Synthesis of highly reactive sorbent from industrial wastes and its CO2 capture capacity
Sun Rongyue Li Yingjie
(1School of Energy and Power Engineering, Shandong University, Jinan 250061, China)(2School of Energy and Power Engineering, Nanjing Institute of Technology, Nanjing 211167, China)
Synthesis of highly reactive sorbent from industrial wastes and its CO2capture capacity
Sun Rongyue1,2Li Yingjie1
(1School of Energy and Power Engineering, Shandong University, Jinan 250061, China)(2School of Energy and Power Engineering, Nanjing Institute of Technology, Nanjing 211167, China)
A kind of industrial solid waste, i.e., carbide slag, was used as CaO precursor to synthesize CO2sorbent. The highly reactive synthetic sorbent was prepared from carbide slag, aluminum nitrate hydrate and glycerol water solution by the combustion synthesis method. The results show that the synthetic sorbent exhibits a much higher CO2capture capacity compared with carbide slag. The CO2capture capacity and the carbonation conversion of the synthetic sorbent are 0.38 g/g and 0.70 after 50 cycles, which are 1.8 and 2.1 times those of carbide slag. The average carbonation conversion and the CO2capture efficiency of the synthetic sorbent are higher than those of carbide slag with the same sorbent flow ratios. The required sorbent flow ratios are lower for synthetic sorbent to achieve the same CO2capture efficiency compared with carbide slag. With the same sorbent flow ratio and CO2capture efficiency, the energy requirement in calciner for the synthetic sorbent is less than that for carbide slag.
carbide slag; synthetic CO2sorbent; CO2capture
A great amount of industrial wastes are generated in industrial production every year, most of which are difficult to recover and are usually landfilled. If handled improperly, industrial wastes will pose a threat to human health, ground water resources and the atmospheric environment. It is of interest to convert those harmful solid wastes to harmless and useful materials[1-3]. The research on the reuse of industrial wastes such as a substitute for limestone has been studied in different fields[1, 4-5]. Some industrial solid wastes have been used to capture CO2[4-5]. Calcium looping, i.e., repetitive carbonation/calcination cycles of CaO, is a feasible CO2capture technology for fossil fuel-fired power plants[6]and H2co-production[7]. Utilization of industrial wastes such as CO2sorbent in calcium looping technology is a promising possibility how to reuse the industrial wastes in the view of circular economy and sustainable development. Some industrial wastes such as carbide slag show favorable CO2capture capacity[1]. However, the carbonation conversion of the industrial wastes decreases with the number of cycles, just like limestone. In order to efficiently capture CO2in fossil fuel combustion and H2production, it is necessary to improve the CO2capture capacity of industrial wastes.
Many methods to raise CO2capture capacity of calcium-based sorbent in the calcium looping cycles have been summarized[8]. The CO2capture performances of the synthetic calcium-based sorbents prepared by dispersing CaO precursors across calcium aluminates (Ca12Al14O33[9], Ca3Al2O6[10], Ca9Al6O18[11], Ca3Al10O18[12]) as support materials have been investigated. The supporters can effectively stabilize the pore structure and increase the sintering resistance of the synthetic sorbents. The synthetic CO2sorbents containing various calcium aluminates are determined by the synthesis routes (the wet mixing method, precipitation method, the sol-gel-combustion-synthesis (SGCS) method and the template method), raw materials such as CaO precursor, Al2O3precursor and dispersant[9, 13]. However, the raw materials used in the existing process such as nano CaCO3, calcium acetate, and calcium-naphthenate, Ca(C6H5O7)2are costly and some solvents and dispersants such as 2-propanol, citric acid and xylene are not cheap. In this paper, we use a type of industrial solid waste, i.e., carbide slag, as CaO precursor and glycerol water as solvent to synthesize a new type of CO2sorbent. The synthetic CO2sorbent is prepared from carbide slag, aluminum nitrate hydrate and glycerol water solution by the combustion synthetic method. Carbide slag and aluminum nitrate hydrate can be dissolved in the glycerol water solution. The glycerol is highly combustible. The synthetic sorbent is synthesized by Ca2+and Al3+in the glycerol water solution by the combustion of the glycerol, so the combustion process of the solution is also the synthesis process of the synthetic sorbent. The glycerol as a byproduct is obtained from the preparation of the biodiesel fuel[14]and carbide slag is also almost free. Thus, the prepared synthetic sorbent is low-cost. The CO2capture performance of the synthetic sorbent is investigated. The average carbonation conversion, CO2capture efficiency and energy requirement in calciner for the post-combustion CO2capture system using a synthetic sorbent are calculated.
1 Experimental
1.1 Sorbent preparation
The carbide slag (sieved to size<0.125 mm) mainly composed of Ca(OH)2was sampled from a chlor-alkali plant located in Shandong Province, China. The chemical components of the carbide slag were analyzed by X-ray fluorescence (XRF) as shown in Tab.1. The aluminum nitrate hydrate (analytical grade Al(NO3)3·9H2O) and the glycerol (analytical grade C3H8O3) were also used as the materials in the preparation of the synthetic sorbent. First, 50 mL glycerol was dissolved in 50 mL deionized water which was stirred at 25 ℃ for 20 min, and then, the solution was heated from 25 to 80 ℃. Secondly, 10 g carbide slag and some Al(NO3)3·9H2O were added into the solution and stirred at 80 ℃. The mass ratios of CaO derived from the carbide slag to Al2O3derived from Al(NO3)3·9H2O were 90∶10 (defined as the best ratio by previous works[15]). After the carbide slag and Al(NO3)3·9H2O were completely dissolved in the glycerol water solution, it was sent to a muffle furnace for the combustion synthesis process. Subsequently, the solution was combusted in the muffle furnace (800 ℃) under air for 1 h. Synthetic sorbents were obtained after the combustion and sieved to size<0.125 mm. The XRD result shows that only CaO and Ca3Al2O6are found in the synthetic sorbent, as shown in Fig.1. It reveals that CaO reacts with all Al2O3to generate Ca3Al2O6.
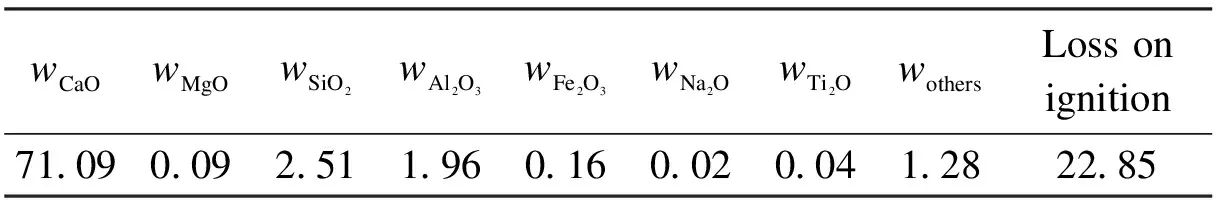
Tab.1 Chemical components in carbide slag %
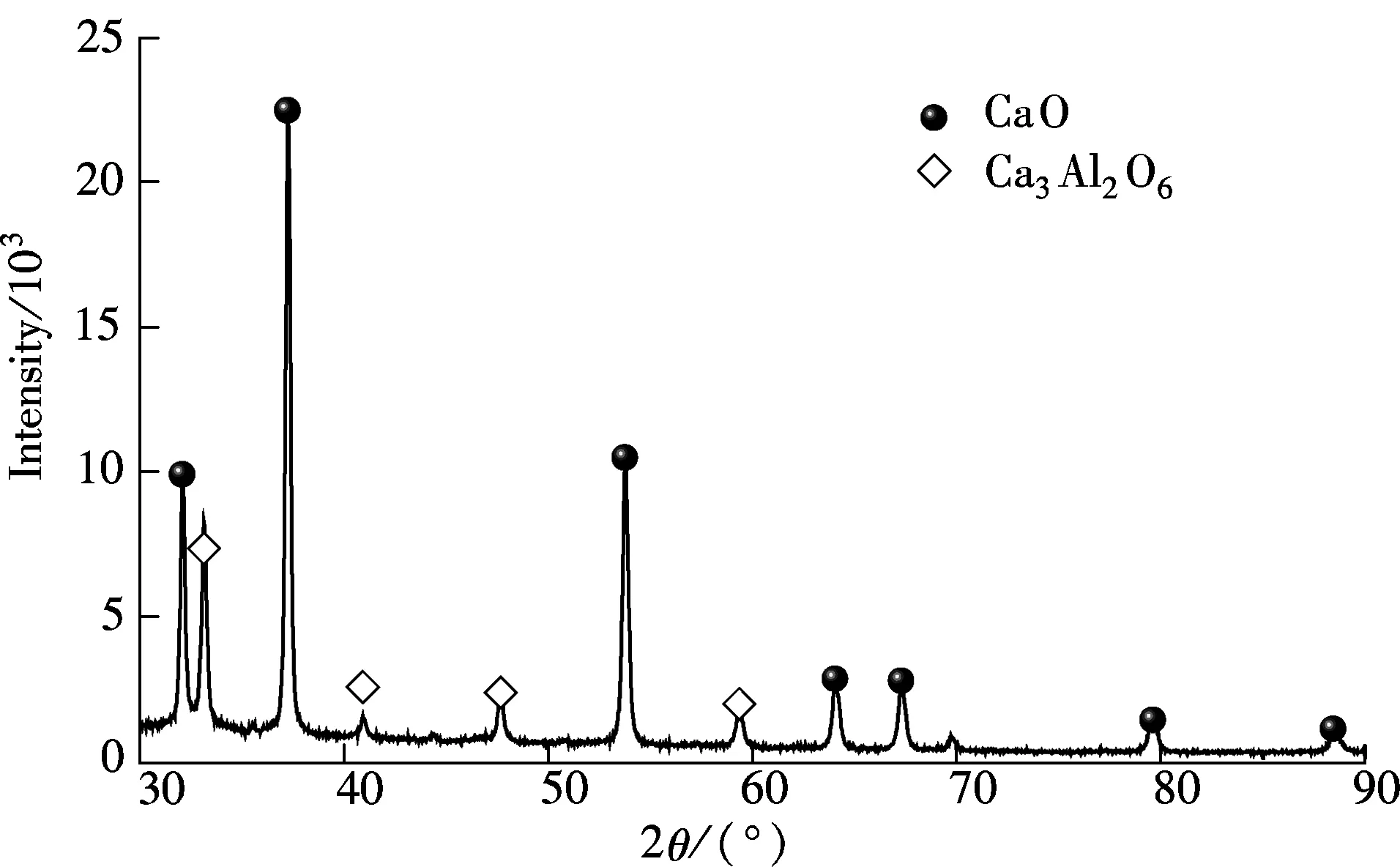
Fig.1 XRD spectra of synthetic sorbent
1.2 Cyclic CO2capture tests
A dual fixed-bed reactor operated under atmospheric pressure, the details of which were given somewhere else, was used to determine the CO2capture behavior of the synthetic sorbents in the carbonation/calcination cycles. The carbonation temperature was 700 ℃, and the carbonation time was 30 min. The calcinations temperature was 850 ℃, and the calcinations time was 10 min. Two parameters including CO2capture capacityCNand carbonation conversionXNwere used to characterize the CO2capture performance of the synthetic sorbent as follows:
(1)
(2)
wheretdenotes the carbonation time, min;CNis the CO2capture capacity of samples attin theN-th cycle, which indicates CO2adsorption amount per unit mass of sample, g/g;XNis the carbonation conversion of samples attin theN-th cycle, which indicates the fractional conversion of CaO derived from sample to CaCO3, mol/mol;m0is the mass of initial sample, g;cis the mass percent of CaO in initial sample, %;mcal,Nis the sample mass after complete calcination in theN-th cycle, g;mcar,N(t) is the sample mass after carbonation attin theN-th cycle, g;MCaOandMCO2are the molar mass of CaO and CO2, respectively, g/mol.
1.3 Average carbonation conversion, CO2capture efficiency and energy requirement in calciner
An equation is employed to fit the carbonation conversion curve for the calcium-based sorbent as follows[16]:
(3)
whereb,fmandfware the fitting constants.
The CO2capture efficiencyECO2is defined as[17]
(4)
whereF0is the makeup flow rate of fresh sorbent, kmol/s;FRis the flow rate of recycled sorbent excluding fresh makeup, kmol/s;FCO2is the flow rate of CO2produced by coal combustion entering the carbonator, kmol/s;Xaveis the average carbonation conversion.
The average carbonation conversion is defined as
(5)
whererkis the mass fraction of CaO derived from the fresh calcium-based sorbent entering the carbonator inF0+FR(kmol/s) afterkcycle;Xkis the carbonation conversion afterkcycles.
The average carbonation conversion of the calcium-based sorbent can be described by
(6)
and the CO2capture efficiency can be calculated by
(7)
The regeneration of CaO is an endothermic reaction, and the energy requirement in the calciner is an important parameter. The heat requirement for heating unreacted CaO, formed CaCO3, Ca3Al2O6and the inert mass from carbonation temperature to calcinations temperature are defined as
H1=[cp,CaOFR(1-Xave)+cp,CaCO3FRXave+cp,inertFR,inert+cp,Ca3Al2O6FR,Ca3Al2O6]ΔTcalc-carb
(8)
wherecpis the heat capacity, J/kmol;ΔTcalc-carbis the temperature difference between the calciner and carbonator, K.
The heat requirement for the calcination of CaCO3formed during carbonation is calculated as
H2=FRXavehCaCO3
(9)
wherehCaCO3is the calcination reaction heat of CaCO3, kJ/mol.
The fresh sorbent filled into the calciner is heated to the calcinations temperature from the ambient temperature and the heat requirement is calculated as
H3=(cp,CaOF0+cp,Ca3Al2O6F0,Ca3Al2O6+cp,inertF0,inert)ΔTcalc-fresh
(10)
where ΔTcalc-freshis the temperature difference between the calciner and fresh sorbent, K.
According to Tab.1, it is easily obtained that
F0,Ca3Al2O6=0.077F0
(11)
FR,Ca3Al2O6=0.077FR
(12)
Incorporating Eqs.(8) to (10), the energy requirementHreqin the calciner is obtained as
Hreq=H1+H2+H3
(13)
Then, the energy requirement per molar captured CO2is calculated as
(14)
2 Results and Discussion
2.1 CO2capture performance of synthetic sorbent
Fig.2 shows the CO2capture capacity and carbonation conversion of the synthetic sorbent and carbide slag having undergone 50 cycles. It can be seen that the synthetic sorbent shows much higher CO2capture capacity and carbonation conversion compared with carbide slag.C50andX50of the synthetic sorbent are 0.38 g/g and 0.70, which are 1.8 and 2.1 times those of carbide slag. Also, the synthetic sorbent demonstrates the slight decay in CO2capture capacity and carbonation conversion with the number of cycles. The glycerol is soluble in the water. At the same time, carbide slag and Al(NO3)3·9H2O can be dissolved in the glycerol water solution. Thus, the obtained solution is the homogeneous solution. Ca2+and Al3+are distributed uniformly in the synthetic sorbent, which is helpful for Ca3Al2O6to play a better role in the synthetic sorbent as support materials. On the other hand, CO2and water vapour are quickly released from the synthetic sorbent due to the rapid burning of the glycerol water solution at high temperatures, which can lead to the formation of the porous structure. This is a possible reason that glycerol addition enhances the cyclic CO2capture capacity of the synthetic sorbent.
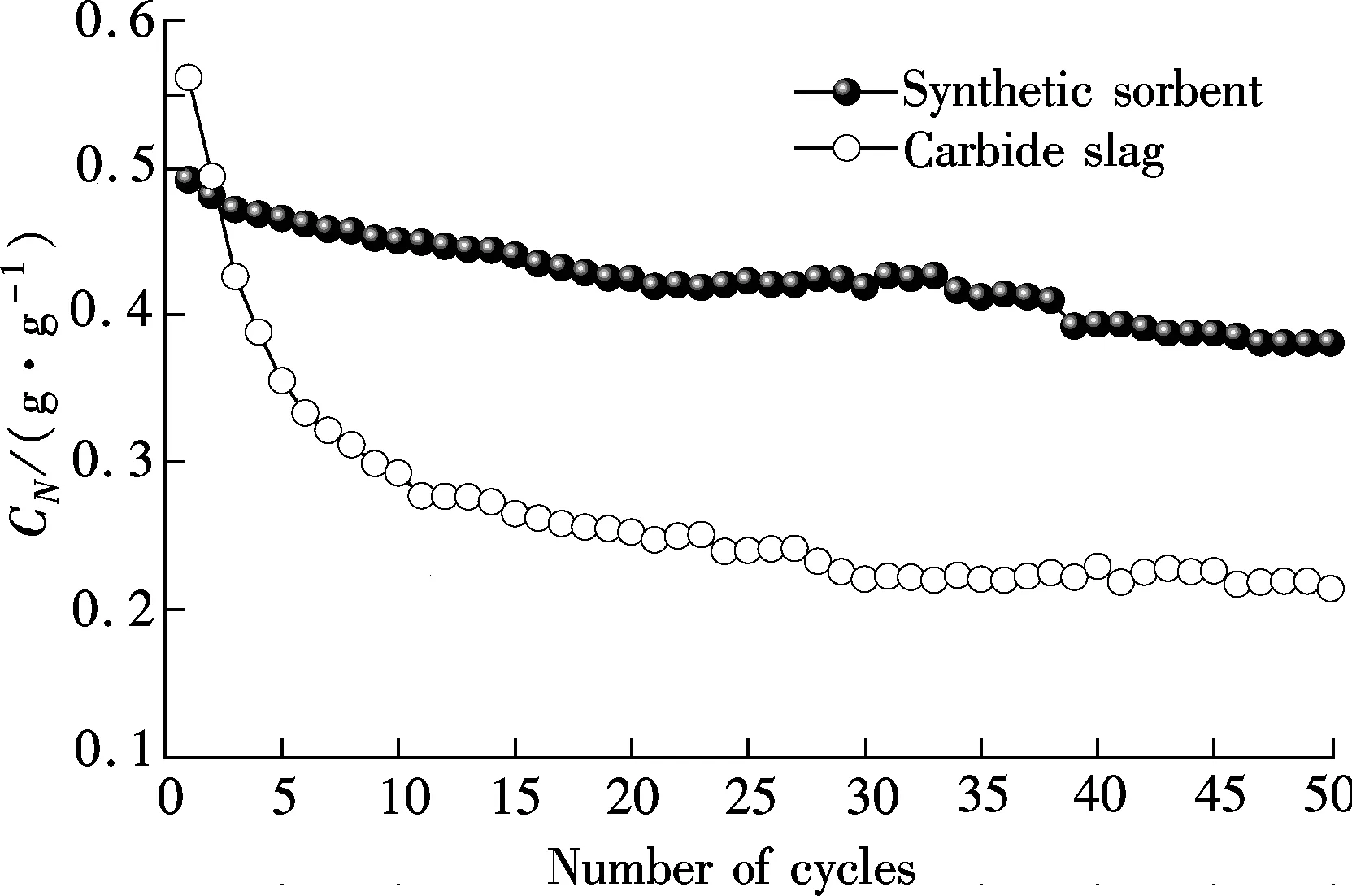
(a)

(b)
2.2 Average carbonation conversion of synthetic sorbent and carbide slag
Eq.(3) is employed to fit the carbonation conversion data of the synthetic sorbent and carbide slag. The fitting curves are shown in Fig.2(b) and the fitting results are shown in Tab.2. Then, the average carbonation conversion can be calculated according to Eq.(6), as shown in Fig.3. The sorbent flow ratios, such asF0/FCO2andFR/FCO2, have a comparable effect on the average carbonation conversion. For both synthetic sorbent and carbide slag,Xaveincreases with the increase ofF0/FCO2. WhenF0/FCO2is a constant,Xavedecreases with the increase ofFR/FCO2. This is mainly because the carbonation conversion of recycled sorbent is lower than that of the fresh one. It can be seen that theXaveof the synthetic sorbent is higher than that of carbide slag with the sameF0/FCO2andFR/FCO2. For example, whenF0/FCO2=0.05 andFR/FCO2=1.5, theXaveof the synthetic sorbent is 0.80, which is 1.9 times that of carbide slag. ForFR/FCO2=1.5, theXaveof the synthetic sorbent is 0.67 whenF0/FCO2=0.01, while theXaveof carbide slag is only 0.54 whenF0/FCO2=0.2.

Tab.2 Fitting results of the carbonation conversion data of synthetic sorbent and carbide slag by Eq.(3)

Fig.3 Effect of flow ratios on average carbonation conversion of synthetic sorbent and carbide slag
2.3 CO2capture efficiency of synthetic sorbent and carbide slag
The effect of flow ratios, includingF0/FCO2andFR/FCO2on CO2capture efficiency can be obtained according to Eq.(7), as shown in Fig.4.ECO2increases clearly with the increase ofF0/FCO2andFR/FCO2. TheECO2of the synthetic sorbent is higher than that of carbide slag with the sameF0/FCO2andFR/FCO2. For example, whenF0/FCO2=0.05 andFR/FCO2=1.0, theECO2of the synthetic sorbent is 86.0%, and theECO2of carbide slag is only 46.4%. The synthetic sorbent can achieve a higher CO2capture efficiency with the sameF0/FCO2andFR/FCO2, compared with carbide slag. ForFR/FCO2=1.5, theECO2of the synthetic sorbent is close to 100% whenF0/FCO2is 0.01. For carbide slag, theECO2of 100% was achieved whenF0/FCO2is as high as 0.14 andFR/FCO2is 2.0. The requiredF0/FCO2andFR/FCO2are lower for the synthetic sorbent to achieve the sameECO2compared with carbide slag.
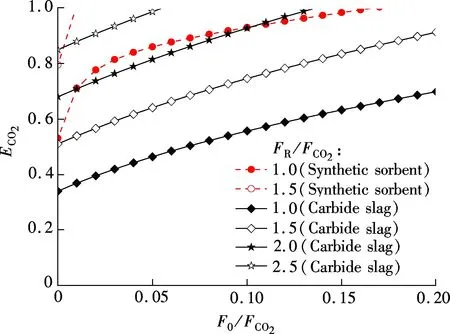
Fig.4 Effect of flow ratios on CO2 capture efficiency of synthetic sorbent and carbide slag
2.4 Energy requirement in calciner
For synthetic sorbent and carbide slag, increasingF0/FCO2andFR/FCO2means a higher CO2capture efficiency. However, higherF0/FCO2andFR/FCO2lead to an increase of energy requirement in the calciner. As shown in Fig.5, for both synthetic sorbent and carbide slag,Hreq/FCO2increase with the increase ofF0/FCO2andFR/FCO2. Also, it has a linear relationship betweenHreq/FCO2andF0/FCO2in the range of the calculated data. With the sameF0/FCO2andFR/FCO2, the energy requirement for the synthetic sorbent is higher than that for carbide slag. That is mainly due to the higher concentration of CaCO3in the synthetic sorbent. Fig.6 shows the effect ofECO2on energy requirement in the calciner. It can be seen thatHreq/FCO2increases with the increase ofECO2, for both synthetic sorbent and carbide slag. With the same flow ratio andECO2,Hreq/FCO2for the synthetic sorbent is less than that for carbide slag. It reveals that less energy is required when using a synthetic sorbent as CO2sorbent in the calcium looping process.
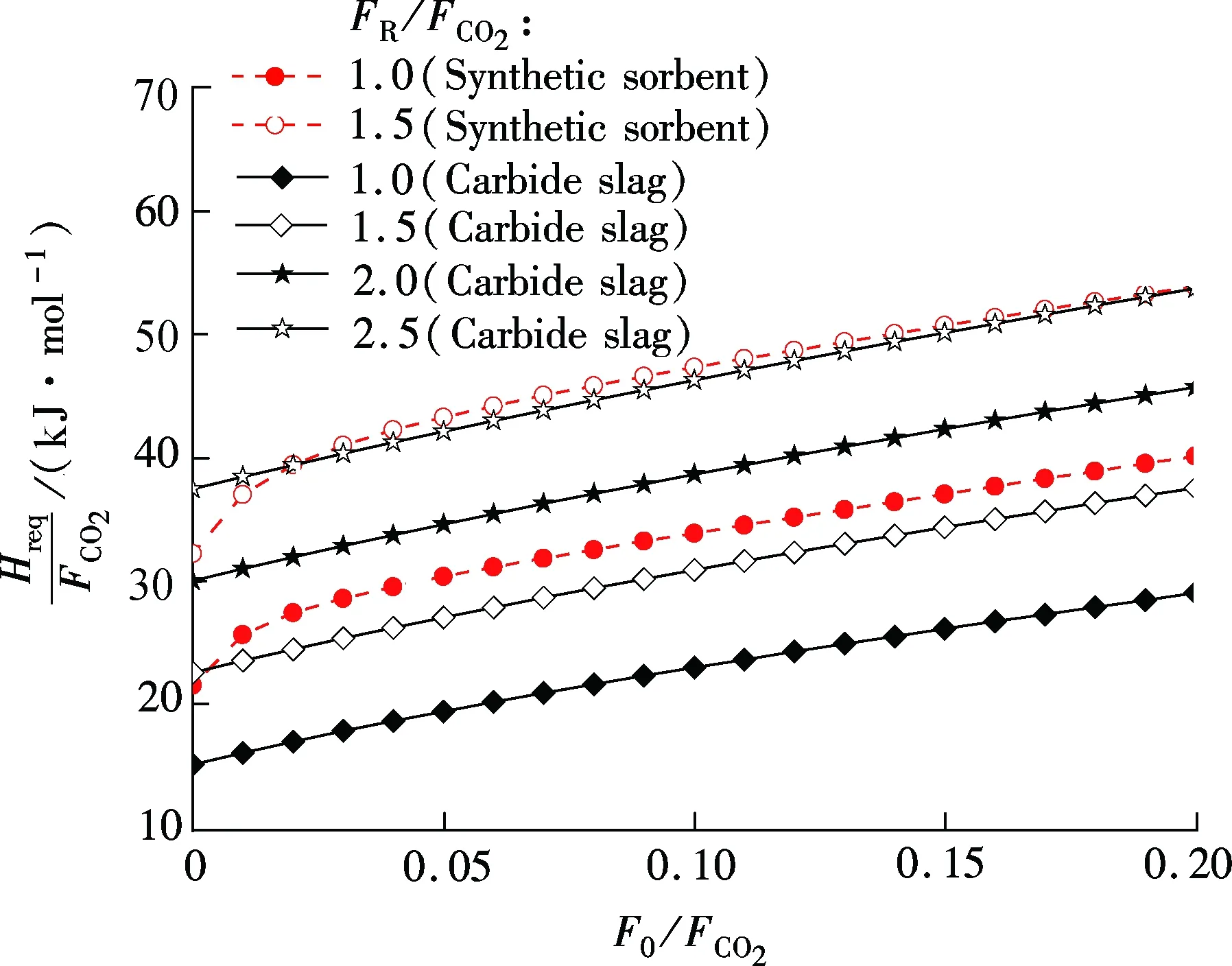
Fig.5 Effect of flow ratios on energy requirement in calciner
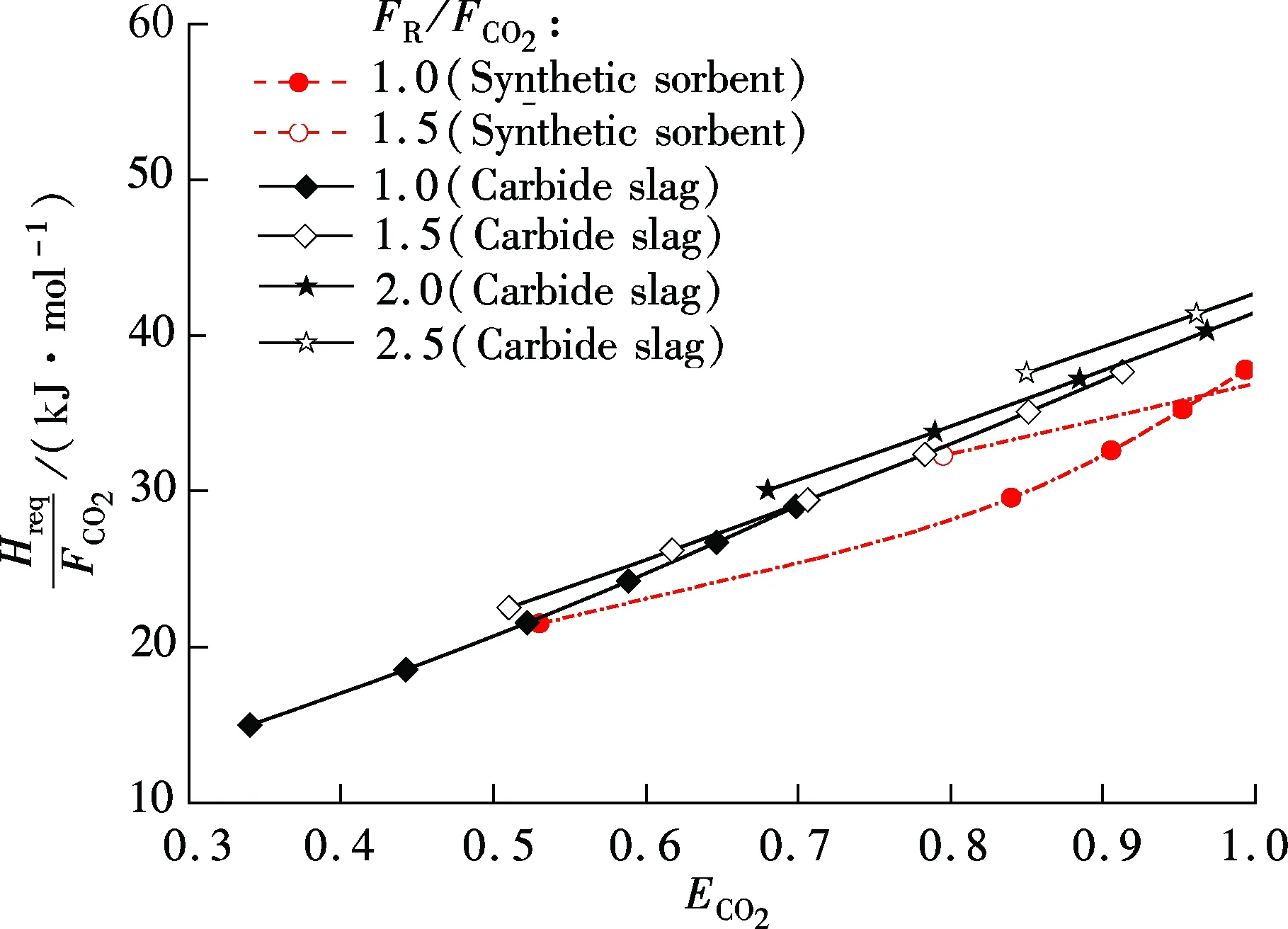
Fig.6 Effect of ECO2 on energy requirement in calciner
3 Conclusion
A type of highly reactive synthetic CO2sorbent is prepared from carbide slag, aluminum nitrate hydrate and glycerol water solution by the combustion synthesis method. The CO2capture performance of the synthetic sorbent is investigated. The average carbonation conversion, CO2capture efficiency and energy requirement in a calciner for the post-combustion CO2capture system using synthetic sorbent are calculated. The synthetic sorbent shows a much higher CO2capture capacity and carbonation conversion compared with carbide slag. The uniform distribution of Ca2+and Al3+in the synthetic sorbent and the formation of the porous structure due to the rapid burning of the glycerol water solution may be two possible reasons for the high cyclic CO2capture capacity of the synthetic sorbent. TheXaveandECO2of synthetic sorbent are higher than those of carbide slag with the sameF0/FCO2andFR/FCO2. The requiredF0/FCO2andFR/FCO2are lower for the synthetic sorbent to achieve the sameECO2compared with carbide slag. With the same sorbent flow ratio andECO2,Hreq/FCO2for the synthetic sorbent is less than that for carbide slag. The synthetic sorbent is more suitable as a CO2sorbent in the calcium looping process compared with carbide slag.
[1]Li Y J, Sun R Y, Liu C T, et al. CO2capture by carbide slag from chlor-alkali plant in calcination/carbonation cycles [J].IntJGreenhouseGasControl, 2012, 9:117-123.
[2]Miró L, Navarro M E, Suresh P, et al. Experimental characterization of a solid industrial by-product as material for high temperature sensible thermal energy storage (TES) [J].ApplEnergy, 2014, 113:1261-1268.
[3]Sharma V K, Fortuna F, Mincarini M, et al. Disposal of waste tyres for energy recovery and safe environment [J].ApplEnergy, 2000, 65(1/2/3/4):381-394.
[4]Sanna A, Dri M, Hall M R, et al. Waste materials for carbon capture and storage by mineralisation (CCSM)—a UK perspective [J].ApplEnergy, 2012, 99:545-554.
[5]Bobicki E R, Liu Q, Xu Z, et al. Carbon capture and storage using alkaline industrial wastes [J].ProgEnergyCombustSci, 2012, 38(2):302-320.
[6]Valverde J M, Sanchez-Jimenez P E, Perez-Maqueda L A, et al. Role of crystal structure on capture by limestone derived CaO subjected to carbonation/recarbonation/calcination cycles at Ca-looping conditions [J].ApplEnergy, 2014, 125:264-275.
[7]Lin S Y, Harada M, Suzuki Y, et al. Process analysis for hydrogen production by reaction integrated novel gasification (HyPr-RING) [J].EnergyConversManage, 2005, 46(6):869-880.
[8]Liu W, An H, Qin C, et al. Performance enhancement of calcium oxide sorbents for cyclic CO2capture—a review [J].EnergyFuels, 2012, 26(5):2751-2567.
[9]Ridha F N, Manovic V, Macchi A, et al. The effect of SO2on CO2capture by CaO-based pellets prepared with a kaolin derived Al(OH)3binder [J].ApplEnergy, 2012, 92:415-420.
[10]Zhang M, Peng Y, Sun Y, et al. Preparation of CaO-Al2O3sorbent and CO2capture performance at high temperature [J].Fuel, 2013, 111:636-642.
[11]Zhou Z, Qi Y, Xie M, et al. Synthesis of CaO-based sorbents through incorporation of alumina/aluminate and their CO2capture performance [J].ChemEngSci, 2012, 74:172-180.
[12]Chen H, Zhao C, Yu W. Calcium-based sorbent doped with attapulgite for CO2capture [J].ApplEnergy, 2013, 112:67-74.
[13]Chen H C, Zhao C S, Yang Y M, et al. CO2capture and attrition performance of CaO pellets with aluminate cement under pressurized carbonation [J].ApplEnergy, 2012, 91:334-340.
[14]García E, Laca M, Pérez E, et al. New class of acetal derived from glycerin as a biodiesel fuel component [J].EnergyFuels, 2008, 22(6): 4274-4280.
[15]Liu C T. Cyclic carbonation characteristics of modified carbide slag as a CO2sorbent in high temperature [D]. Jinan: School of Energy and Power Engineering, Shandong University, 2014. (in Chinese)
[16]Li Y J, Zhao C S, Chen H C, et al. CO2capture efficiency and energy requirement analysis of power plant using modified calcium-based sorbent looping cycle [J].Energy, 2011, 36(3):1590-1598.
[17]Abanades J C. The maximum capture efficiency of CO2using a carbonation/calcination cycle of CaO/CaCO3[J].ChemEngJ, 2002, 90(3):303-306.
基于钙基废弃物的高活性吸收剂合成及其循环捕集CO2性能
孙荣岳1,2李英杰1
(1山东大学能源与动力工程学院, 济南 250061)
(2南京工程学院能源与动力工程学院, 南京 211167)
利用一种典型的钙基废弃物电石渣作为CaO源合成CO2吸收剂.该高活性吸收剂由电石渣、九水硝酸铝和甘油水溶液经燃烧合成法合成.结果表明,该合成吸收剂表现出明显好于电石渣的CO2捕集性能.50次循环后,合成吸收剂的CO2吸收量和碳酸化转化率为0.38 g/g和0.70,分别是电石渣相同循环次数时的1.8和2.1倍.当吸收剂补充率相同时,合成吸收剂的平均碳酸化转化率和CO2捕集效率均高于电石渣.为取得相同的CO2捕集效率,合成吸收剂所需吸收剂的补充率小于电石渣.当吸收剂补充率和CO2捕集效率相同时,用合成吸收剂作为CO2吸收剂时煅烧炉内所需的能量小于用电石渣作为CO2吸收剂时所需能量.
电石渣;合成CO2吸收剂; CO2捕集
TQ534
Foundation item:The National Natural Science Foundation of China (No.51376003).
:Sun Rongyue, Li Yingjie.Synthesis of highly reactive sorbent from industrial wastes and its CO2capture capacity[J].Journal of Southeast University (English Edition),2015,31(2):209-214.
10.3969/j.issn.1003-7985.2015.02.009
10.3969/j.issn.1003-7985.2015.02.009
Received 2015-01-05.
Biographies:Sun Rongyue (1986—), male, graduate; Li Yingjie (corresponding author), male, doctor, associate professor, liyj@sdu.edu.cn.
 Journal of Southeast University(English Edition)2015年2期
Journal of Southeast University(English Edition)2015年2期
- Journal of Southeast University(English Edition)的其它文章
- Adaptive modulation in MIMO optical wireless communication systems
- An improving energy efficiency cooperation algorithm based on Nash bargaining solution in selfish user cooperative networks
- Performance analysis of an O2/CO2 power plantbased on chemical looping air separation
- Model of limestone calcination/sulfation under oxy-fuel fluidized bed combustion
- A novel carbon trap sampling systemfor coal-fired flue gas mercury measurement
- Applicability of Markov chain-based stochastic modelfor bubbling fluidized beds
