Chinese herbal formula Tongluo Jiunao injection protects against cerebral ischemia by activating neurotrophin 3/tropomyosin-related kinase C pathway
Peiman Alesheikh, Arezou Mashouf, Hui-ling Tang, Wei Zhang, Bo Di, Yang-yang Yan, Peng-tao Li, Yan-shu Pan,
1 Research Center of Natural Product Health, North Khorasan University of Medical Science, Bojnourd, North Khorasan, Iran
2 Department of Pathology, Preclinical Medical School, Beijing University of Chinese Medicine, Beijing, China
3 Dongzhimen Hospital Affliated to Beijing University of Chinese Medicine, Beijing, China
Chinese herbal formula Tongluo Jiunao injection protects against cerebral ischemia by activating neurotrophin 3/tropomyosin-related kinase C pathway
Peiman Alesheikh1, Arezou Mashouf1, Hui-ling Tang2, Wei Zhang2, Bo Di2, Yang-yang Yan2, Peng-tao Li3, Yan-shu Pan2,*
1 Research Center of Natural Product Health, North Khorasan University of Medical Science, Bojnourd, North Khorasan, Iran
2 Department of Pathology, Preclinical Medical School, Beijing University of Chinese Medicine, Beijing, China
3 Dongzhimen Hospital Affliated to Beijing University of Chinese Medicine, Beijing, China
The Chinese herbal formulaTongluo Jiunao, containing the active componentsPanax notoginsengandGardenia jasminoides, has recently been patented and is in use clinically. It is known to be neuroprotective in cerebral ischemia, but the underlying pathway remains poorly understood. In the present study, we established a rat model of cerebral ischemia by occlusion of the middle cerebral artery, and administeredTongluo Jiunao, a positive control (Xuesai Tong, containingPanax notoginseng) or saline intraperitoneally to investigate the pathway involved in the action ofTongluo Jiunaoinjection. 2,3,5-Triphenyltetrazolium chloride (TTC) staining showed that the cerebral infarct area was signifcantly smaller in model rats that receivedTongluo Jiunaothan in those that received saline. Enzyme-linked immunosorbent assay revealed significantly greater expression of neurotrophin 3 and growth-associated protein 43 in ischemic cerebral tissue, and serum levels of neurotrophin 3, in theTongluo Jiunaogroup than in the saline group. The reverse transcription polymerase chain reaction and immunohistochemical staining showed that after treatment withTongluo JiunaoorXuesai Tong, tropomyosin-related kinase C gene expression and immunoreactivity were signifcantly elevated compared with saline, with the greatest expression observed afterTongluo Jiunaotreatment. These fndings suggest thatTongluo Jiunaoinjection exerts a neuroprotective effect in rats with cerebral ischemia by activating the neurotrophin 3/ tropomyosin-related kinase C pathway.
neural regeneration; cerebral ischemia; Chinese herbal formula; Tneurotrophic factor; ongluo Jiunao injection; nerve growth factor receptor; Xuesai Tong; neuroprotection; NSFC grant; neuroprotection; neural regeneration
Funding:This work was supported by grants from the National Basic Research Program (973 Program), No. 2012CB518602, the National Natural Science Foundation of China, No. 30830120, and a grant from Beijing University of Chinese Medicine in China.
Alesheikh P, Mashoufi A, Tang HL, Zhang W, Di B, Yan YY, Li PT, Pan YS (2015) Chinese herbal formula Tongluo Jiunao injection protects against cerebral ischemia by activating neurotrophin 3/ tropomyosin-related kinase C pathway. Neural Regen Res 10(3)∶445-450.
Introduction
After central nervous system injury, activation of certain intracellular pathways can reduce infarct size and damage to the brain. The neurotrophin-tropomyosin-related kinase (Trk) signaling pathway is important in this process (Bachis et al., 2002; Barnabe-Heider and Miller, 2003). Neurotrophin 3 (NT3), one of the most recently discovered members of the neurotrophin family, acts on several intracellular pathwaysviathe TrkC receptor (Kahn et al., 1999; Yamauchi et al., 2003). The NT3/TrkC pathway is fundamental to neuroregeneration as it stimulates the growth and activity of glial cells (Postigo et al., 2002; Hess et al., 2007). Furthermore, NT3 is not only a mediator for TrkC, but also upregulates the expression of TrkA and TrkB on neuronal membranes (Lewis et al., 2006). In the present study, we usedXuesai Tong (XST) as a positive control drug. XST promotes blood circulation, making it useful for the treatment of unstable angina pectoris (Liu et al., 2008). In addition, anin vivostudy showed that XST decreases thrombosis (Chen et al., 2007). This is achieved by the inhibition of platelet activationviaa reduction in arachidonic acid release (Wang et al., 2004). A clinical trial revealed that continuous infusion of XST can improve nerve conductivity in patients with diabetic peripheral neuropathy (Han, 2003). In stroke, XST has several therapeutic effects; it promotes blood circulation during ischemic stroke and reduces tissue infammation following ischemia (Ai et al., 2004; He and Xu, 2006). XST therapy improves learning and memory, and increases neurotrophin expression in a rat model of cerebral ischemia/reperfusion injury (He et al., 2004, 2005; Chuang et al., 2008; Son et al., 2009; Li et al., 2009).
The Chinese herbal formulaTongluo Jiunao(TLJN) has recently been patented (Li et al., 2007; Qing et al., 2007; Si et al., 2009; Wang et al., 2009). TLJN protects endothelial cells in cerebral blood vessels against ischemic attacks (Li et al.,2007; Qing et al., 2007), suggesting that it may be useful in protecting the blood-brain barrier. TLJN injection promotes the proliferation and differentiation of neural stem cells in the rat cortex following devascularization, by upregulating endogenous nerve growth factors (Si et al., 2009). TLJN also exhibits protective effects following ischemic injuryin vivo(Wang et al., 2009). The compound is a combination of two active components,Panax notoginsengandGardenia jasminoides. In Chinese medicine, stroke is represented by a toxic heat in the head and brain collaterals; thus, any herb that can clear this toxic heat and promote blood fow is regarded as an effective therapy.Panax notoginsengandGardenia jasminoidesare known herbs with this function.
The purpose of the present study was to investigate the effects of TLJN injection on the NT3/TrkC pathway in a rat model of cerebral ischemia induced by middle cerebral artery occlusion (MCAO).
Materials and Methods
Animals
A total of 312 male, specifc pathogen-free Sprague-Dawley rats, 4 months old and weighing 283 ± 36 g, were purchased from the Experimental Animal Center of Beijing Vital River, China (License No. SCXK (Jing) 2007-0001). The study was approved by the Experimental Animal Ethics Committee of Dongzhimen Hospital Affliated to Beijing University of Chinese Medicine in China. The rats were randomly and equally divided into four groups: normal (naïve control), model (MCAO only), TLJN (MCAO + TLJN), and XST (MCAO + XST). Four time points were used for observation: 0.5 days (n= 15), 1 day (n= 21), 3 days (n= 21), and 7 days (n= 21) following the onset of ischemia.
Establishment of rat MCAO models
Focal cerebral ischemia was induced using the reversible flament occlusion model as previously described (Longa et al., 1989). In brief, animals were anesthetized with 10% chloral hydrate (0.4 g/kg intraperitoneally (i.p.)). The left external carotid artery was carefully dissected through a median incision in the neck. A piece of nylon suture, with a diameter of 0.25 mm and one end rounded by heat into a ball with a diameter of < 0.3 mm, was introduced into the transected lumen of the external carotid artery and gently advanced into the internal carotid artery to block the origin of the left middle cerebral artery. The retracted soft tissues were then replaced, the incision sutured, and the rats were returned to their home cages. Body temperature was maintained at 37°C with a heat lamp and heating pad during and after surgery, until the rats regained consciousness.
Neurological evaluation was performed before and 2 hours after surgery according to a previously described method (Bederson et al., 1986). Neurological scores prior to surgery were 0 for all rats. Following surgery, rats with a neurological score of ≥ 2 were selected for the study, and rats with a score of < 2 were considered to be unsuccessful MCAO candidates.
drug treatment
Concentrated TLJN solution (approval No. 2004L01620) was provided by Tianjin Hongri Pharmaceutical Co. (Tianjin, China) and contained 4.95 mg/mL geniposide, 1.02 mg/mL ginsenoside Rg1, and 1.73 mg/mL geniposidic acid. TLJN was administered daily at a dose of 3 mL/kg i.p., equivalent to the clinical dose (Si et al., 2009). Concentrated XST solution (approval No. Z53020662) was provided by Kunming Pharmaceutical Corp. (Kunming, China) and contained 25 mg/mL ginsenoside Rg1. XST was administered daily at a dose of 1.4 mL/kg (i.p.), equivalent to the clinical dose. In the model group, an equivalent volume of saline solution was injected instead of drugs. The frst injection of each solution was given 2 hours after MCAO.
Infarct volume percentage
Rats were anesthetized and decapitated 1, 3, and 7 days after surgery (n= 6 in each group per time point). Brains were rapidly removed and cut into coronal sections (2-mm thick). Slices were stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO, USA) in 0.1 M PBS (pH 7.4) for 30 minutes at room temperature, and fxed in 10% buffered formalin overnight. The sections were photographed with an HPIAS-1000 image acquirement system (Beijing Kong Hai, China). Infarct volumes were quantifed using the Cavalieri principle (Gundersen, 1986; Yang, 1990). Quantification was carried out directly by summing the infarct volumes of all sections, or indirectly, correcting for brain edema, by subtracting the volume of undamaged ipsilateral hemisphere from contralateral hemisphere. The infarct volume percentage was calculated by dividing the infarct volume by the total tissue volume and multiplying by 100%. All quantifcation was carried out by an investigator blind to experimental grouping.
Brain tissue and serum enzyme-linked immunosorbent assay (ELISA)
At 0.5, 1, 3, and 7 days after MCAO, rats (n= 5—6 per group per time point) were deeply anesthetized with 10% chloral hydrate, and blood samples were collected from the abdominal aorta. The brains were removed and immediately dissected on ice. The cerebral hemisphere ipsilateral to the MCAO was dissected out and stored at -80°C. Subsequently, brain samples were homogenized in PBS buffer, the homogenate was centrifuged for 45 minutes at 9,727 ×gat 4°C, and the supernatant was collected. Concentrations of brain lysate and serum NT3, as well as brain levels of growth-associated protein 43 (GAP-43), were quantifed using a commercially available NT3 and GAP-43 ELISA kit (Ever Systems Biology Laboratory, Sacramento, CA, USA). Protein concentrations were determined using the bicinchoninic acid method (Ye et al., 2007).
RT-PCR
RT-PCR was used to measure TrkC gene expression in the cerebral tissue ipsilateral to the MCAO (n= 3 rats per group per time point). TrkC mRNA primer sequences were designed using Primer Premier 5.0 software (Premier, Vancouver, Canada) as follows: rat TrkC019248 251 bp: upstream, 5′-CAA GCC CAC CCA CTA CAA C-3′; downstream, 5′-AGA GGA CCA CCA GAA GGA C-3′; rat β-actin NM_031144.2 150 bp: upstream, 5′-CCC ATC TAT GAG GGT TAC GC-3′;downstream, 5′-TTT AAT GTC ACG CAC GAT TTC-3′. Band density was determined using Gel-Pro 32-bit image analysis software (Media Cybernetics, Silver Spring, MD, USA), and TrkC densities were divided by corresponding β-actin values, resulting in relative expression levels of TrkC mRNA.
Immunohistochemical staining
Rats in each group were anesthetized and decapitated at 0.5, 1, 3, and 7 days after MCAO (n= 5 rats per time point). The brains were immersed in silicone gel and 8-µm thick coronal sections were cut using a freezing microtome (Leica Microsystems, Wetzlar, Germany) for immunohistochemistry. Sections were dehydrated through an increasing ethanol gradient and stored at -20°C until use. The sections were rinsed in 20 mM PBS and incubated in 3% H2O2/10% ethanol for 20 minutes at room temperature to block endogenous peroxidase. The sections were then boiled in 0.01 M citrate buffer for 10 minutes for antigen retrieval, and incubated overnight in rabbit anti-TrkC polyclonal antibody (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by biotinylated goat anti-rabbit IgG (Zhongshan Golden Bridge Biotechnology, Beijing, China) at 1:1,000 dilution in 2% bovine serum albumin, for 1 hour. Finally, diaminobenzidine was used for coloration. PBS was used instead of the primary antibody as a negative control. TrkC-stained sections were digitized using a mounted camera on an Eclipse 50i microscope (Nikon, Tokyo, Japan) and an image analysis system (Nikon). To quantify TrkC expression, five high-magnification (× 100) visual fields were selected in the peri-infarct zone, and the area of positive expression was assessed compared with the total area of each feld. Immunohistochemical analysis was performed blind to experimental grouping.
Statistical analysis
Data are expressed as the mean ± SD. One-way analysis of variance, followed bypost-hocanalysis using the Student-Newman-Keuls multiple comparison test, was performed using SPSS 11.0 software (SPSS, Chicago, IL, USA).P< 0.05 was regarded as statistically signifcant.
Results
XST and TLJN reduced ischemic infarct size in rats with MCAO
TTC staining showed that the infarct volumes in the TLJN and XST groups were smaller than in the model group (Figure 1). One day after MCAO, total infarct volume in the TLJN group was smaller than that in the model group (P<0.05). On days 3 (P< 0.01) and 7 (P< 0.05), infarct volume was significantly smaller in both treatment groups than in the model group, with no signifcant difference between the XST and TLJN groups.
TLJN treatment increased GAP-43 in the ipsilateral cerebral hemisphere in rats with MCAO
A markedly lower level of GAP-43 was observed at all time points in the model group than in the normal (naïve control) group (P< 0.01). One day after MCAO, the level of GAP-43 in the TLJN group was signifcantly higher than that in the model and XST groups (P< 0.05). At 0.5 (P< 0.05), 3 (P< 0.01) and 7 (P< 0.01) days after surgery, GAP-43 levels in the XST group were signifcantly greater than those in the model group (Figure 2).
TLJN treatment increased NT3 in the serum and ipsilateral cerebral hemisphere of rats with MCAO
ELISA revealed that serum NT3 peaked 1 day after surgery in MCAO model rats, but no significant differences were observed between the model and normal groups throughout the experiment (Figure 3A). In the brain, NT3 level was signifcantly lower in the model group than in the normal group 7 days after surgery (P< 0.05) (Figure 3B). Rats that had received TLJN showed signifcantly higher serum NT3 levels than rats in the model group 7 days after MCAO (P< 0.05) (Figure 3A). Rats that received XST had significantly lower brain NT3 levels than those in the model group at 0.5 days (P< 0.01) and 1 day (P< 0.05). At 3 days after MCAO, brain NT3 level in the TLJN group was significantly greater than that in the model and XST groups (P< 0.01) (Figure 3B).
TLJN treatment increased TrkC gene expression in the ipsilateral cerebral hemisphere of rats with MCAO
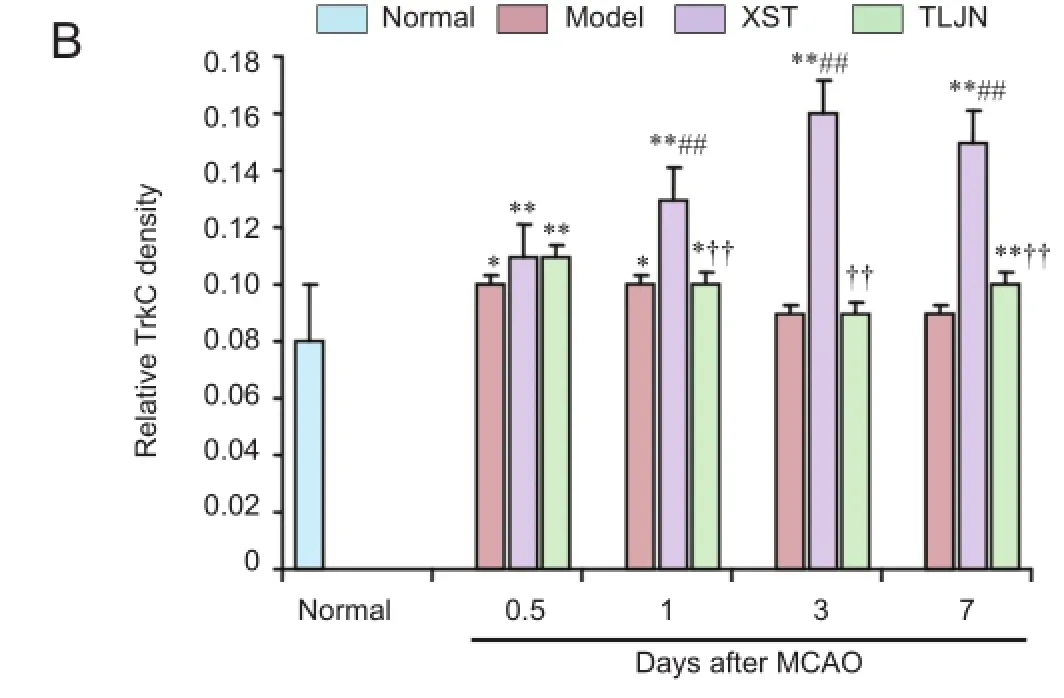
RT-PCR showed that at 0.5 (P< 0.01), 1 (P< 0.05), and 7 (P< 0.01) days after MCAO, TrkC gene expression was signifcantly lower in the model group than in the normal group (Figure 4). In the XST group, TrkC gene expression peaked 0.5 days after MCAO and was signifcantly greater than that in the normal and model groups (P< 0.01) (Figure 4). At 1 day after MCAO, TrkC gene expression was significantly greater in the TLJN group than in the model and XST groups (P< 0.01 andP< 0.05 respectively).
TLJN treatment did not alter TrkC immunoreactivity in the ipsilateral cerebral hemisphere of rats with MCAO
Compared with the normal group, rats with MCAO showed greater TrkC immunoreactivity at 0.5 days and 1 day after surgery (P< 0.05). Brain TrkC immunoreactivity was signifcantly higher in the XST group than in the model group from 1 to 7 days after MCAO (P< 0.01). TrkC immunoreactivity was not signifcantly different between the TLJN and model groups (Figure 5).
discussion
Nerve growth factor (NGF)/TrkA, brain-derived neurotrophic factor (BDNF)/TrkB, and NT-3/TrkC pathways are known to be important in healing and regeneration after central nervous system injuries. In the present study, we evaluated the effects of TLJN injection on the NT3/TrkC pathway. Our results suggest that TLJN promotes recovery after ischemic injury by increasing NT3 levels, reducing the necrotic area, and upregulating brain GAP-43.
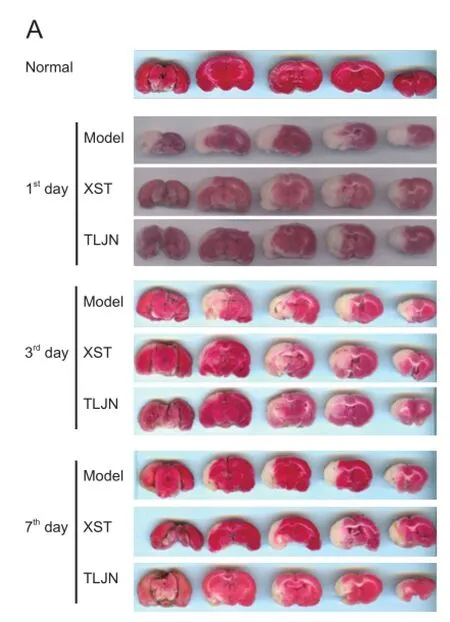
Figure 1Tongluo Jiunao(TLJN) injection treatment reduced the ischemic infarct size detected by 2,3,5-triphenyltetrazolium chloride staining.
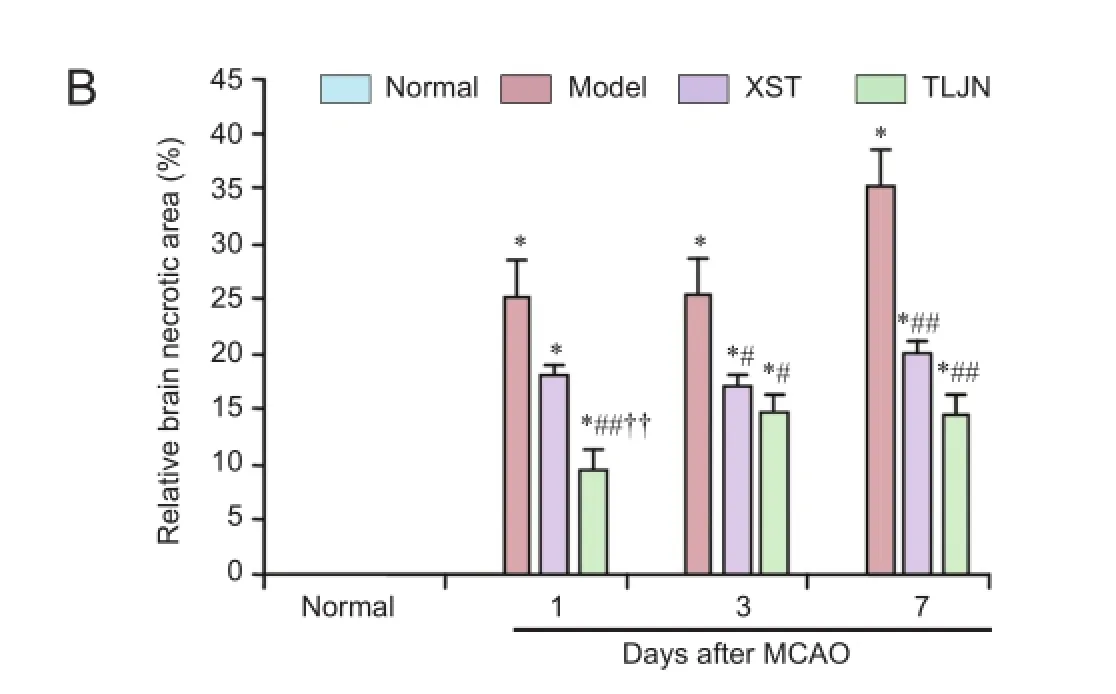
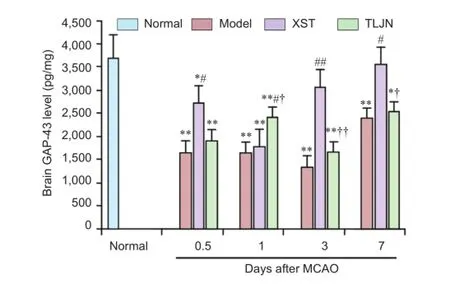
Figure 2 Growth-associated protein 43 (GAP-43) levels, detected by enzyme-linked immunosorbent assay, in rats with middle cerebral artery occlusion (MCAO) followed by daily injections ofTongluo Jiunao(TLJN),Xuesai Tong(XST) or saline (Model).
Hypoxia can increase NT3 and TrkC gene expression in central nervous system pericytes, which increases the tolerance of neurons to hypoxia (Arimura et al., 2012). There is evidence that injection of exogenous NT3 into the brain after MCAO in rats can alleviate brain injury by decreasing TrkC expression (Wyatt et al., 1999; Zhang et al., 1999). In the present study, serum NT3 level did not change after MCAO. In addition, brain NT3 decreased on the 7thday after MCAO. Brain NT3 levels in the XST group remained low throughout the period of observation, indicating that the therapeutic effects attributed to XST are not related to an effect on NT3. However, in the TLJN group, NT3 levels were particularly high during the later stages of the observation period.

Figure 4 Relative TrkC gene expression levels, detected by reverse transcription polymerase chain reaction, in rats with cerebral ischemia induced by middle cerebral artery occlusion (MCAO) followed by daily injections ofTongluo Jiunao(TLJN group),Xuesai Tong(XST group) or saline (model group).
TTC staining is a nonspecifc method used to evaluate tissue necrosis (Hua et al., 2008; Musiolik et al., 2010), whereas GAP-43 is a specific neuronal marker and plays a role in sprouting and branching (Yuan et al., 2009; Korshunova and Mosevitsky, 2010). In the present study, infarct volume was smaller in model rats that received TLJN than in those that received saline, indicating that TLJN protects brain tissue against necrosis after an ischemic attack. In addition, GAP-43 expression was elevated after TLJN, suggesting that it may improve brain function following ischemic injury. However, expression of NT3, but not TrkC, was elevated after TLJN injections. This suggests that the TLJN strengthened the NT3/ TrkC pathway by increasing NT3 expression, thereby resulting in better recovery from the ischemic attack.
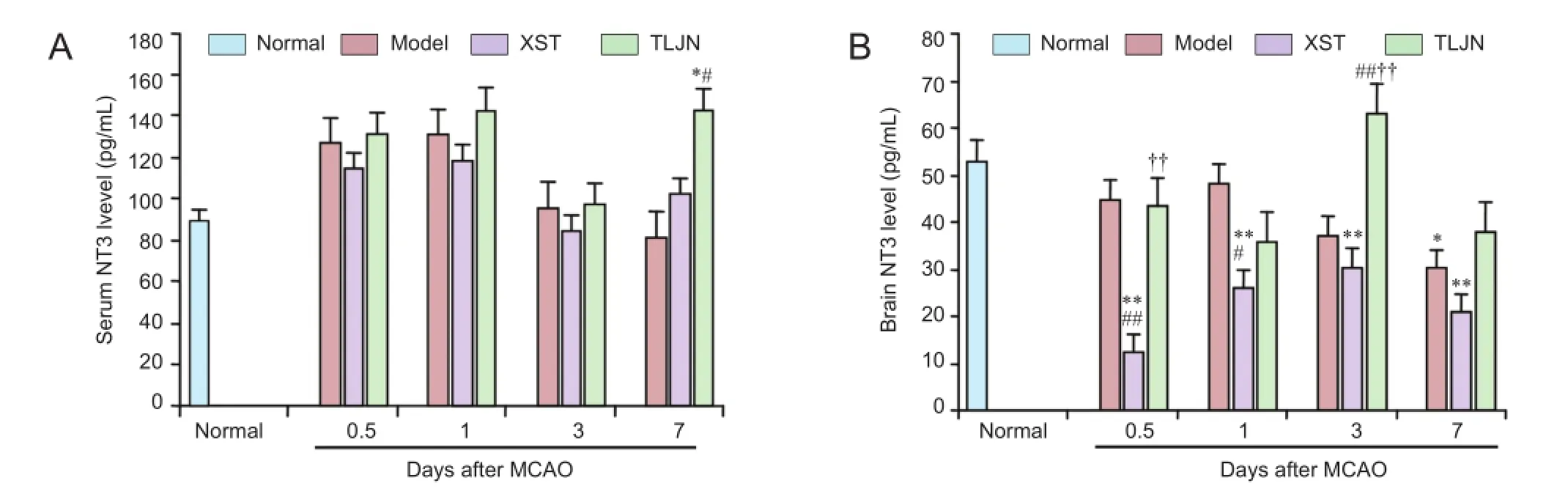
Figure 3 Serum (A) and brain (B) neurotrophin 3 (NT3) levels, detected by enzyme-linked immunosorbent assay, in rats with cerebral ischemia induced by middle cerebral artery occlusion (MCAO) followed by daily injections ofTongluo Jiunao(TLJN group),Xuesai Tong(XST group), or saline (model group).

Figure 5 Tropomyosin-related kinase C (TrkC) distribution and immunoreactivity, determined by immunohistochemical staining, in the ipsilateral cerebral cortex in rats with cerebral ischemia induced by middle cerebral artery occlusion (MCAO) followed by daily injections ofTongluo Jiunao(TLJN group),Xuesai Tong(XST group) or saline (model group).
NT3 release occurs in both the central and peripheral ner-vous systems, so we measured levels of NT3 in the brain and serum. In the TLJN group, brain NT3 levels peaked on day 3 in the brain but day 7 in serum, indicating that the systemic increase is secondary to central NT3 changes. We conclude, therefore, that TLJN increases brain NT3, and since XST can markedly lower NT3 in the brain, its mechanism of action is independent of the NT3/TrkC pathway.
Our findings confirm that TLJN and XST cause upregulation of TrkC gene expression. However, overexpression of TrkC after XST, particularly in the later stages of ischemic injury, may be counteracted by certain regulatory mechanisms, resulting in more extensive necrosis; this is avoided with TLJN. Further studies are needed to determine the effects of TLJN following brain ischemia in humans.
Author contributions:PA, HLT, YSP, BD, AM, WZ, YYY, and PTL were responsible for conception and design of the study. PA, HLT, YSP, BD, WZ, YYY, and PTL participated in the definition of intellectual content and the experimental studies. PA, HLT, YSP, BD, AM, WZ, and PTL were in charge of data acquisition. HLT, YSP, AM, WZ, YYY, and PTL were responsible for literature retrieval, data analysis, statistical analysis and manuscript review. PA, YSP, AM, and WZ were in charge of manuscript preparation and editing and were the guarantors of the manuscript. All authors approved the final version of the manuscript.
Conficts of interest:None declared.
Ai WB, Chen YH, Yang QJ (2004) Clinical observation on effect of xuesaitong injection as auxiliary treatment of severe craniocerebral injury. Zhongguo Zhong Xi Yi Jie He Za Zhi 24:213-215.
Arimura K, Ago T, Kamouchi M, Nakamura K, Ishitsuka K, Kuroda J, Sugimori H, Ooboshi H, Sasaki T, Kitazono T (2012) PDGF receptor β signaling in pericytes following ischemic brain injury. Curr Neurovasc Res 9:1-9.
Bachis A, Rabin SJ, Del Fiacco M (2002) Gangliosides prevent excitotoxicity through activation of TrkB receptor. Neurotox Res 4:225-234.
Barnabe-Heider F, Miller FD (2003) Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. J Neurosci 23:5149-5160.
Bederson JB, Pitts LH, Tsuji M (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17:472-476.
Chen YH, Zhang SF, Sun JN (2007) Effect of Xuesaitong drop pills on experimental thrombosis and thrombolysis in rats. Zhongguo Zhongyao Zazhi 32:253-256.
Chuang CM, Hsieh CL, Lin HY, Lin JG (2008) Panax notoginseng Burk attenuates impairment of learning and memory functions and increases ED1, BDNF and beta-secretase immunoreactive cells in chronic stage ischemia-reperfusion injured rats. Am J Chin Med 36:685-693.
Gundersen HJ (1986) Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc 143:3-45.
Han HG (2003) The infuence of XueSai Tong Injection on the speed of neuro-conductivity in patients with diabetic peripheral neuropathy. Zhongguo Zhongyi Xinxi Zazhi 10:20-21.
He K, Liu Y, Yang Y (2005) A dammarane glycoside derived from ginsenoside Rb3. Chem Pharm Bull 53:177-179.
He W, Xu XJ (2006) Attenuation of brain infammatory response after focal cerebral ischemia/reperfusion with Xue saitong injection in rats. Chin J Integr Med 12:203-206.
He W, Zhu Z, Liu J (2004) Study on therapeutic window of opportunity for Panax notoginseng saponins following focal cerebral ischemia/ reperfusion injury in rats. Zhong Yao Cai 27:25-27.
Hess DM, Scott MO, Potluri S, Pitts EV, Cisterni C, Balice-Gordon RJ (2007) Localization of TrkC to Schwann cells and effects of neurotrophin-3 signaling at neuromuscular synapses. J Comp Neurol 501:465-482.
Hua Q, Zhu X, Li P (2008) Refned Qing Kai Ling, traditional Chinese medicinal preparation, reduces ischemic stroke-induced infarct size and neurological defcits and increases expression of endothelial nitric oxide synthase. Biol Pharm Bull 31:633-637.
Kahn MA, Kumar S, Liebl D, Chang R, Parada LF, De Vellis J (1999) Mice lacking NT-3, and its receptor TrkC, exhibit profound defciencies in CNS glial cells. Glia 26:153-165.
Korshunova I, Mosevitsky M (2010) Role of the growth-associated protein GAP-43 in NCAM-mediated neurite outgrowth. Adv Exp Med Biol 663:169-182.
Lewis MA, Hunihan L, Franco D, Robertson B, Palmer J, Laurent DR, Balasubramanian BN, Li Y, Westphal RS (2006) Identification and characterization of compounds that potentiate NT-3-mediated Trk receptor activity. Mol Pharmacol 69:1396-1404.
Li H, Deng CQ, Chen BY, Zhang SP, Liang Y, Luo XG (2009) Total saponins of Panax notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J Ethnopharmacol 121:412-418.
Li WH, Li PT, Hua Q (2007) Influence of different endothelial cells conditioned medium on the function of mitochondria of cortical neurons and the protective effect of Tongluo Jiunao Injection. Zhongguo Zhong Xi Yi Jie He Za Zhi 27:131-134.
Liu X, Li J, Yang G, Wang J (2008) Study on effect of promoting blood circulation drugs components in treating unstable angina in patients with blood stasis infammatory levels. Zhongguo Zhong Yao Za Zhi 33:2950-2953.
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
Musiolik J, van Caster P, Skyschally A, Boengler K, Gres P, Schulz R, Heusch G (2010) Reduction of infarct size by gentle reperfusion without activation of reperfusion injury salvage kinases in pigs. Cardiovasc Res 85:110-117.
Postigo A, Calella AM, Fritzsch B, Knipper M, Katz D, Eilers A, Schimmang T, Lewin GR, Klein R, Minichiello L (2002) Distinct requirements for TrkB and TrkC signaling in target innervation by sensory neurons. Genes Dev 16:633-645.
Qing XM, Li PT, Hu JH, Li WH, Hou JC, Du H, Wang B, Suo L (2007) Conditioned mediums of different rat cerebral microvascular endothelial cells against damage of ischemia and ischemia/reperfusion neurons. Zhong Xi Yi Jie He Xue Bao 5:183-188.
Si YC, Li XG, Li HY (2009) Effects of cortical devascularization on vimentin, GFAP positive cells located on SVZ of adult rat and the role of Tongluojiunao Injection. Zhonghua Zhongyiyao Zazhi 24:709-712.
Son HY, Han HS, Jung HW, Park YK (2009) Panax notoginseng attenuates the infarct volume in rat Ischemic brain and the infammatory response of microglia. J Pharmacol Sci 109:368-379.
Wang J, Xu J, Zhong JB (2004) Effect of Radix notoginseng saponins on platelet activating molecule expression and aggregation in patients with blood hyperviscosity syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi 24:312-316.
Wang J, Li PT, Hou JC (2009) Effect of Tongluojiunao injection on MIP-1 secretion of cerebral micro vascular endothelial cells in rats with oxygen glucose deprivation. Tianjin Zhongyiyao 26:12-15.
Wyatt S, Middleton G, Doxakis E, Davies AM (1999) Selective regulation of trkC expression by NT3 in the developing peripheral nervous system. J Neurosci 19:6559-6570.
Yamauchi J, Chan JR, Shooter EM (2003) Neurotrophin 3 activation of TrkC induces Schwann cell migration through the c-Jun N-terminal kinase pathway. Proc Natl Acad Sci U S A 100:14421-14426.
Yang ZW (1990) Estimation of volume surface arer length thickness and curvature by stereology. Chuanbei Yixueyuan Xuebao 5:43-52.
Ye XM, Tian SJ, Li HY (2007) Determination of surfactant protein in porcine pulmonary surfactant and its lyophilized powder by BCA method. Zhongguo Yaopin Biaozhun 8:28-31.
Yuan Q, Hu B, Su H, So KF, Lin Z, Wu W (2009) GAP-43 expression correlates with spinal motoneuron regeneration following root avulsion. J Brachial Plex Peripher Nerve Inj 4:18.
Zhang WR, Hayashi T, Wang JM, Sasaki C, Sakai K, Warita H, Shiro Y, Suenaga H, Ohmae H, Tsuji S, Itoh T, Nishimura O, Nagasaki H, Abe K (1999) Reduction of tyrosine kinase B and tyrosine kinase C inductions by treatment with neurotrophin-3 after transient middle cerebral artery occlusion in rat. Neurosci Lett 276:161-164.
Copyedited by Slone Murphy J, Raye W, Li CH, Song LP, Zhao M
*
.
10.4103/1673-5374.153694
http://www.nrronline.org/
Accepted: 2015-01-06
- 中国神经再生研究(英文版)的其它文章
- RAFting the rapids of axon regeneration signaling
- TAM receptors: two pathways to regulate adult neurogenesis
- Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration?
- Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light
- Novel advancements in threedimensional neural tissue engineering and regenerative medicine
- Functional regeneration of the brain: white matter matters

