CaCl2催化NaBH4还原氧化石墨烯
杨真真,郑庆彬,邱汉迅,李 静,杨俊和
(上海理工大学 材料科学与工程学院,上海 200093)
1 Introduction
Graphene,as a two-dimensional carbon material with only one atomic layer,has attracted significant research efforts on its fabrication method owing to its exceptional electrical,thermal,optical and mechanical properties[1-3].Among all the fabrication methods,chemical oxidation of graphite followed by reduction was regarded as a cost-effective approach and promising for a large scale production.Graphene oxide (GO),produced by oxidation and exfoliation of graphite,is hydrophilic and electrically insulating because a large number of oxygen-containing functional groups are bonded with carbon atoms[4].In order to recover the electrical conductivity of graphene,GO should be reduced to graphene-like sheet,named reduced GO (RGO),by partially removing the oxygen-containing functional groups and recovering the sp2carbon atoms.RGO was considered to be a chemically converted graphene due to the residue functional groups and the defects.
The reduction of GO has been carried out by thermal treatment,microwave irradiation and chemical reagent reduction[5].Several kinds of reducing agent have been studied,such as HI[6],hydrazine and hydrazine derivate[7],Al[8],vitamin C[9]and NaBH4[10].Among the reducing agents,NaBH4was considered as one of the efficient,nontoxic,noncorrosive,inexpensive agents.NaBH4has been frequently used as a reagent to reduce aldehydes and ketones to alcohols[11],where the C=O species can be reduced effectively,while the C—O species are tend to remain.Similarly,the majority of carbonyl and epoxy groups can be changed into hydroxyl groups in the reduction of GO by NaBH4[10].To increase the reducing ability of NaBH4,AlCl3was used as a catalyst and part of the hydroxyl groups can be removed at a reaction temperature below 150 ℃in DMF solvent[12].
In this study,reduction of GO was carried out in aqueous solution at ambient temperature using NaBH4-CaCl2reagent system,aiming at developing a facile and environmental-friendly reduction procedure.
2 Experimental
2.1 Synthesis of graphene oxide
GO was prepared by modified hummers method[13].The graphite intercalated compound (supplied by Asbury Graphite Mills,USA)was heated at 1 050 ℃ for 30 s to produce worm-like expanded graphite.1 g expanded graphite and 200 mL sulfuric acid (H2SO4,98%)were mixed for half an hour in a beaker.10 g KMnO4was added to the beaker while stirring.The temperature was then raised to 60 ℃and kept for 24 h.After the oxidation,the mixture was cooled in an ice-bath and then 100 mL deionized (DI)water,30 mL H2O2and 20 mL HCl were added sequentially.The GO suspension was washed by DI water several times until the pH value became around 6.
2.2 Reduction of graphene oxide
As prepared GO was diluted in DI water to form a suspension of 0.5 mg/mL.NaBH4(2.28 g)and CaCl2(4.4 mg~1.78 g)was added to a 200 mL GO suspension.The mixture was kept stirring at room temperature for 12 h to obtain RGO.Next,the RGO was filtered and washed by DI water until neutralization.The as-prepared RGO was referred to as CRGO-x (x represented the molar concentration of CaCl2in the unit of mmol/L).For comparison,GO reduced by NaBH4without the presence of CaCl2was referred as N-RGO.
2.3 Characterizations
Atomic force microscopic (AFM)images were obtained in a non-contact mode using an Auto-Probe CP/MT scanning probe microscope (XE-100,PSIA).The sample for AFM was prepared by depositing dispersion of GO on a clean silicon wafer.Transmission electron microscopic (TEM)images and selected area electron diffraction (SAED)patterns were obtained using a Tecnai G2 F30 operating at an accelerating voltage of 300 kV.For TEM sample preparation,GO/RGO was dispersed in ethanol and sonicated for 5 min,which was deposited on a copper grid with porous carbon film.Attenuated total reflectance Fourier transform infrared (ATR-FT-IR)analysis was performed on a Spectrum 100 FT-IR spectrometer (Perkin-Elmer)over a wave length range from 500 to 4 000 cm-1at a resolution of 4 cm-1.The elemental compositions and the assignments of the carbon peaks were characterized using Xray photoelectron spectroscopy (XPS,PHI 5000C ESCA System),which was equipped with a monochromated Al-kα (1 486.6 eV)X-ray source operated at 250 W (14 kV)in a residual vacuum of 5 ×10-9Torr.The pass energy was 93.9 eV for survey scans and 23.5 eV for high-resolution scans.The high-resolution spectra were analyzed with XPS Peak4.1 software (provided by Raymund W.M.Kwok (The Chinese University of Hong Kong,China)),using the containment carbon (C1s=284.6 eV)for calibration and Shirley method for background subtraction.The GO and RGO samples were evaluated by UV-Vis spectroscopy (Perkin-Elmer Lambda 750).The sheet resistance of the RGO papers with a constant thickness was measured using a four-point probe method.RGO papers were obtained by filtrating 300 mL RGO aqueous dispersion(0.03 mg/mL)on a filter membrane with a sand core funnel and no extra binder was used during filtration.
3 Results and discussion
Fig.1a shows the AFM image of GO.The wrinkled surface of GO made it fluctuating in the thickness of GO,but the thickness can be estimated to be around 1.5 nm according to the height profile given in Fig.1a.Because of the oxygen-containing functional groups bonded on both sides of graphene basal plane,the thickness of GO was anticipated to be around 1 nm[14].The thickness of GO in our samples was from 0.86 to 1.77 nm,indicating a monolayer or bilayer of carbon atoms.The TEM morphology of GO is shown in Fig.1b.The GO sheet showed a relative flat surface and large lateral size compared with the CRGO sheets shown in Fig.1c and d.The reduction of GO was accompanied by an increased roughness and a reduced lateral size of graphene sheets due to a significant atomic rearrangement[15].Agglomerated C-RGO was observed due to its poor dispersibility after the reduction.In the inset of Fig.1b,a typical diffraction ring with some bright spots from GO was observed by the SAED pattern,implying a partial amorphization of GO with some residual graphitic crystalline[16,17].The SAED pattern of C-RGO,as an inset of Fig.1d,was similar to the diffraction pattern of GO,indicating a lack of perfect crystallinity of the hexatomic ring after the reduction.
Fig.1e shows the aqueous suspensions of GO,N-RGO and C-RGO-50 with a concentration of 1 mg/mL.The suspensions were left undisturbed for 24 h after 2 h sonication.The GO suspension showed a brown color with a semi-transparency,while the color of N-RGO and C-RGO-50 turned into black after the chemical reduction.The C-RGO-50,reduced with the presence of CaCl2,formed a black precipitation and settled to the bottom due to the decreased surface functionality.The uniform suspension of GO in DI water was held for months due to the large amounts of oxygen containing functional groups.The N-RGO suspension was also stable because the amount of hydrophilic functional groups on N-RGO were more than that on C-RGO-50.This would be proved by the XPS and FTIR results shown in Fig.2 and 3.With the reduction of GO,the polar functionality on the graphene sheet decreased.Consequently,the graphene layers with all the carbon atoms exposed to the surface tended to form aggregates by Van der Waals force,resulting in the sedimentation of CRGO-50 suspension.To produce a stable suspension of RGO in water,efforts have been made to modify the RGO surface either by covalent bonds or by noncovalent bonds.A microwave assisted titanate coupling agent modification will be employed in this project and reported separately.
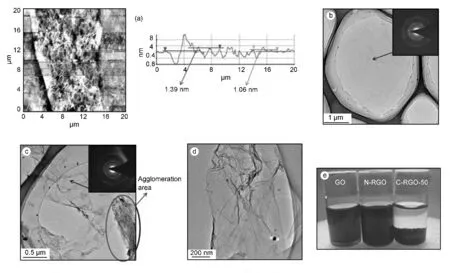
Fig.1 (a)AFM image of the GO film with the height profiles;(b)TEM image of GO and SAED pattern in the inset;(c,d)TEM images of C-RGO and SAED pattern in the inset of (c,e)dispersion of GO,N-RGO and C-RGO-50 in water.
The C/O atomic ratio of GO,N-RGO and CRGO-x,obtained by XPS analysis,is shown in Table 1.The C/O ratio increased from 2.42 of GO to 5.38 of C-RGO-50.With the increasing of CaCl2concentration,C/O atomic ratio increased dramatically.The highest value of C/O atomic ratio was 5.38 at a CaCl2concentration of 50 mmol/L.The N-RGO obtained without CaCl2as the catalyst had a medium C/O atomic ratio of 3.09,indicating a moderate degree of reduction.It is evident that the presence of CaCl2promoted deoxygenation of GO by NaBH4.Although the highest C/O ratio of 5.38 was slightly lower than the reported value of 5.58 in a similar work[12],the emphasis of our work was on the environmentalfriendly reduction procedure performed at the ambient temperature with DI water as the solvent.
The electrical resistances of N-RGO and C-RGOx are listed in Table 1.The electrical resistance of NRGO was over two orders of magnitudes higher than C-RGO-x.The lowest electrical resistance of 18.6 kΩ/sq was obtained at the CaCl2concentration of 50 mmol/L,in accordance with the results of C/O ratio.However,the electrical resistance of C-RGO-X was not simply dependent on the C/O ratio.The electrical resistance of C-RGO-80 was higher than that of C-RGO-0.5 although C-RGO-80 exhibited a higher C/O ratio as listed in Table 1.The electrical resistivity of RGO was mainly relied on the recovery of longrange conjugated lattice[5,18],which was affected by many factors,not only the removal of oxygen containing functional groups,but also the density and types of defects generated during the oxygen reduction as well as the final distribution of different types of C—C and C—O bonds[15].
The C1s XPS spectra were fitted with four components:non-oxygenated C,the C in C—O bonds,the carbonyl C and the carboxylate C,as shown in Fig.2.Area fractions of the contributing peaks are listed in Table 1.
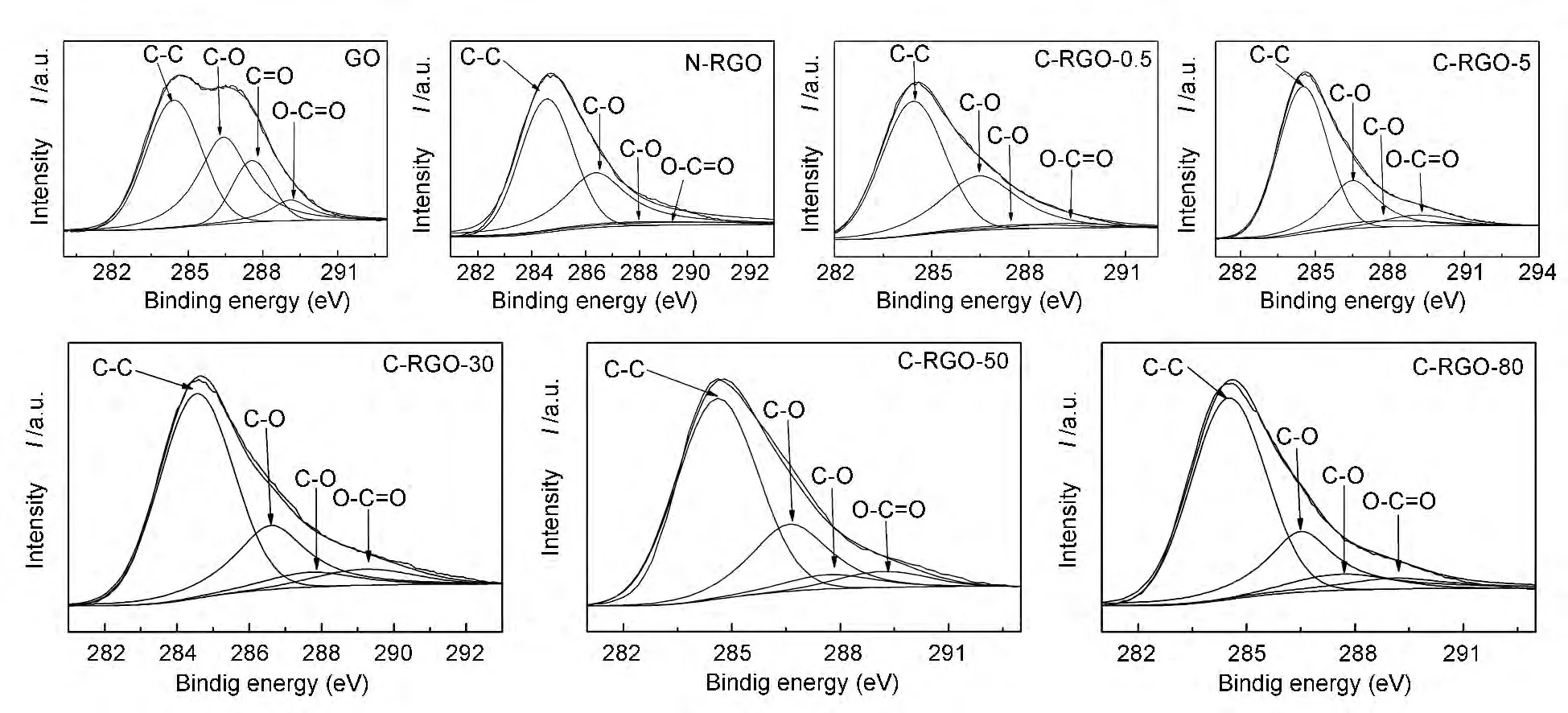
Fig.2 Deconvoluted curve fitting of C1s XPS spectra.
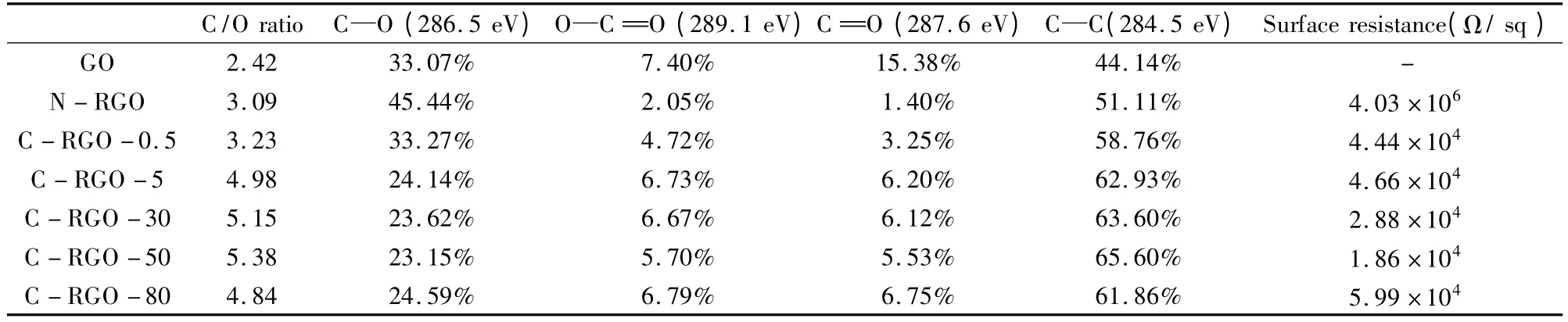
Table 1 The elemental composition,relative atomicpercentage of various functional groups and surface resistance of GO,N-RGO and C-RGO-x.
Most of the carbonyl groups and the carboxylic groups were reduced for N-RGO,while the percentage of the hydroxyl groups was even higher than that in GO.Consistent with its reactivity in organic synthesis[11],NaBH4is most effective for reducing C=O species and has a moderate capability in reduction of carboxylic acids and a poor ability for reduction of hydroxyl groups.Thus,the hydroxyl groups on N-RGO had two sources:the hydroxyl groups inherited from GO and that from the reduction of C=O and O— C=O species.Using NaBH4-CaCl2reagent system,part of hydroxyl groups can be removed,in addition to the reduction of the carbonyl groups and the carboxylic groups.Similar to the reduction of organics,the reduction ability of NaBH4was increased by the addition of CaCl2.With the increase of the CaCl2concentration,the removal of hydroxyl groups and the restoration of C—C bonds were facilitated until the concentration of CaCl2reached 50 mmol/L.
Fig.3 illustrates the FT-IR spectra of GO,NRGO and C-RGO-x.GO exhibited peaks at 3 395 cm-1,1 734 cm-1,1 634 cm-1,1 217 cm-1,1 099 cm-1corresponding to —OH stretch,stretching vibration of — C=O,a romatic C=C,—OH bending and epoxy C—O stretch,respectively[19].The spectrum of N-RGO showed peaks at 3 385 cm-1,1 599 cm-1,1 373 cm-1,1 091 cm-1corresponding to—OH stretch,a romatic C=C,C—OH stretch and epoxy C—O stretch,respectively[12],while the peaks of carboxyl and carbonyl were removed upon the reduction.Compared with GO and N-RGO,C-RGO-x exhibited less peak number,indicating an effective elimination of the functional groups.The remaining peaks for C-RGOx appearing at 1 720 cm-1,1 568 cm-1,1 200 cm-1were assigned to — C=O,a romatic C=C and —OH bending,respectively.

Fig.3 FT-IR spectra of GO,N-RGO and C-RGO-x.
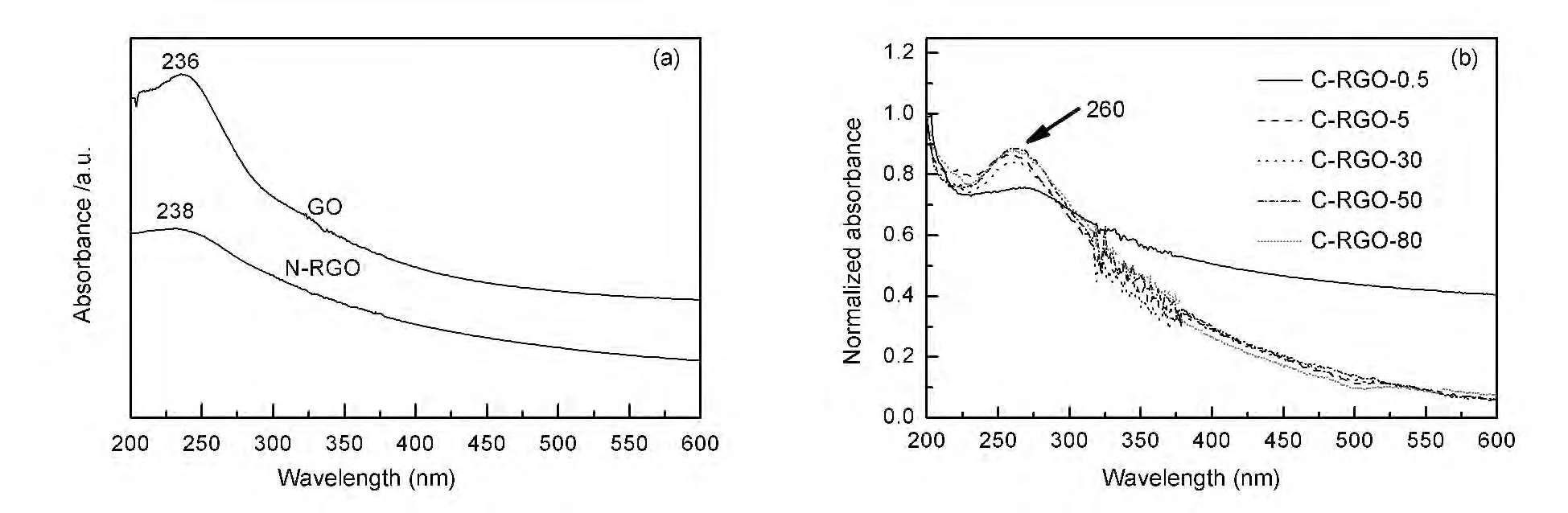
Fig.4 UV-Vis absorption spectra of (a)GO,N-RGO and (b)C-RGO-x (normalized).
Fig.4 shows the UV-Vis spectra of GO,NRGO,C-RGO-x.The absorption peak of GO suspension appeared at a wavelength of 236 nm.After the reduction,it was shifted to 238 and 260 nm for NRGO and C-RGO-x,respectively.The red shift observed can be ascribed to a restoration of electronic conjugation within the graphene sheets upon the reduction[20].
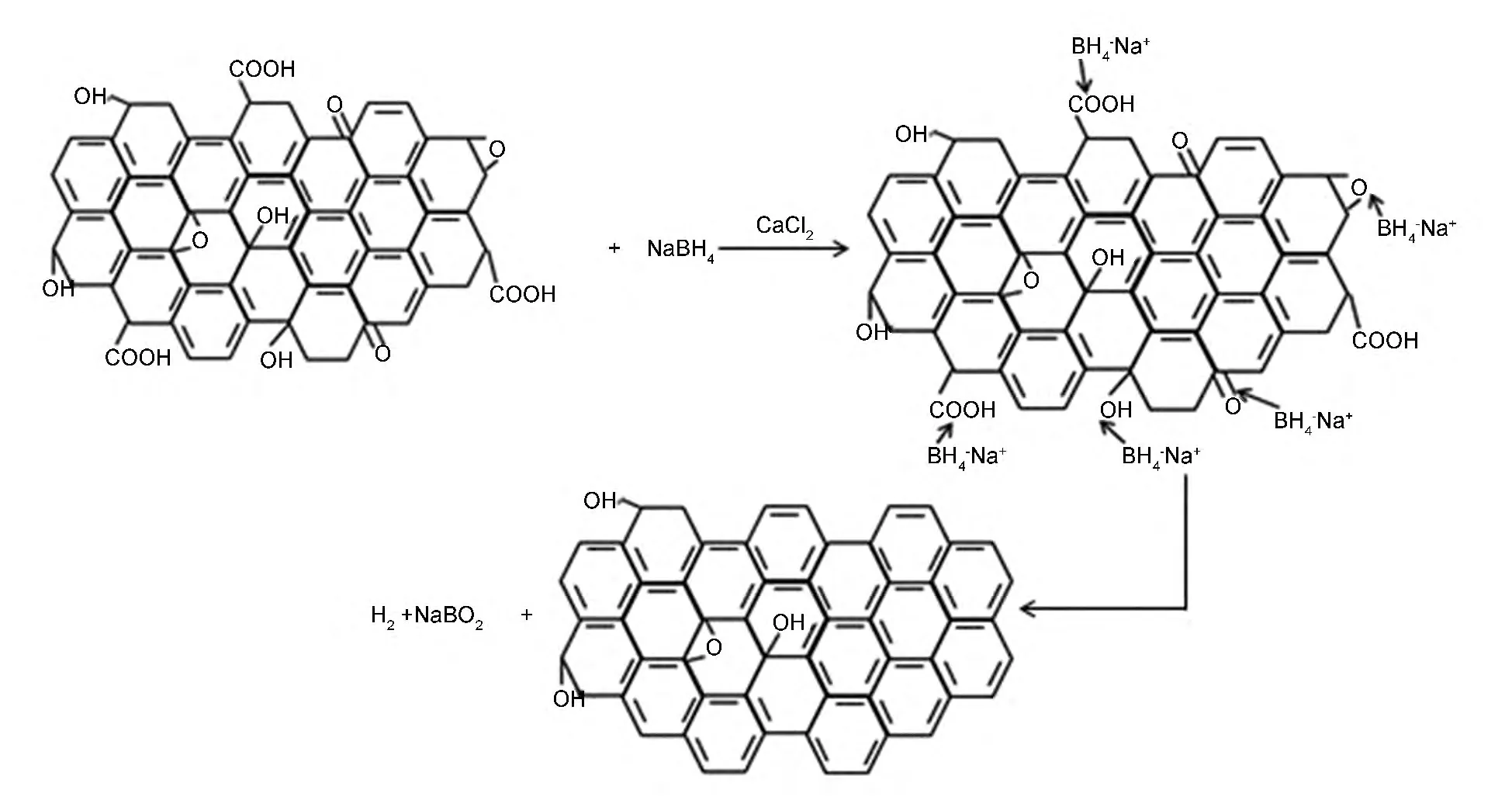
Fig.5 Schematics of the reducing reaction.
The red shift observed for C-RGO-x was more obvious than that for N-RGO.The shoulder at around 300 nm for GO was ascribed to the n→π* transition of carbonyl groups[21].After the reduction,the shoulder disappeared for N-RGO and C-RGO-x,which implied an elimination of carbonyl groups.
The use of CaCl2activated NaBH4for the reduction of various oxygen-containing functional groups in accordance with the catalysis effect of metal halide in organic synthesis,which had the benefits of mild reaction temperature,high reduction yields and environmental-friendly solvent.The use of metal halides to activate NaBH4has been extensively studied for organic synthesis.The activation effect has been proved by an increased production yielding and an extended scope of reducible species[11,22].A catalytic hydrogenation mechanism was proposed for the metal halides-NaBH4reagent through the in situ generation of borane complexes[22,23].A possible reduction reaction was proposed in Fig.5,which was an extensive simplification of real case.In real reaction,the NaBH4also reacted with DI water and generate hydrogen bubbles.Therefore,excessive amount of NaBH4was added in the reagent system.For comparison,the asprepared GO was reduced by hydrazine hydrate with microwave assistance[24],which gave a similar C/O ratio of 5.28.In this paper,we emphasize on the nontoxic reducing agent and the ambient reaction temperature.
4 Conclusions
We reported a simple procedure for the reduction of GO,which was performed at ambient temperature,using NaBH4as a reducing agent,CaCl2as a catalysis and DI water as a solvent.CaCl2can improve the reduction efficiency of NaBH4and facilitate the elimination of the hydroxyl groups and restoration of electronic conjugation of graphene.The RGO prepared by NaBH4-CaCl2reagents achieved a higher C/O atomic ratio,a lower electrical resistance and less oxygen functional groups than the RGO prepared without CaCl2.The lowest C/O ratio of 5.38 and lowest resistance of 18.6 kΩ/sq were obtained simultaneously at a CaCl2concentration of 50 mmol/L.
[1]Geim A K,Novoselov K S.The rise of graphene[J].Nature Materials,2007,6:183-191.
[2]Rao C N R,Biswas K,Subrahmanyam K S,et al.Graphene,the new nanocarbon[J].Journal of Materials Chemistry,2009,19:2457-2469.
[3]Soldano C,Mahmood A,Dujardin E,et al.Production,properties and potential of grapheme[J].Carbon,2010,48(8):2127-2150.
[4]He H,Klinowski J,Forster M,et al.A new structural model for graphite oxide[J].Chemical Physics Letters,1998,287(1-2):53-56.
[5]Pei S,Cheng H M.The reduction of graphene oxide[J].Carbon,2012,50(9):3210-3228.
[6]Moon K,Lee J,Ruoffand S R,et al.Reduced graphene oxide by chemical graphitization[J].Nature Communications,2010,1:73-78.
[7]Stankovich S,Dikin D A,Piner R D,et al.Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide[J].Carbon,2007,45(7):1558-1565.
[8]Fan Z,Wang K,Wei T,et al.An environmentally friendly and efficient route for the reduction of graphene oxide by aluminum powder[J].Carbon,2010,48(5):1686-1689.
[9]FernJandez-Merino M J,Guardia L,Paredes J I,et al.Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions[J].Journal of Chemical Physics,2010,114(14):6426-6432.
[10]Shin H J,Kim K K,Benayad A,et al.Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance[J].Advanced Functional Materials,2009,19(12):1987-1992.
[11]Periasamy M,Thirumalaikumar M.Methods of enhancement of reactivity and selectivity of sodium borohydride for applications in organic synthesis[J].Journal of Organometallic Chemistry,2000,609(1-2):137-151.
[12]Li J,Lin H,Yang Z,et al.A method for the catalytic reduction of graphene oxide at temperatures below 150 ℃[J].Carbon,2011,49(9):3024 -3030.
[13]Zheng Q,Ip W H,Lin X,et al.Transparent conductive films consisting of ultralarge graphene sheets produced by langmuirblodgett assembly[J].ACS Nano,2011,5(7):6039-6051.
[14]Schniepp H C,Li J L,McAllister M J,et al.Functionalized single graphene sheets derived from splitting graphite oxide[J].Journal of Chemical Physics,2006,110(17):8535-8539.
[15]Bagri A,Mattevi C,Alik M,et al.Structural evolution during the reduction of chemically derived graphene oxide[J].Nature Chemistry,2010,2(7):581-587.
[16]Mkhoyan K A,Contryman A W,Silcox J,et al.Atomic and electronic structure of graphene-oxide[J].Nano Letter,2009,9(3):1058-1063.
[17]Gao W,Alemany L B,Ci L,et al.New insights into the structure and reduction of graphite oxide[J].Nature Chemistry,2009,1(5):403-408.
[18]Erickson K,Erni R,Lee Z,et al.Determination of the local chemical structure of graphene oxide and reduced graphene oxide[J].Advanced Materials,2010,22(40),4467-4472.
[19]Liu K,Zhang J,Yang G,et al.Direct electrochemistry and electrocatalysis of hemoglobin based on poly(diallyldimethylammonium chloride)functionalized graphene sheets/room temperature ionic liquid composite film[J].Electrochemistry Communications,2010,12(3):402-405.
[20]Marcano D C,Kosynkin D V,Berlin J M,et al.Improved synthesis of graphene oxide[J].ACS Nano,2010,4(8):4806 -4814.
[21]Pei S F,Zhao J P,Du J H,et al.Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids[J].Carbon,2010,48(15):4466-4474.
[22]Robert C,Wade R C.Catalyzed reduction of organofunctional groups with sodium borohydride[J].Journal of Molecular Catalysis,1983,18(3):273-297.
[23]Suzuki Y,Kaneno D,Tomoda S.Theoretical study on themechanism and diastereoselectivity of NaBH4reduction[J].The Journal of physical chemistry A,2009,113(11):2578-2583.
[24]Li J,Yang Z,Qiu H,et al.Microwave-assisted simultaneous reduction and titanate treatment of graphene oxide[J].Jounal of Material Chemistry A,2013,1(37):11451-11456.
——材料科学与工程

