Effect of CD74 on the prognosis of patients with resectable pancreatic cancer
Jun-Feng Zhang, Rong Hua, De-Jun Liu, Wei Liu, Yan-Miao Huo and Yong-Wei Sun
Shanghai, China
Effect of CD74 on the prognosis of patients with resectable pancreatic cancer
Jun-Feng Zhang, Rong Hua, De-Jun Liu, Wei Liu, Yan-Miao Huo and Yong-Wei Sun
Shanghai, China
BACKGROUND:CD74 is known as a type II transmembrane glycoprotein that is associated with the major histocompatibility complex class II α and β chains. Recent studies have demonstrated that the expression of CD74 is also linked to some forms of tumors. The present study was to assess the effect of CD74 expression on the prognosis of resectable pancreatic ductal adenocarcinoma (PDAC).
METHODS:Forty-six patients who had received a curative resection of primary PDAC and postoperative chemotherapy were included in this study. Immunohistochemical staining was conducted of CD74 on paraffin-embedded tumor sample slices. The patients were grouped according to CD74 staining: CD74 (-): CD74 positive tumor cells <25%; and CD74 (+): CD74 positive tumor cells ≥25%. The correlation of CD74 expression level with clinicopathological features and cumulative survival rate was calculated.
RESULTS:The numbers of CD74 (+) and (-) patients were 32 and 14, respectively. CD74 (+) patients showed a high rate of perineural invasion (P=0.007). The 3- and 5-year cumulative survival rates of CD74 (-) patients were significantly higher than those of CD74 (+) patients (62% and 41% vs 9% and 0%, P=0.000). Multivariate analysis showed that CD74 expression and lymphatic permeation were the independent prognostic indicators.
CONCLUSIONS:The overexpression of CD74 is a key factor associated with perineural invasion. Lower-stage (I and II) PDAC patients with CD74 overexpression have a poor prognosis even if they receive a curative resection. CD74 can be used as a prognostic indicator for resectable PDAC.
(Hepatobiliary Pancreat Dis Int 2014;13:81-86)
CD74;
pancreatic ductal adenocarcinoma; perineural invasion;
cumulative survival rate;
prognosis
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies of the digestive system.[1]Only 20% of the diagnosed patients have the opportunity to receive surgery because of locally advanced state or metastasis.[2]Even after a R0 curative resection, the patients still have a poor outcome because of the high rate of local recurrence and systemic metastasis. Clinicopathologic studies[3, 4]revealed that perineural invasion is one of the poor prognostic factors for PDAC patients. And overexpression of CD74, the γ invariant chain of human leukocyte antigen-DR (HLADR), may be strongly associated with perineural invasion.
Understanding the molecular mechanism of this invasion is considered to be the crucial step to sort out prognostic markers and therapeutic targets for PDAC therapy. The present study was undertaken to clarify the relationship between CD74 and perineural invasion. The expression of CD74 was retrospectively analyzed by immmunohistochemistry in 46 PDAC patients who had received surgical therapy; the relationship was evaluated between the expression of CD74, clinicopathological traits and prognosis.
Methods
Patients
Forty-six patients who had received a curative (R0) resection of primary PDAC from January 1, 2008 to December 31, 2009 were included in this study. All patients underwent operation at the Department of General Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University. According to the tumornode metastasis (TNM) staging system (7th Edition) of the American Joint Commission on Cancer (AJCC),[5]these patients were of stage I and II (T1-3, N0-1, M0). Those of stage III and IV with locally advanced unresectable foci (T4) and/or systemic metastases (M1) were excluded. The patients comprised 22 men and 24 women with ages ranging from 42 to 83 years (median 68). All patients were followed up until December 31, 2012 or death, ranging from 2 to 58 months (median 12.5). The survived patients received postoperative chemotherapy according to the following protocol: six times of intravenous infusion of gemcitabine (1000 mg/ m2, total dose: 6000 mg/m2), six months of oral administration of tegafur-gimeracil-oteracil capsules (80 mg/d, 3 weeks/month, total dose: 10 080 mg, according to the dose of tegafur). The level of serum CA19-9 was measured and abdominal ultrasonography and/or computed tomography was performed every 3 months for monitoring tumor recurrence. The study protocol was approved by the Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiaotong University.
Tissue samples
Resected specimens of PDAC were fixed in 10% formalin and then embedded in paraffin. Histological diagnoses were made by two senior pathologists according to the WHO criteria.[6]Perineural invasion was defined as PDAC cell infiltration of the perineural space between the perineurium and endoneurium of the peripheral nerve in directly contact with the endoneurium (Fig. 1A) and intraneural invasion (Fig. 1B).[4]
Immunohistochemical expression of CD74
Sections with the largest number of fixed PDAC cells were used for immunohistochemical staining. Each section was deparaffinized, rehydrated and incubated with 0.3% hydrogen peroxide in methanol for 30 minutes at 25 ℃. After washing with PBS, the section was heated in 10 mmol/L citrate buffer (pH=6) for 10 minutes. After blocking with endogenous peroxidase, the section was incubated with mouse monoclonal anti-CD74 antibody (Abcam Co., Cambridge, UK) at a concentration (v/v) of 1% overnight at 4 ℃. After washing with PBS, the section was incubated with secondary antibody for 30 minutes at 25 ℃. Normal pancreatic duct showed negative immunostaining for CD74 (Fig. 2A). Lymphocytes showed uniform strong immunostaining and served as a positive control (Fig. 2B). Positive staining was defined as equal to or stronger than positive control. According to the percentage of positive cancer cells, patients were divided into two groups: 1, CD74 positive PDAC cells <25% (Fig. 2C); and 2, CD74 positive PDAC cells ≥25% (Fig. 2D). Negative control staining (Fig. 2E) is essential for determining specificity.
Statistical analysis
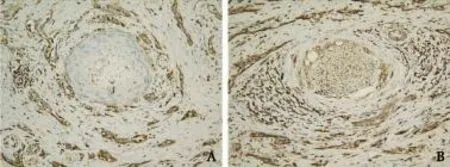
Fig. 1.Two patterns with perineural invasion (original magnification ×200).A: PDAC cell infiltrated the perineural space between the perineurium and endoneurium of the peripheral nerve in directly contact with the endoneurium;B: PDAC cell penetrated the endoneurium of the peripheral nerve.
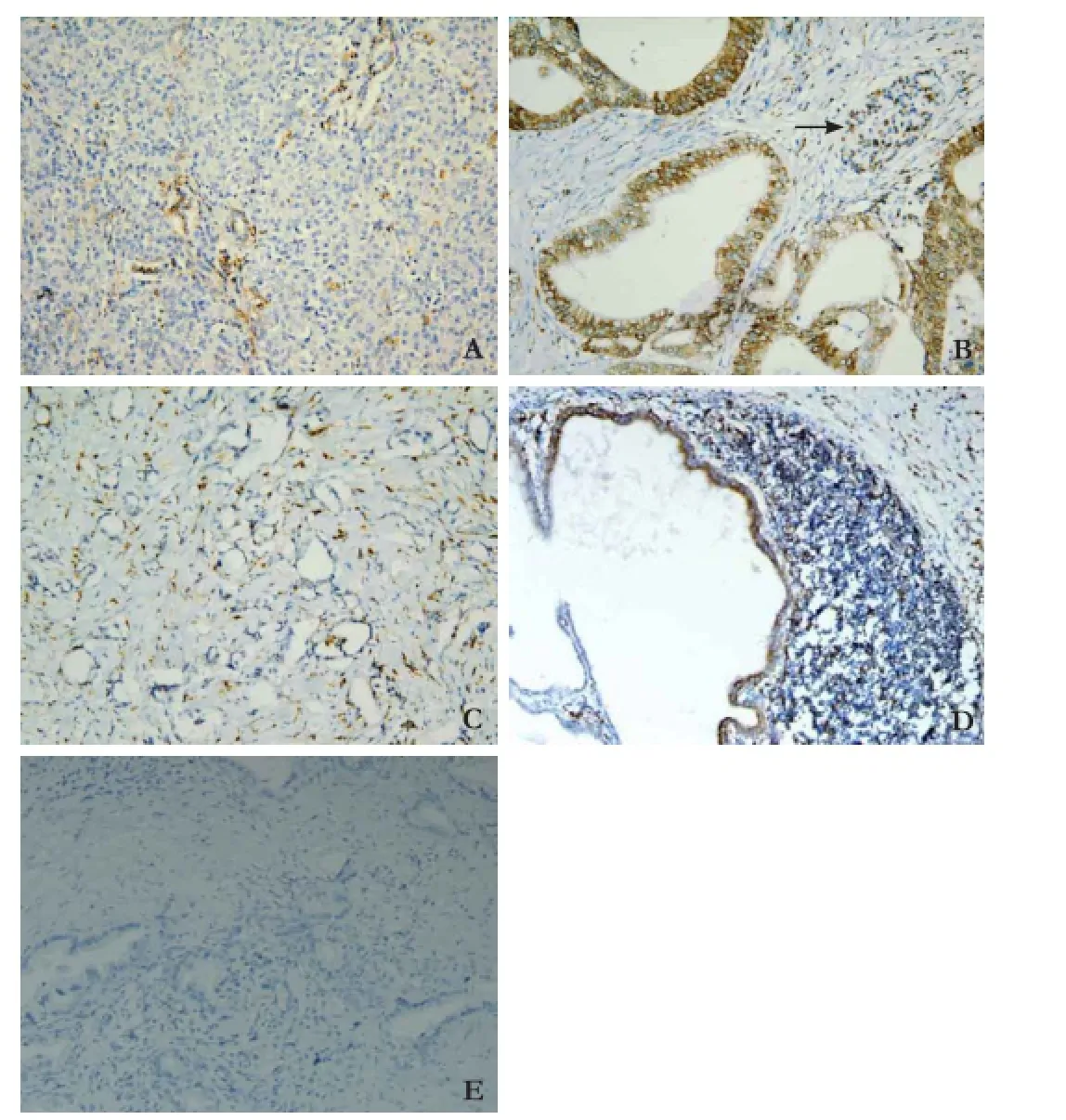
Fig. 2.Immunohistochemical expression of CD74 (original magnification ×200).A: Normal pancreatic tissue, CD74 staining is negative with weak, limited labeling of normal pancreatic duct cells;B: positive control, lymphocytes showed uniform strong expression of CD74 (arrow);C: CD74 positive PDAC cells <25%;D: CD74 positive PDAC cells ≥25%;E: CD74 negative control staining of PDAC cells.
SPSS 17.0 software package (SPSS, Chicago, IL, USA) was used to perform statistical analyses. The correlation between CD74 expression and clinicopathologicalfeatures was analyzed by the Chi-square test and Fisher's exact test. The Kaplan-Meier and life table methods were used to calculate the cumulative survival rate, and the difference in survival curves was evaluated using the log-rank test. Cox's proportional hazards regression model in a stepwise manner was used to analyze the independent prognostic factors. A P value of less than 0.05 was considered to be statistically significant.
Results
Followed-up outcomes
One patient died of sepsis secondary to pancreatic leakage during her hospital stay (61 days after operation). The remaining 45 patients were followed up until December 31, 2012 or death. In these patients, 35 died of pancreatic cancer, 3 from other causes (myocardial infarction in 1, ketoacidosis in 1 and leukopenia in 1), and 7 were alive at the end of the study. Forty-six patients were followed up for more than 36 months. Tumor recurrence was confirmed in 36 patients (78.3%), including 12 patients with local recurrence, 14 patients with metastasis to other organs or systems and 10 patients with both. The 3- and 5-year cumulative survival rates were 25% and 15%, respectively.
Immununohistochemical staining results of CD74
In all the 46 PDAC patients, 32 patients (69.6%) present strong CD74 staining in ≥25% of cancer cells, which were defined as CD74 positive. The remaining 14 patients (30.4%) with CD74 positive staining in <25% of cancer cells were regarded as CD74 negative.
Correlation between CD74 expression and clinicopathological features in PDAC
The histological types of samples from the 46 patients were papillary (29 patients) or tubular (17). Adenocarcinomas were grouped morphologically as well differentiated (G1, 2 patients), moderately differentiated (G2, 30), and poorly differentiated (G3, 14). The pathological TNM staging of carcinomas showed stage I in 15 patients, and stage II in 31. The information about the relationship between CD74 expression and clinicopathological features is shown in Table 1.
CD74 expression and prognosis of PDAC
Patients with CD74 negative expression had much higher 3- and 5-year cumulative survival rates than those with CD74 positive expression (3-year cumulative survival rates: 62% vs 9%; 5-year: 41% vs 0%; P=0.000; Fig. 3). Univariate analysis demonstrated that age(P=0.030), perineural invasion (P=0.008), lymphatic permeation (P=0.048) and pTNM (P=0.002) were the clinicopathological factors with prognostic significance (Table 2). However, multivariate analysis showed that only CD74 expression and lymphatic permeation were identified as independent prognostic indicators (Table 3).

Fig. 3.Cumulative survival rate of PDAC patients with positive and negative CD74 expression (P=0.000).
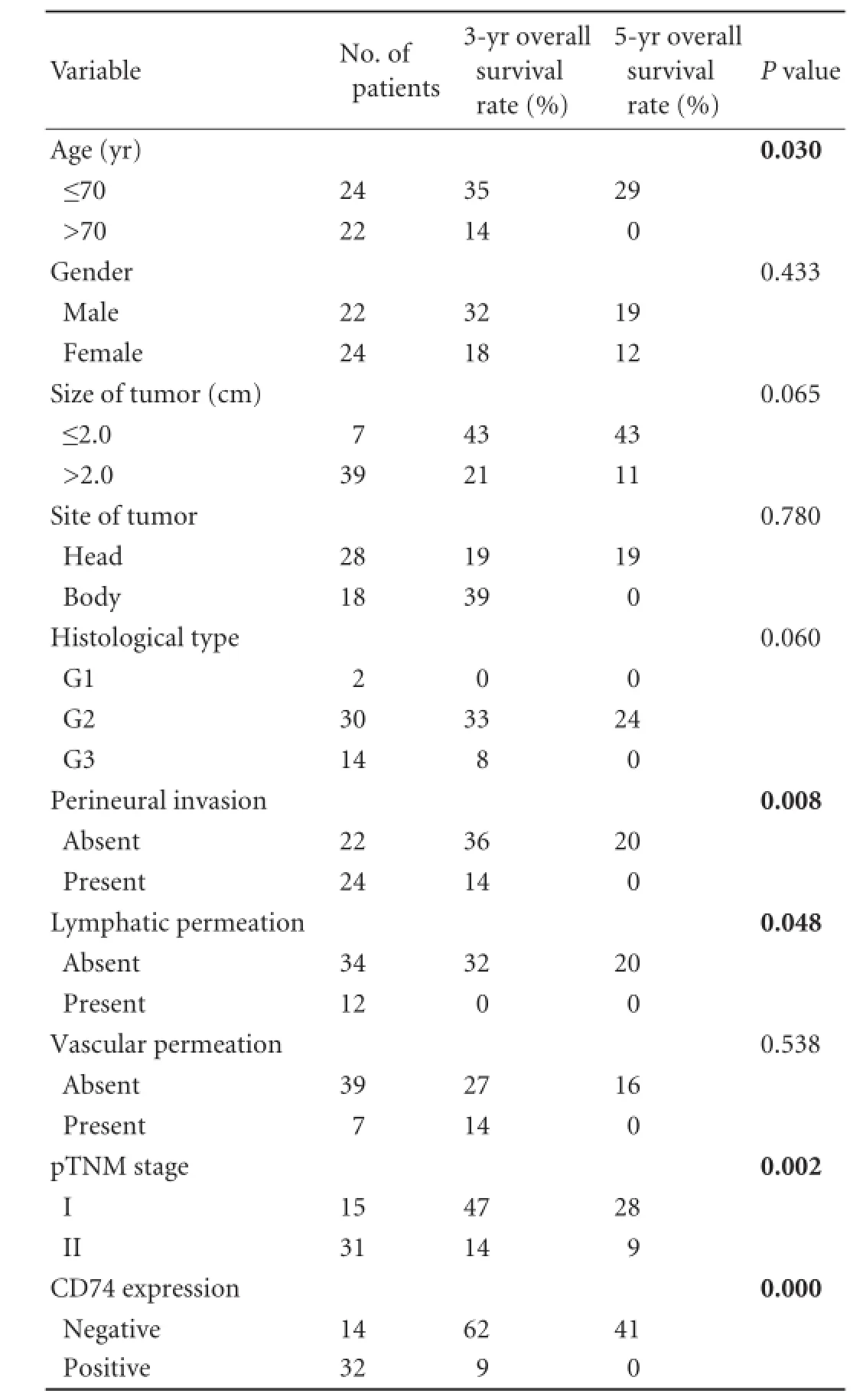
Table 2.Univariate analysis of clinicopathological features for the cumulative survival rate of 46 PDAC patients
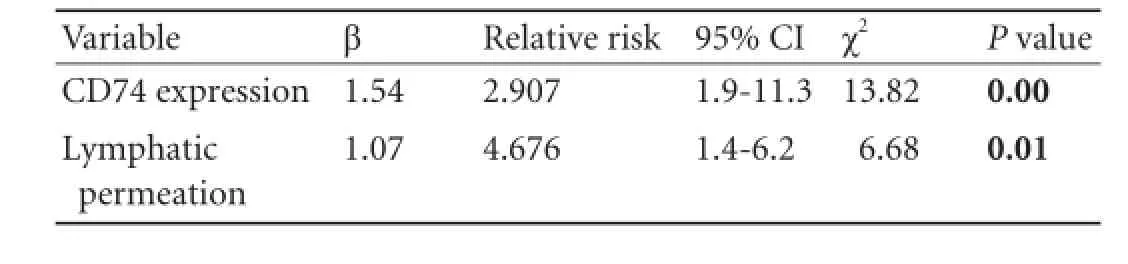
Table 3.Multivariate analysis of clinicopathological features for the cumulative survival rate of 46 PDAC patients
Discussion
Despite numerous individual or cooperative studies, PDAC remains a deadly disease with a low surgical resection rate but a high mortality rate. Even in patients who have received a curative resection, the survival rate is still very low.[7]Although the AJCC staging system is the best tool to provide the prognostic information at present, further studies on predictive factors are helpful to improve the prognosis and to optimize the treatment.[8, 9]
CD74, as a type II transmembrane glycoprotein that is associated with the major histocompatibility complex (MHC) class II α and β chains, is involved in several steps in the immune system, including antigen presentation, B-lymphocyte differentiation and inflammatory signaling.[10]Recent studies[11-14]have found that the expression of CD74 is also linked to some forms of tumors. The overexpression of CD74 on some malignant cells suggests that CD74 expression may prevent presentation of tumor antigens and suppress the host immune responses.[15]In hematological malignancies like non-Hodgkin's lymphoma and multiple myelomas, CD74 is confirmed as an attractive and promising therapeutic target by its high expression and rapid turnover on the cell surface.[16]
Nagata et al[17]reported the relationship between the expression of CD74 and the prognosis of 68 PDAC patients who underwent extended pancreaticoduodenectomy. In that study, patients with CD74 (+) expression showed a higher rate of lymphatic permeation (P=0.04) and perineural invasion (P=0.001) compared with those with CD74 (-) expression. The study also found that patients with CD74 (-) had a significantly higher 5-year survival rate than those with CD74 (+) (69% vs 0%, P=0.003). Multivariate analysis revealed that CD74 expression level and vascular permeation of carcinoma were independent prognostic indicators. But all patients included in this study received neoadjuvant chemoradiotherapy and showed the absence of disease progression before curative resection. Although we have no clinical findings to support that preoperative remedies may affect CD74 expression of tumor cells, several studies have shown neoadjuvant chemoradiotherapy may influence the status of perineural invasion and resectability in borderline pancreatic cancer.[18-20]And exclusion of patients with disease progression may result in sample selection bias. Thus, no preoperative chemoradiotherapy was delivered to patients and only those with lesions resectable (defined as stage I and II by the 7th edition AJCC) were included in our study.
Because of the lack of recognized standard, the predictive value of the overexpression of CD74 on theprognosis of PDAC is still inconclusive. In our study, the cutoff point of positive CD74 expression was defined as more than 25% of PDAC cells which were of significant prognostic significance. CD74 overexpression in PDAC has been observed in several experimental and clinical studies.[4, 21]Although the definitive mechanism is still unknown, CD74 has been reported as a critical factor associated with perineural invasion of PDAC, and may have the potential to become a prognostic marker of tumor-related clinical outcome.[4]The present study showed that the incidence of perineural invasion in CD74 (+) patients was significantly higher than that in CD74 (-) patients (66% vs 21%, P=0.007), which clearly demonstrated the relationship between CD74 and perineural invasion in resectable PDAC. In another unfinished study (data not shown), we found that synthesis of nerve growth factor (NGF) increased in high perineural invasion PDAC cell lines. The cell lines are consistent with the findings of Peng's study in C6 glioma cells.[22]Our unpublished data suggest that CD74 may regulate the perineural invasion via NGF.
The 7th edition of the AJCC Cancer Staging Manual provides an objective and clear classification of "resectable" PDAC (stage I and II).[5]Our study showed that patients received a curative resection and postoperative chemotherapy also had a poor prognosis (3- and 5-year cumulative survival rate: 25% and 15%, respectively). Further grouping of patients upon CD74 expression displayed that lack of CD74 expression might indicate a better outcome (3- and 5-year cumulative survival rate: 62% and 41%, respectively). On the contrary, those with overexperssion of CD74 had a poor prognosis (3- and 5-year cumulative survival rate: 9% and 0, respectively). Moreover, because of the high risk of recurrence or metastasis, patients with CD74 (+) might need more aggressive neoadjuvant or postoperative chemoradiotherapy.
In conclusion, the overexpression of CD74 is a key factor for perineural invasion. Lower-stage (I and II) PDAC patients with CD74 overexpression have a significant poor prognosis although they have received a curable resection. These patients need more aggressive adjuvant chemoradiotherapy. CD74 has been confirmed as a prognostic indicator for resectable PDAC.
一种STC89C51单片机控制按摩眼罩的设计………………………………卢莉蓉,王小明,李慧贤,等(46)
Acknowledgements:The authors thank the pathologists, Drs. Qiang Liu and Yan-Yin Shen for their technical help.
Contributors:SYW proposed the study. ZJF and HR performed research and wrote the first draft. LDJ, LW and HYM collected and analyzed the data. ZJF and LDJ contributed equally to this article. All authors contributed to the design and interpretation of the study and to further drafts. SYW is the guarantor.
Funding:This study was supported by a grant from Shanghai Health Bureau (20114213).
Ethical approval:This study protocol was approved by the Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiaotong University.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96.
3 Hirai I, Kimura W, Ozawa K, Kudo S, Suto K, Kuzu H, et al. Perineural invasion in pancreatic cancer. Pancreas 2002;24: 15-25.
4 Koide N, Yamada T, Shibata R, Mori T, Fukuma M, Yamazaki K, et al. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clin Cancer Res 2006;12:2419-2426.
5 Edge SB, Byrd DR, Carducci M, Compton CC, Fritz AG. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual (7th ed.). New York: Springer; 2009.
6 Hamilton SR, Aaltonen LA. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000.
7 Gebhardt C, Meyer W, Reichel M, Wünsch PH. Prognostic factors in the operative treatent of ductal pancreatic carcinoma. Langenbecks Arch Surg 2000;385:14-20.
8 Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg 2004;240:293-298.
9 Wasif N, Ko CY, Farrell J, Wainberg Z, Hines OJ, Reber H, et al. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Ann Surg Oncol 2010;17:2312-2320.
10 Borghese F, Clanchy FI. CD74: an emerging opportunity as a therapeutic target in cancer and autoimmune disease. Expert Opin Ther Targets 2011;15:237-251.
11 Binsky I, Haran M, Starlets D, Gore Y, Lantner F, Harpaz N, et al. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci U S A 2007;104:13408-13413.
12 Datta MW, Shahsafaei A, Nadler LM, Freeman GJ, Dorfman DM. Expression of MHC class II-associated invariant chain (Ii;CD74) in thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol 2000;8:210-215.
13 McClelland M, Zhao L, Carskadon S, Arenberg D. Expression of CD74, the receptor for macrophage migration inhibitory factor, in non-small cell lung cancer. Am J Pathol 2009;174: 638-646.
14 Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol 2006;177:8730-8739.
15 Stein R, Mattes MJ, Cardillo TM, Hansen HJ, ChangCH, Burton J, et al. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res 2007; 13:5556s-5563s.
16 Berkova Z, Tao RH, Samaniego F. Milatuzumab - a promising new immunotherapeutic agent. Expert Opin Investig Drugs 2010;19:141-149.
17 Nagata S, Jin YF, Yoshizato K, Tomoeda M, Song M, Iizuka N, et al. CD74 is a novel prognostic factor for patients with pancreatic cancer receiving multimodal therapy. Ann Surg Oncol 2009;16:2531-2538.
18 Barbier L, Turrini O, Grégoire E, Viret F, Le Treut YP, Delpero JR. Pancreatic head resectable adenocarcinoma: preoperative chemoradiation improves local control but does not affect survival. HPB (Oxford) 2011;13:64-69.
19 Stokes JB, Nolan NJ, Stelow EB, Walters DM, Weiss GR, de Lange EE, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619-627.
20 Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833- 848.
21 Hustinx SR, Cao D, Maitra A, Sato N, Martin ST, Sudhir D, et al. Differentially expressed genes in pancreatic ductal adenocarcinomas identified through serial analysis of gene expression. Cancer Biol Ther 2004;3:1254-1261.
22 Peng CH, Huang CN, Hsu SP, Wang CJ. Penta-acetyl geniposide-induced apoptosis involving transcription of NGF/p75 via MAPK-mediated AP-1 activation in C6 glioma cells. Toxicology 2007;238:130-139.
Received March 1, 2013
Accepted after revision September 4, 2013
Author Affiliations: Department of General Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200127, China (Zhang JF, Hua R, Liu DJ, Liu W, Huo YM and Sun YW)
Yong-Wei Sun, MD, Department of General Surgery, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200127, China (Tel: 86-21-68383773; Email: syw0616@126.com) © 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60011-4
 Hepatobiliary & Pancreatic Diseases International2014年1期
Hepatobiliary & Pancreatic Diseases International2014年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Samaritan donor interchange in living donor liver transplantation
- Hepatic ischemic preconditioning increases portal vein flow in experimental liver ischemia reperfusion injury
- Graft cholangiopathy: etiology, diagnosis, and therapeutic strategies
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Graft-to-recipient weight ratio lower to 0.7% is safe without portal pressure modulation in right-lobe living donor liver transplantation with favorable conditions
- Platelet count reduction and outcomes in living liver donors
