,29]. This has inspired much interest in antioxidant activity of phytochemicals. Our results haveshown that methanol extract ofR. vesicariusL. displayed strong antioxidant activity. Antioxidant activity ofRumexcorroborates the findings of El-Bakry,et al[7]. In addition, the mechanism by whichR. vesicariusmight inhibit oxidative damage involves intracellular and extracellular pathways by scavenging free radicals, preventing nucleophilic sites of DNA, inhibiting the uptake of mutagen or their precursors[29].
The chemical analysis of extracts ofR. vesicariusshowed the presence of various phyto-constituents. The results of the present study also supplement the folkloric usage of the studied plant, which possesses several known and unknown bioactive compounds with bioactivity. HPTLC finger printing is a valuable quality assessment tool for the evaluation of botanical materials. It allows for the analysis of a broad number of compounds both efficiently and cost effectively. HPTLC studies have shown that it is more versatile than ordinary TLC methods as the spots are well resolved. The HPTLC method is simple, rapid, accurate, reproducible, selective and economic, can be used for quality control analysis and for quantitative determination of the plant material[13,30]. The HPTLC finger print profiles of methanolic extract ofR. vesicariusshowed the presence of eight major compounds, out of these compound No. 4 was found in maximum concentration.
The obtained results of chemical examinations of successive extractives solvents study showed a positive relationship between chemical compositions of successive extractives solvents and the obtained results of antioxidant activity studies. It was found that, methanolic extract ofRumexwas rich in flavonoids, total phenol and proanthocyanidin and these substances were known as antioxidant agents (such as quercetin and naringenin) which may be the reason for the highly antioxidant activities obtained by using these extracts. These results were in harmony with previous findings[17,18,31-33], as were found that flavonoids and polyphenols were good antioxidant agents sinceR. vesicariusis rich in polyphenols. Therefore it can be found that there was a positive relationship between chemical composition and the obtained biological activity results of this plant. The presence or absence of flavonoids may be a determining factor of this biological activity. The medicinal importance of this plant is a reflection to its chemical composition since this plant contains many bioactive substances[34].
The phenolic compounds and flavonoids are associated with antioxidative action in biological systems, acting as scavengers of singlet oxygen and free radicals[35]. In this present study the antioxidant activity was investigated by DPPH scavenging assay. The DPPH antioxidant assay is based on the ability of DPPH, a stable free radical to decolorize in the presence of antioxidants.
Therefore, it would seem likely that methanol solvents were able to extract those compounds, which are responsible for the antioxidant activity of the plant. This activity could partly explain why the plant is used in the traditional medicine practice of the Kingdome of Saudi Arabia.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are thankful to Deanship of Scientific Research, Salman Bin Abdulaziz University, Al-Kharj Saudi Arabia for providing the funds to carry out this study under research grants No. 4/1432.
Comments
Background
Reactive oxygen species have been involved in many diseases including cancer. In this present study authors evaluated for radical scavenging activity of acetone, ethyl acetate,n-butanol, and methanol extracts ofR. vesicarius. HPTLC fingerprint profiling ofR. vesicariusmethnolic extract was also done.
Research frontiers
Different extracts ofR. vesicariuswere studied for inhibition of the level of lipid peroxidation induced by Fe(++)/ascorbate, DNA sugar damage, scavenging of hydrogen peroxide, DPPH radical scavenging activity, total phenolic content, total flavonoids content and total proanthocyanidin. The methanolic extract showed maximum efficacy. On the basis of the result HPTLC fingerprint of methanolic extract was also done.
Related reports
No similar reports were found in the literature regarding inhibition of the level of lipid peroxidation induced by Fe(++)/ascorbate, DNA sugar damage, scavenging of hydrogen peroxide.
Innovations and breakthroughs
This study was planned to assessin vitroantioxidant activity of different extracts and perform HPTLC fingerprint analysis of most active fraction (methanolic) ofR. vesicarius. It showed the presence of eight major compounds, out of these compounds No. 4 was found in maximum concentration. These compounds especially compound present in higher concentration may be responsible for its biological activities (antioxidant activities). It recommended that authors should identify these compounds by nuclear magnetic resonance or other techniques in further studies.
Applications
This paper can be used to access activity of traditional medicine claimming for their antioxidant activities and identify and isolate those compounds that are responsible for the antioxidant activity.
Peer review
This manuscript is well written, contains advanced methodological qualitative analysis. The materials and methods are written precisely and well referenced. Manuscript contains good data about the use of natural products against the free radical mediated damage.
[1] Khan TH, Prasad L, Anuradha, Sultana S. Soy isoflavones inhibits the genotoxicity of benzo(a)pyrene in Swiss albino mice. Hum Exp Toxicol 2005; 24(3): 149-155.
[2] Khan TH, Sultana S. Apigenin induces apoptosis in Hep G2 cells: possible role of TNF-alpha and IFN-gamma. Toxicology 2006; 217(2-3): 206-212.
[3] Khan TH, Sultana S. Antioxidant and hepatoprotective potential of Aegle marmelos Correa. against CCl4-induced oxidative stress and early tumor events. J Enzyme Inhib Med Chem 2009; 24(2): 320-327.
[4] Khan TH, Sultana S. Effect of Aegle marmelos on DEN initiated and 2-AAF promoted hepatocarcinogenesis: a chemopreventive study. Toxicol Mech Methods 2011; 21(6): 453-462.
[5] Khan TH. Soy diet diminish oxidative injure and early promotional events induced by CCl4in rat liver. Int J Pharmacol 2012; 8(1): 30-38.
[6] Adekunle AS, Aline AB, Afolabi OK, Rocha JBT. Determination of free phenolic acids, flavonoid contents and antioxidant capacity of ethanolic extracts obtained from leaves of mistletoe (Tapinanthus globiferus). Asian J Pharm Clin Res 2012; 5(3): 36-41.
[7] El-Bakry AA, Mostafa HAM, Alam EA. Antioxidant activity of Rumex vesicarius l. at the vegetative stage of growth. Asian J Pharm Clin Res 2012; 5(4): 111-117.
[8] Asrar AA. Seed germination induction of Hommaidh (Rumex vesicarius L.) by gibberellic acid and temperature applications. American-Eurasian J Agric Environ Sci 2011; 10(3): 310-317.
[9] Al-Yahya MA, Al-Meshal IA, Mossa JS, Al-Badr AA, Tariq M. Saudi plants: a phytochemical and biological approach. Riyadh: King Saud University Press; 1990.
[10] Chaudhury RR. Herbal medicine for human health. New Delhi: World Health Organization, Regional Office for South-East Asia; 1992, p. 1-80.
[11] WHO. Quality control methods for medicinal plant material. Geneva: WHO; 1998, p. 1-15.
[12] Aktar MW, Poi R, Bhattacharyya A. Status of sennosides content in various Indian herbal formulations: method standardization by HPTLC. Bangladesh J Pharmacol 2008; 3: 64-68.
[13] Pawar RK, Sharma S, Singh KC, Sharma Rajeev KR. Physicochemical standardisation and development HPTLC method for the determination of plumbagin in Kalmegh Navayas Loha-an ayurvedic formulation. Int J Curr Pharm Res 2011; 3(1): 43-48.
[14] Al-Rumaih MM, Al-Saad FA, Warsy AS. Seasonal variation in mineral content of different organs during development of Rumex vesicarius L. Saudi J Biol Sci 2002; 9(1): 69-79.
[15] Alfawaz MA. Chemical composition of hummayd (Rumex vesicarius) grown in Saudi Arabia. J Food Compost Anal 2006; 19: 552-555.
[16] Barbosa-Filho JM, Alencar AA, de Andrade Tomaz AC, Sena-Filho JG, Athayde-Filho PF, Silva MS, et al. Sources of alpha-, beta-, gamma-, delta- and epsilon-carotenes: a twentieth century review. Brazil J Pharma 2008; 18(1): 135-154.
[17] Rao BN. Bioactive phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac J Clin Nutr 2003; 12(1): 9-22.
[18] Matkowski A. Plant in vitro culture for the production of antioxidants-a review. Biotechnol Adv 2008; 26: 548-560.
[19] Wright JR, Colby HD, Miles PR. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys 1981; 206(2): 296-304.
[20] Halliwell B, Gutteridge JM. Formation of thiobarbituric-acidreactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett 1981; 128: 347-352.
[21] Liu F, Ng TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci 2000; 66: 725-735.
[22] Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 1995; 28(1): 25-30.
[23] Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 1999; 299: 152-178.
[24] Ordoñez AAL, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem 2006; 97: 452-458.
[25] Sun JS, Tsuang YH, Chen IJ, Huang WC, Hang YS, Lu FJ. An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns 1998; 24(3): 225-231.
[26] Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. 3rd ed. Berlin: Springer; 1998.
[27] Wagner H, Baldt S. Plant drug analysis: a thin layer chromatography atlas. Berlin: Springer; 1996.
[28] Steenkamp V, Stewart MJ, Chimuka L, Cukrowska E. Uranium concentrations in South African herbal remedies. Health Phys 2005; 89: 679-683.
[29] Hamilton KL. Antioxidants and cardioprotection. Med Sci Sports Exerc 2007; 39(9): 1544-1553.
[30] Yamunadevi M, Wesely EG, Johnson M. Chromatographic finger print analysis of steroids in Aerva lanata L by HPTLC technique. Asian Pac J Trop Biomed 2011; 1: 428-433.
[31] Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents 2005; 26: 343-356.
[32] Park BS, Lee HK, Lee SE, Piao XL, Takeoka GR, Wong RY, et al. Antibacterial activity of Tabebuia impetiginosa Martius ex DC (Taheebo) against Helicobacter pylori. J Ethnopharmacol 2006; 105: 255-262.
[33] Stević T, Šavikin K, Ristić M, Zdunić G, Janković T, Krivokuća-Đokić D, et al. Composition and antimicrobial activity of the essential oil of the leaves of black currant (Ribes nigrum L.) cultivar Čačanska crna. J Serbian Chem Soc 2010; 75(1): 35-43.
[34] Prasad PSH, Ramakrishnan N. In vitro lipid peroxidation assay of Rumex vesicarius L. Int J Pharm Pharm Sci 2012; 4(Suppl 1): S368-S370.
[35] Saha MR, Alam MA, Akter R, Jahangir R. In vitro free radical scavenging activity of Ixora coccinea L. Bangladesh J Pharmacol 2008; 3: 90-96.
10.12980/APJTB.4.2014C1168
*Corresponding author: T.H. Khan, Department of Pharmacology, College of Pharmacy, Salman Bin Abdulaziz University, P.O. Box 173, Al-Kharj, 11942, Kingdom of Saudi Arabia.
Tel: 00966 11 5886035
E-mail: tajdarhamdard@gmail.com
Foundation Project: Supported by Deanship of Scientific Research, Salman bin Abdulaziz University, Al-Kharj Saudi Arabia (Grant No.4/1432).
Article history:
Received 20 May 2014
Received in revised form 22 May, 2nd revised form 29 May, 3rd revised form 5 Jun 2014
Accepted 15 Jun 2014
Available online 28 Jul 2014
Methods:In the present study, acetone, ethyl acetate, n-butanol, and methanol extracts of R. vesicarius were evaluated for radical scavenging activity by studying the inhibition of the level of lipid peroxidation induced by Fe(++)/ascorbate, DNA sugar damage, scavenging of hydrogen peroxide, diphenylphosphine DPPH radical scavenging activity, total phenolic content, total flavonoids content and total proanthocyanidin. High performance thin layer chromatography finger print profiling of R. vesicarius L. was also done.
Results:Lipid peroxidation induced by the iron/ascorbate system, hydrogen peroxide, diphenylphosphine and DNA sugar damage were inhibited by the addition of different extract of R. vesicarius. Among them, methanolic extract showed maximum efficacy. The methanolic extract showed the highest total phenolic, total flavonoids and total proanthocyanidin contents.
Conclusions:The results suggest that the extracts can be a vital source of phytochemical antioxidants.
 Asian Pacific Journal of Tropical Biomedicine2014年7期
Asian Pacific Journal of Tropical Biomedicine2014年7期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antimicrobial activity against periodontopathogenic bacteria, antioxidant and cytotoxic effects of various extracts from endemic Thermopsis turcica
- Proteomics analysis of antimalarial targets of Garcinia mangostana Linn.
- The presence of eucalyptol in Artemisia australis validates its use in traditional Hawaiian medicine
- Jeju seaweeds suppress lipopolysaccharide-stimulated proinflammatory response in RAW 264.7 murine macrophages
- Cytotoxicity screening of Melastoma malabathricum extracts on human breast cancer cell lines in vitro
- In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines
