Optimising repetitive transcranial magnetic stimulation for neural circuit repair following traumatic brain injury
Optimising repetitive transcranial magnetic stimulation for neural circuit repair following traumatic brain injury
While it is well-known that neuronal activity promotes plasticity and connectivity, the success of activity-based neural rehabilitation programs remains extremely limited in human clinical experience because they cannot adequately control neuronal excitability and activity within the injured brain in order to induce repair. However, it is possible to non-invasively modulate brain plasticity using brain stimulation techniques such as repetitive transcranial (rTMS) and transcranial direct current stimulation (tDCS) techniques, which show promise for repairing injured neural circuits (Henrich-Noack et al., 2013; Lefaucher et al., 2014). Yet we are far from having full control of these techniques to repair the brain following neurotrauma and need more fundamental research (Ellaway et al., 2014; Lefaucher et al., 2014). In this perspective we discuss the mechanisms by which rTMS may facilitate neurorehabilitation and propose experimental techniques with which magnetic stimulation may be investigated in order to optimise its treatment potential.
Since the year of its frst application, interest in rTMS has increased exponentially and it is widely applied as a non-invasive method for brain stimulation in experimental and clinical settings (Pell et al., 2011; Di Lazzaro et al., 2013). During magnetic stimulation, an electric coil induces a magnetic feld which passes through the skull to produce an electric feld in the brain (Pell et al., 2011; Deng et al., 2013). As immediate effects of rTMS can be easily visualised in humans,e.g., stimulation to the motor cortex results in muscle twitches, it is generally accepted that eddy currents induced in the cortex lead to action potential fring. As the magnetic feld deteriorates only with distance from the central point of stimulation (Deng et al., 2013), the discrete stimulated brain regions are surrounded by adjacent cortical and sub-cortical tissue that also receive stimulation albeit at lower intensity (Rodger et al., 2012; Makowiecki et al., 2014), but whose contribution to the effects of rTMS remain ill-defned (Ellaway et al., 20214).
However, in the last few years, there has been mounting evidence that rTMS may not induce reliable and reproducible effects. The high variability within and between subjects, and often-contradictory outcomes of rTMS experiments in different laboratories, has made its use somewhat controversial. Thus in recent years, the viability of rTMS as a therapeutic tool has increasingly come under scrutiny (Di Lazzaro et al., 2013; Lefaucher et al., 2014). This lack of reproducibility refects that rTMS has been used clinically for almost two decades without preceding fundamental animal andin vitroresearch to identify the cellular effects beyond inducing action potentials. Given that human experiments allow limited opportunity to investigate underlying cellular and molecular mechanisms, developing the stimulation tools to conduct rTMS experiments in animals andin vitromodels is critical to allow an improved understanding of the primary actions of rTMS on neurons and neural circuits. This fundamental approach is necessary if we are to successfully manipulate brain stimulation in order to harness the excitability and plasticity that promote optimal recovery following injury.
what rTMS parameters may promote neural repair?:
(1)Activity dependent plasticity—Although we know that rTMS induces action potentials in cortical neurons, the factors that determine whether a magnetic pulse will lead to an action potential remain poorly characterised. Key factors are magnetic feld intensity (directly related to distance from stimulation device) and its focus (Deng et al., 2013). Computational modelling studies suggest that the likelihood of action potential fring may also depend on properties of the neuron (Pell et al., 2011), such as intrinsic excitability, morphology and orientation with respect to the magnetic feld, yet these have never been directly investigated in real neurons. Moreover, the cerebral cortex is a complex heterogeneous tissue, thus rTMS may stimulate a combination of excitatory, inhibitory and neuromodulatory neurons that activate internal regulatory circuits (Pell et al., 2011). This, in turn, will confound interpretation of what any given stimulation paradigm is doing to neural activity, how this may be altered when that circuit is damaged (Ellaway et al., 2014) and thus whether such activation may facilitate repair.
Moreover, as magnetic stimulation induces action potentials, rTMS-induced activity will trigger long term potentiation (LTP) and long term depression (LTD)-like synaptic plasticity. Evidence for this comes indirectly from human studies with long lasting post-stimulation changes in cortical excitability (Pell et al., 2011), but also directly fromex vivoneonatal mouse brain slices in which rTMS induces LTP (Vlachos et al., 2012). Not surprisingly, the effects of magnetic stimulation frequency match those of electrical stimulation observed in classical electrophysiological experiments: low frequency inhibits, and high frequency excites neural circuitsviathe induction of LTD or LTP (Pell et al., 2011).
The frequency-specific aspect of long term rTMS outcomes is a major clinical advantage because treatments can be tailored to specifc dysfunctions: low frequency stimulation has been successful in treating disorders associated with cortical hyper-excitability such as stroke and spinal cord injury, while high frequency stimulation is effective in treating depression (Pell et al., 2011). Human studies have hinted at improved cognitive function and faster reaction times but evidence is patchy and poorly reproducible (Pell et al., 2011). However, recent animal studies reveal that rTMS may have signifcant and lasting impact by reopening developmental critical periods and altering metaplasticity (Makowiecki et al., 2014; Mix et al., 2015). This is a more powerful outcome than a simple change in excitability because it has the potential to facilitate long term structural and functional change, effectively rewiring the brain.
(2)Are action potentials necessary for rTMS effects?-Because human rTMS studies most commonly measure muscle responses to magnetic felds applied at intensities close to those required to activate the motor cortex, the effects of rTMS are generally assumed to be due to induction of action potentials in neurons. However, there is a signifcant body of work showing that low intensity magnetic fields, several orders of magnitude lower than the common rTMS protocols, are also effective at inducing neural modulation. In humans, low intensity rTMS (LI-rTMS) modulates cortical excitability, induces analgesia and alleviates depression (Di Lazzaro et al., 2013). In mice, LI-rTMS induces structural changes incongenitally abnormal brain circuits, resulting in improved behaviour (Rodger et al., 2012; Makowiecki et al., 2014).In vitroexperiments have shown that such stimulation (LI-rMS) does not trigger action potentials, but nonetheless increases intracellular calcium within individual neurons, providing the basis for synaptic plasticity and metaplasticity processes to occur (Grehl et al., 2015). This fnding raises some key questions about the mechanisms underlying rTMS:
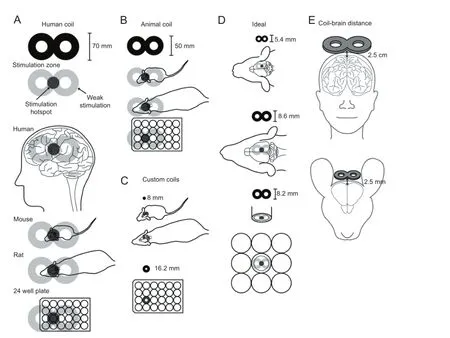
Figure 1 Summary of available and desired coils to deliver magnetic felds to animals andin vitrothat are equivalent to those applied in human repetitive transcranial magnetic stimulation (rTMS).
·Magnetic feld or electric feld?There is evidence of magneto-reception in all vertebrate classes (Wiltschko and Wiltschko, 1995), yet in our focus on induced electric feld and neuronal depolarisation, we forget that the magnetic feld itself may exert a direct effect on cells.
·Neurons are not the only targets.Given that action potential fring may not be a pre-requisite for some aspects of rTMS effectiveness (Grehl et al., 2015), other cells within the brain such as glial cells, vascular endothelial cells, immune cells etc should be considered potential targets of rTMS.
what next? How to optimise rTMS for neural repair:
Although our current knowledge provides tantalising information about the power of magnetic stimulation to modulate brain function, improve dysfunction and potentially repair an injured brain, the appropriate stimulation parameters remain unknown. The current major challenge is how to identify them. It is known in human research that stimulation devices can deliver slightly differing waveforms under the same settings, resulting in diverging corticaleffects over-and-above inter-subject variability (Pell et al., 2011). Unfortunately the effects of magnetic stimulation are based on the combination of several parameters, the impact of which can only be assessed by systematically acquiring data under highly controlled and standardized experimental conditions. This is the strength of animal andin vitromodels, which allow manipulation not only of the external environment, but also of the genetic and pharmacological environment within the brain. However, the stimulation tools we possess at the moment are tailored for the human brain and we need to develop devices to extend our investigations to a wide range of stimulation parameters on a wide range of targets (Figure 1).
(1)Coil design-Although rodent models have revealed key molecular changes following rTMS (Pell et al., 2011; Vlachos et al., 2012; Grehl et al., 2015), most studies use coils that are larger than the rodent brain, such as small commercially available fgure-of-eight or round coils of at least 50 mm outer diameter. Whilst the use of such coils allows for stimulation at the high intensities used in humans (1—2 T), they lack 2 crucial facets: equivalent spatial resolution which confounds correlation of its outcomes to humans; and similar stimulation fields which are determined by the coil-to-target size ratio (Deng et al., 2013). Therefore, animal researchers are increasingly beginning to design small coils tailored to their experimental requirements. However, with decreasing coil size, it is challenging to maintain high stimulation intensities, due to thermal and mechanical stress. Strategies to overcome these problems address the trade-off between stimulus focality and intensity: addition of inbuilt cooling devices in commercial coils, complex coil shapes to improve focus (Deng et al., 2013) or use of low intensity stimulation (Rodger et al., 2012; Makowiecki et al., 2014; Grehl et al., 2015). However, an “ideal”animal coil that accurately reproduces the physical properties of human rTMS in an animal brain has yet to be built (Figure 1). Thus, while a wide range of repetitive magnetic stimulation paradigms can be evaluated experimentally, if we are to understand the type of electric felds induced in human subjects during rTMS, there is an urgent need to develop small coils that deliver focal magnetic stimulation at high intensity in animal models and in culture dishes.
(2)Control of stimulation parameters-In addition to developing appropriate coils, it will be necessary to construct stimulation devices that can deliver the full range of rTMS parameters, controlling frequency, rhythm, number of pulses, intensity, waveform, field orientation, total length of stimulation, etc (Pell et al., 2011). These constraints are necessary to defne the induced electrical feld, which is what acts upon the neuronal tissue. Therefore the current convention of using rTMS intensities of “X % of motor threshold”or “Y % maximal output of the machine” does not permit valid comparison between studies because the induced electric feld remains unknown.
Conclusion:rTMS presents a unique opportunity to modulate brain excitability and plasticity in a precisely controlled manner yet its role for neurorehabilitation remains poorly understood. We propose that rTMS is taken from the bedside back to the bench: the use of appropriate delivery devices in animal andin vitromodels is crucial to provide a practical and theoretical framework to direct how rTMS can be applied following neurotrauma to promote regeneration and rehabilitation of neural circuits.
JR is a NHMRC Senior Research Fellow. We are grateful for CNRS PICS support for our collaboration, scientifc discussions with Stephanie Grehl and Alex Tang that are refected in thiscommentary, and fgure preparation by Marissa Penrose-Menz.
Jennifer Rodger*, Rachel M. Sherrard
Experimental and Regenerative Neuroscience, School of Animal Biology, the University of Western Australia, Perth, Australia (Rodger J)
Sorbonne Universités, UPMC Univ Paris 06 & CNRS, Institut de Biologie Paris Seine-B2A, UMR 8256 Biological Adaptation and Ageing, Paris France (Sherrard RM)
*Correspondence to: Jennifer Rodger, Ph.D., jennifer.rodger@uwa.edu.au.
Accepted:2015-01-16
Deng ZD, Lisanby SH, Peterchev AV (2013) Electric feld depth—focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stim 6:1-13.
Di Lazzaro V, Capone F, Apollonio F, Borea PA, Cadossi R, Fassina L, Grassi C, Liberti M, Paff A, Parazzini M, Varani K, Ravazzani P (2013) A consensus panel review of central nervous system effects of the exposure to low-intensity extremely low-frequency magnetic fields. Brain Stimul 6:469-476.
Ellaway PH, Vásquez N, Craggs M (2014) Induction of central nervous system plasticity by repetitive transcranial magnetic stimulation to promote sensorimotor recovery in incomplete spinal cord injury. Front Integr Neurosci 8:42.
Grehl S, Viola H, Fuller P, Carter KW, Dunlop S, Hool L, Sherrard R, Rodger J (2015) Cellular and molecular changes to cortical neurons following low intensity repetitive magnetic stimulation at different frequencies. Brain Stim 8:114-123
Henrich-Noack P, Voigt N, Prilloff S, Fedorov A, Sabel BA (2013) Transcorneal electrical stimulation alters morphology and survival of retinal ganglion cells after optic nerve damage. Neurosci Lett 543:1-6.
Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipovic SR, Hummel FC, Jaaskelainen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, et al. (2014) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 125:2150-2206
Makowiecki K, Harvey A, Sherrard R, Rodger J (2014) Low-intensity repetitive transcranial magnetic stimulation improves abnormal visual cortical circuit topography and upregulates BDNF in mice. J Neurosci 34:10780-10792.
Mix A, Hoppenrath K, Funke K (2015) Reduction in cortical parvalbumin expression due to intermittent theta-burst stimulation correlates with maturation of the perineuronal nets in young rats. Dev Neurobiol 75:1-11.
Pell GS, Roth Y, Zangen A (2011) Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Infuence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol 93:59-98.
Rodger J, Mo C, Wilks T, Dunlop SA, Sherrard RM (2012) Transcranial pulsed magnetic feld stimulation facilitates reorganization of abnormal neural circuits and corrects behavioral defcits without disrupting normal connectivity. FASEB J 26:1593-1606.
Vlachos A, Muller-Dahlhaus F, Rosskopp J, Lenz M, Ziemann U, Deller T (2012) Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures. J Neurosci 32:17514-17523.
Wiltschko R, Wiltschko W (1995) Magnetic Orientation in Animals. Berlin: Springer Verlag.
10.4103/1673-5374.153676 http∶//www.nrronline.org/
Rodger J, Sherrard RM (2015) Optimising repetitive transcranial magnetic stimulation for neural circuit repair following traumatic brain injury. Neural Regen Res 10(3)∶357-359.
- 中国神经再生研究(英文版)的其它文章
- RAFting the rapids of axon regeneration signaling
- TAM receptors: two pathways to regulate adult neurogenesis
- Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration?
- Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light
- Novel advancements in threedimensional neural tissue engineering and regenerative medicine
- Functional regeneration of the brain: white matter matters

