Cardiac Resynchronization Therapy in 2015:Lessons Learned
Siva Ketha and Fred M. Kusumoto
1Heart Rhythm Service, Department of Cardiovascular Disease, Mayo Clinic, Jacksonville, FL 32224, USA
Introduction
Management and treatment of heart failure has evolved dramatically over the past 40 years. Medications such as angiotensin-converting enzyme inhibitors [1, 2], angiotensin receptor blockers [3,4], beta blockers [5, 6], and aldosterone receptor antagonists [7, 8] have led to signif i cant improvements in both symptom control and survival in patients with heart failure (HF). Additionally,devices such as implantable cardioverter def i brillators (ICDs) are now recommended for primary prevention of sudden cardiac death in selected patients with ischemic and nonischemic cardiomyopathy [9]. Some HF patients benef i t from simultaneous pacing of both ventricles (biventricular pacing) or pacing of one ventricle in patients with bundle branch block, an approach known as cardiac resynchronization therapy (CRT) [10, 11].CRT can be achieved with a device designed only for pacing or can be incorporated into a combination device with an ICD. CRT is now recommended across a spectrum of patients with HF due to systolic dysfunction in association with QRS delay.The rationale for CRT is based on the observation that the presence of a bundle branch block or other intraventricular conduction delay can worsen HF due to systolic dysfunction by causing ventricular dyssynchrony.
Pathophysiology
Cardiomyopathy can result in structural abnormalities of ventricular myocardium that can affect both electrical activation of the ventricles and mechanical contraction [12, 13].
Electrical Dyssynchrony
Under normal conditions, the myocardium is activated by a uniform, high-velocity electrical waveform that propagates through the His-Purkinje system, resulting in synchronized depolarization of the ventricles. In the diseased myocardium, altered electrochemical substrate and impaired conduction fi bers can change the velocity and uniformity of electrical propagation, resulting in areas of delayed activation.This delay manifests itself as lengthening of the QRS complex on the surface 12-lead electrocardiogram.Because the QRS complex represents the summation vector of electrical forces generated by the ventricular myocardium during the course of ventricular systole, a prolonged QRS duration suggests electrical dyssynchrony [14]. Although the prevalence of a prolonged QRS duration (>120 ms) is approximately 20% in the general HF population, it is approximately 35% among patients with symptomatic HF [15].
Mechanical Dyssynchrony
Mechanical dyssynchrony is the result of electrical dyssynchrony and can beintraventriculardyssynchrony within the left ventricle seen commonly in patients with left bundle branch block (LBBB)because of a delay between the relatively earlyactivated interventricular septum and late-activated posterolateral wall,interventriculardyssynchrony between the left and right ventricles that results from sequential activation of the ventricles because of LBBB or right bundle branch block (RBBB), oratrioventricular(AV) dyssynchrony secondary to prolonged or absent AV nodal conduction, potentially coupled with His-Purkinje system dysfunction [14]. Mechanical dyssynchrony can prolong the periods of isovolumic contraction and isovolumic relaxation and consequently decrease cardiac pumping eff i ciency. Additionally, a dyssynchronous dilated left ventricle can result in mitral regurgitation because of lack of leaf l et coaptation and papillary muscle dysfunction [16]. Although a prolonged QRS duration is the best marker for dyssynchrony, some evidence suggests that mechanical dyssynchrony can be present in the absence of QRS duration prolongation [17]. Because QRS morphology and duration are inf l uenced only when a signif i cant amount of the myocardium is involved,regional discrepancies represented by small vectors are often not evident on the surface electrocardiogram. Therefore, small areas of impaired contractility can produce mechanical dyssynchrony without any detectable electrical conduction disturbance.However, as outlined later, there are no clinical data to suggest that treating mechanical dyssynchrony in the setting of a narrow QRS morphology with currently available CRT devices is benef i cial, and may even be associated with adverse outcomes. Longstanding cardiac dyssynchrony leads to remodeling that manifests itself clinically as dilation of the left ventricle, worsening systolic and diastolic function,and progressive HF.
History of CRT
In 1979, temporary biventricular pacing was used to assess tachyarrhythmias due to intraventricular reentry [18]. A decade later, in 1989, Grines et al.[19] described how LBBB reduced the diastolic filling time and the septal contribution to left ventricular (LV) ejection. By the 1990s, a link had emerged between electrical dyssynchrony and impairment of LV function, and it became apparent that LV pacing was more hemodynamically favorable than right ventricular (RV) pacing. The concept of “biventricular pacing,” primarily aimed at HF treatment was developed by Morton Mower, who conceived a method of pacing both ventricles after a predetermined AV interval by connecting two electrodes in series, one in the right ventricle and the other around the free wall of the left ventricle [20]. Proof of this concept came in 1993 when Bakker et al. [21]treated 12 patients with end-stage congestive HF,sinus rhythm, and complete LBBB with biventricular stimulation and showed an improvement in functional capacity, improved systolic and diastolic LV function, and a decrease in mitral regurgitation during 2- and 3-year follow-up. Soon thereafter, Cazeau and colleagues described a four-chamber pacing system that reduced pulmonary capillary wedge pressure and increased cardiac output in a patient with New York Heart Association (NYHA) class IV HF[22] and later described a transvenous CRT implantation method [23]. These initial studies paved the way for the pivotal clinical trials of CRT described herein and have led to their widespread use.
Evidence of Clinical Benefit
Several landmark prospective multicenter studies (Table 1) on the effectiveness of biventricular pacing – namely, Pacing Therapies for Congestive Heart Failure (PATH-CHF) [24], Multisite Stimulation in Cardiomyopathies (MUSTIC) [25], Multicenter InSync Randomized Clinical Evaluation(MIRACLE) [26], Multicenter InSync ICD Randomized Clinical Evaluation II (MIRACLE-ICD)[27], Cardiac Resynchronization Therapy for the Treatment of HF in Patients with Intraventricular Conduction Delay and Malignant Ventricular Tachyarrhythmias (CONTAK-CD) [28], Comparison of Medical Therapy, Pacing, and Def i brillation in Heart Failure (COMPANION) [10], and Cardiac Resynchronization-Heart Failure (CARE-HF)[11] – were performed between 2000 and 2005.These studies consistently showed that CRT safely improved the patient’s quality of life, NYHA functional class, exercise capacity, LV ejection fraction(LVEF; absolute improvement of 5–15%) and, in COMPANION and CARE-HF, reduced mortality.
Later clinical trials – Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction(REVERSE) [29], Multicenter Automatic Def i brillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) [30], and Resynchronization for Ambulatory Heart Failure Trial (RAFT)[31] – focused on whether CRT was benef i cial in HF patients with reduced LVEF, a wide QRS complex, and milder symptoms (NYHA class I or II). In these studies, patients were randomized to receive an ICD or an ICD with CRT capabilities (CRT-D),and CRT-D was associated with signif i cant reverse remodeling [29], lower rates of hospitalization [30],and improved survival [31].
As would be expected, the magnitude of mortality benef i t (Figure 1) conferred by CRT was much higher in the earlier clinical trials COMPANION and CARE-HF (which compared CRT with optimal medical management in patients with severe HF) than in the later trials MADIT-CRT and RAFT (which compared CRT with ICD in patients with less severe HF).
Mechanism of Benefit
Although several randomized controlled trials have shown improved outcomes with CRT in appropriately selected patients with systolic HF who have an intraventricular conduction delay or LBBB, the molecular basis for these mechanical changes is not well understood. One experimental model suggests that CRT reduces regional and global molecular remodeling, generating more homogeneous activation of stress kinases and reducing apoptosis [32]. Potential mechanisms of benef i t include improved contractile function and reverse ventricular remodeling manifested as reductions in LV chamber size and measures of mitral regurgitation.Other hemodynamic and clinical benef i ts during short-term or long-term biventricular or LV pacing include an increase in cardiac index and a reduction in pulmonary capillary wedge pressure, when compared with normal sinus rhythm or RV pacing[22, 33–35], the ability to tolerate more aggressive medical therapy and neurohormonal blockade, particularly with improved tolerance of beta blockers[36], improved diastolic function among responders to CRT [37], and an improvement in heart rate variability [9].
Device Implantation
Initially, an epicardial lead for LV pacing or a transvenous lead that was not specifically designed and tested for long-term LV pacing was used in clinical trials [28, 38]. Daubert et al. [23] described transvenous coronary sinus lead placement for long-term LV pacing, and this wholly transvenous approach has simplif i ed the implantation procedure and reduced operative risk. However, implantation via the coronary sinus may result in perforation or dissection and other complications, and should be performed only by experienced operators. The most common complication with transvenous CRT implantation is the inability to implant the LV pacing lead successfully in the coronary vein. Other infrequently encountered complications include coronary sinus or coronary vein trauma, pneumothorax, diaphragmatic/phrenic nerve pacing, and infection [10, 11, 39, 40]. Although a theoretical concern existed about a proarrhythmic effect due to alterations in depolarization and repolarization sequences [41], randomized controlled trials have not suggested any excess risk of sudden death or noncardiac death in CRT device recipients.
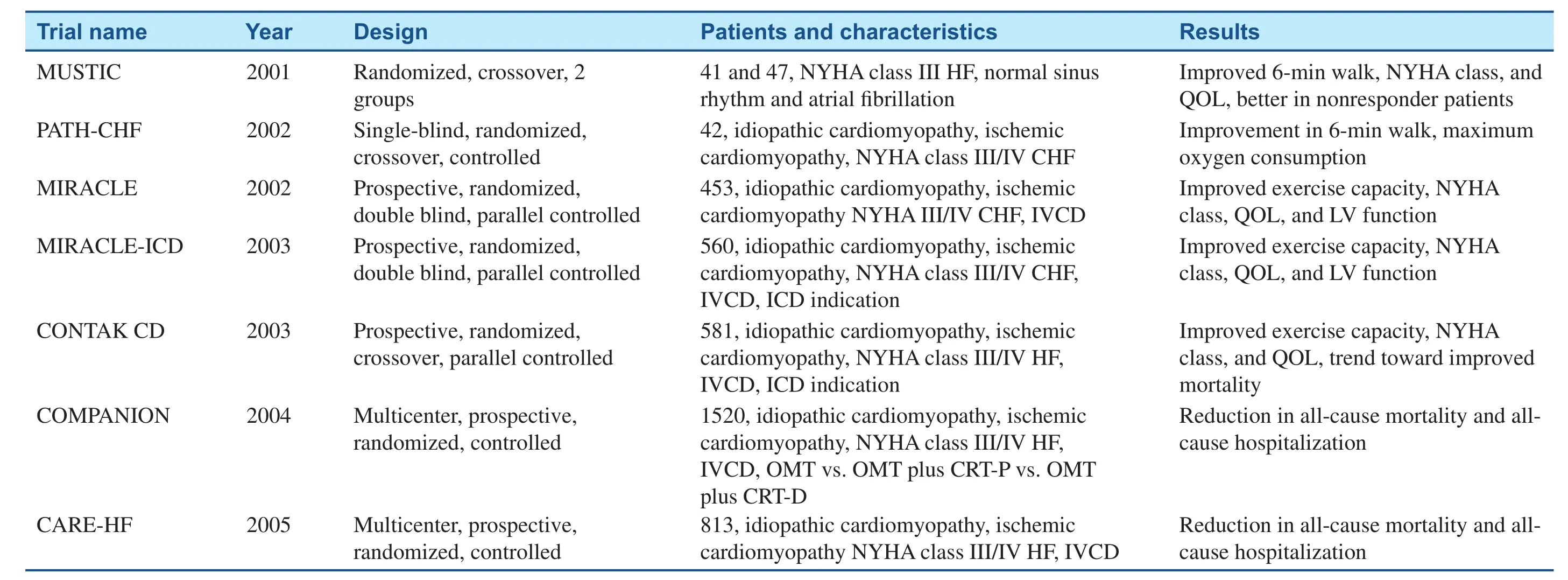
Table 1 Pivotal cardiac resynchronization therapy trials (2001–2005).

Figure 1 Summary of 2-year Mortality Rates for Cardiac Resynchronization Therapy (CRT) Studies that had Mortality End Points and Relative Percentages of Different New York Heart Association Heart Failure Classi fi cation Patients in the Study Populations.
Follow-up
Once implanted, CRT devices need careful followup and at times in-off i ce device reprogramming,which may be more frequent than in patients with standard pacemakers or ICDs. However, one report from the Insync ICD Italian Registry indicated a marked reduction in the number of interrogations requiring reprogramming between the first 6 months of follow-up and subsequent periods, as pacing and CRT delivery parameters were usually optimized soon after implantation and were maintained unmodif i ed thereafter [42]. Therefore, remote follow-up appears to be an acceptable alternative in these patients, especially with the use of algorithms that automatically adjust device settings such as LV pacing output on the basis of daily threshold measurements [43].
Nonresponders
About a third of patients do not achieve the expected clinical benef i ts after CRT device implantation [44, 45]. Several theories, including inconsistent pacing, myocardial scar burden, lack of mechanical dyssynchrony, and suboptimal lead positioning, have been proposed to explain CRT nonresponse and are bring actively investigated(Figure 2). Although the cause of CRT nonresponse is an area of active investigation, one difficulty in assessing a prognostic response to CRT appears to be the lack of an adequate surrogate. LV reverse remodeling [46], peak Vo2, and natriuretic peptides [47] have been considered but have signif i cant limitations that render them inadequate as potential surrogates.
Controversies, Areas of Ongoing Research
Patient Selection
CRT in Atrial Fibrillation
An important subset of HF patients are those with atrial fi brillation (AF), who make up 25%–30% of HF patients, and are overrepresented among HF patients with more advanced symptoms. In HF patients with AF, CRT appears to be less effective than in patients in sinus rhythm, which may be due to competition between ventricular depolarization generated by the CRT device and intrinsic ventricular depolarization from the heart’s own electrical conduction system. RAFT [48] included more patients with permanent AF than all other published studies combined, and failed to demonstrate a clear improvement in any clinical or surrogate outcome by CRT in patients with permanent AF, despite a trend toward reduction of HF hospitalization rates.It has been hypothesized that the reduced benef i t might be attributed to suboptimal delivery of CRT since only a third of the patients received more than 95% ventricular pacing. Because a CRT device paces only when the patient’s intrinsic rate is lower than the programmed rate, in patients with AF who are prone to rapid ventricular response, their tachycardia may limit the percentage of paced beats and thereby lead to decreased effectiveness of the CRT.Ablation of the AV node to increase the percentage of pacing in AF is likely to increase CRT response,and this strategy is being prospectively evaluated in the Cardiac Resynchronization Therapy and AV Nodal Ablation Trial in Atrial Fibrillation Patients(CAAN-AF) study.

Figure 2 Top row: Chest radiography in a patient who initially received a cardiac resynchronization therapy (CRT) device with additional de fi brillator capabilities that used epicardial leads placed apically (posteroanterior view) and anteriorly (best seen in the lateral view). Because of continued symptoms despite CRT, the patient underwent device revision with placement of an endovascular lead placed in a lateral vein via the coronary sinus (arrowheads). The patient’s heart failure symptoms improved after device revisions. Second row: The 12-lead electrocardiogram obtained with the epicardial electrodes is characterized by a predominantly negative QRS complex in lead aVL (suggesting an anterolateral location) Third row: The 12-lead electrocardiogram obtained with the endocardial electrode has a later precordial transition (positive QRS complex in V2), which suggests a more basal pacing site.
CRT in Narrow QRS Complex Patients
Yu et al. [49] found that LV systolic and diastolic mechanical dyssynchrony is common even in patients with HF with narrow QRS complexes(<120 ms) and proposed that the QRS complex duration is not a determinant of systolic asynchrony,but rather that the assessment of intraventricular synchronicity is probably more important than QRS duration. This early premise formed the foundation for subsequent research evaluating whether CRT is benef i cial in patients with a QRS duration less than 120 ms. Although preliminary data from small single-center studies were encouraging [50–52],two contemporary multicenter randomized controlled studies, The Evaluation of Resynchronization Therapy for Heart Failure (LESSER-EARTH)trial [53] and the Echocardiography Guided Cardiac Resynchronization Therapy (EchoCRT) study [54],failed to show a mortality benef i t from addition of CRT to an ICD in patients with HF, reduced LVEF and a narrow QRS complex (≤120 ms for LESSEREARTH and ≤130 ms for EchoCRT). In the LESSER-EARTH trial, CRT did not improve clinical outcomes or induce LV reverse remodeling and adversely affected exercise tolerance [53]. Unlike the LESSER-EARTH trial, patients enrolled in the EchoCRT study had echocardiographic evidence for cardiac dyssynchrony. However, even with this additional enrollment criterion, the EchoCRT study was stopped prematurely for futility on the basis of the recommendation of the data and safety monitoring board because patients who were randomized to the CRT-D group not only did not have reduced rates of death or hospitalization for HF but were also found to have increased mortality [54]. At this point CRT should not be considered in patients with a narrow QRS complex unless they will require substantial ventricular pacing due to AV block (see later).
CRT in RBBB
The use of CRT in patients with a wide QRS complex without LBBB is not well established. A metaanalysis of randomized CRT trials with a total of 5356 patients, of which 1233 patients had non-LBBB conduction abnormalities and were randomly assigned to receive CRT or no CRT, found that there was no reduction in the rate of clinical events in the non-LBBB patient category [55].Post hoc analysis of MADIT-CRT has shown that subgroups with non-LBBB QRS morphology do not derive signif i cant benef i t from CRT [56]. This analysis was designed to determine whether QRS morphology identif i es patients who benef i t from CRT-D and whether it inf l uenced the risk of primary and secondary end points in patients enrolled in MADIT-CRT. The combined end point of HF event or death was the primary end point of the trial. Death, HF event, ventricular tachycardia, and ventricular fi brillation were secondary end points.Among 1817 patients with available sinus rhythm electrocardiograms at the baseline, there were 1281(70%) with LBBB, 228 (13%) with RBBB, and 308(17%) with nonspecific intraventricular conduction disturbances. The latter two groups were def i ned as non-LBBB groups. Hazard ratios for the primary end point for comparisons of CRT-D patients versus patients who received only an ICD were signifi cantly lower in LBBB patients (0.47; P <0.001)than in non-LBBB patients (1.24; P = 0.257). The risk of ventricular tachycardia, ventricular fi brillation, or death was decreased signifi cantly in CRT-D patients with LBBB but not in non-LBBB patients.Echocardiographic parameters showed signifi cantly greater reduction in LV volumes and improvement in ejection fraction with CRT-D in LBBB patients than in non-LBBB patients (absolute increase in LVEF 12% vs. 9% respectively; P < 0.001). The study authors concluded that no clinical benef i t was observed in patients with a non-LBBB QRS pattern.
The recently completed Pacing Affects Cardiovascular Endpoints in Patients with Right Bundle-Branch Block (PACE-RBBB) trial prospectively investigated whether resynchronization by RV pacing alone is equivalent to biventricular pacing in patients with HF and RBBB. The study was completed in August 2014 but no results have been presented.
CRT in AV Block Regardless of LVEF
The effects of CRT have also been evaluated in patients who require pacemaker implantation for AV block irrespective of LVEF. The Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BIOPACE) study [57] and the Biventricular versus RV Pacing in Patients with Left Ventricular Dysfunction and Atrioventricular Block (BLOCK-HF) study [58] focused on this subgroup of patients. Prior studies, including the Dual Chamber and VVI Implantable Def i brillator(DAVID) [59] and Mode Selection Trial (MOST)[60], showed that RV pacing worsens long-term ventricular function and outcomes. The BLOCKHF study randomized 691 patients with AV block requiring pacing support with an LVEF of 50% or less to CRT or RV pacing. After an average followup of 3 years, CRT signifi cantly reduced the combined primary end point of mortality, HF-related urgent care, and increase in LV end-systolic vol-ume by 26% compared with RV pacing, primarily by reducing LV end-systolic volume. The improvement, however, came at the cost of a higher number of adverse events (83 patients versus 30 patients for CRT and RV pacing, respectively), mostly due to LV lead implant and postimplant issues. In a preliminary presentation of the BIOPACE trial presented at the European Society for Cardiology Congress 2014, after enrollment of 1810 patients with a mean follow-up of 5.6 years, biventricular pacing failed to signifi cantly improve the primary combined outcome of time to death or first HF hospitalization when compared with RV pacing, although a nonsignif i cant trend in favor of biventricular pacing over RV pacing was detected (hazard ratio 0.87; 95% conf i dence interval 0.75–1.01; P = 0.08)(http://www.escardio.org/The-ESC/Press-Office/Press-releases/Last-5-years/Biventricular-pacingdisappoints-in-BIOPACE-trial). Implant failure was more common in the CRT group (14.8%) than in the RV pacing group (0%).
Procedural
Site(s) of Pacing
The currently used technique of LV lead placement via the coronary sinus for CRT implantation has remained mostly unchanged since its first description in 1998 by Daubert et al. [23]. A good angiographic result requires the LV lead to be in the posterolateral position with acceptable pacing parameters and no diaphragmatic stimulation(Figure 2). Apical position of the LV lead has been found to have worse outcomes [61]. However, the response to CRT is variable even when the LV lead is in a “good” position. This led to the concept of targeting LV segments with the most delayed activation in order to improve response. The Speckle Tracking Assisted Resynchronization Therapy for Electrode Region (STARTER) trial [62] showed that deploying LV leads in late-activated segments by echocardiographic guidance reduced the risk of death or HF hospitalization (hazard ratio 0.48; 95%conf i dence interval 0.28–0.82; P = 0.006). However, exact concordance between late-activated segments and LV lead position was achieved in only 30% of patients. Furthermore, segments with likely scar were regarded as missing data. Therefore, it remains unclear whether the benef i ts of echocardiographic guidance were due to avoidance of scar or targeting of late-activated segments. In the Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy (TARGET) trial,Khan et al. [63] also showed signifi cantly improved response (70% vs. 55%), clinical status, and lower rates of combined death and HF-related hospitalizations with the use of speckle-tracking echocardiography to the target LV lead placement .
Viability of the paced LV segment could also inf l uence the CRT outcome. Leyva et al. [64] used cardiac magnetic resonance imaging to avoid scar and demonstrated improvement in the response to CRT. Similarly, in the TARGET trial, patients with an LV lead placed in a region of scar had poorer outcomes and a higher rate of HF hospitalizations [63].The benef i ts of targeting a specific location for lead placement still needs further evaluation, and with current technology will be constrained by venous anatomy, but it seems reasonable to place the electrodes in a late-activating site and to avoid regions of dense scar if technically feasible.
Endocardial LV Pacing
Placing leads in the venous tributaries of the heart leads to initial epicardial depolarization, and investigators have proposed using LV endocardial pacing to maximize the benef i t of CRT, but this strategy has not translated into a clinical benef i t in early studies[65]. The Wireless Stimulation Endocardially for CRT (WiSE-CRT) study demonstrated the feasibility of providing endocardial stimulation for CRT with a leadless ultrasound-based technology [66]. Transeptal/transmitral and transapical endocardial LV pacing approaches remain experimental, but their use can be considered in unique clinical situations.
Multisite LV Pacing
Leclercq et al. [67] evaluated the role of multipolar LV leads in CRT. Potential advantages of multisite LV pacing include avoidance of diaphragmatic stimulation and availability of multiple pacing vectors.This technology is already being widely adopted and will largely replace leads that use only one or two electrodes. There are some isolated case reports that suggest the potential utility of simultaneous pacing from several spatially separate LV locations (e.g.,the anterolateral and posterolateral walls).
Device Optimization
The fi nding that LV function varies according to AV delays [34] has led to several approaches for device optimization. Echocardiography is commonly used to identify the AV delay yielding optimal LV filling. One frequently used Doppler parameter for echocardiography-guided optimization is the aortic velocity-time integral [48, 68], In one single-blind randomized trial the impact of AV delay optimization based on the aortic velocity-time integral was evaluated and showed improvement in NYHA functional class in optimized versus control patients [37]. Many single-center studies have shown improvement in cardiac output by tailored Doppler echocardiography – guided AV delay optimization [11, 25, 34].
In addition to programming the AV delay, all currently available CRT devices allow individual programming of the interval between LV and RV pacing stimuli (VV) and which stimulus will be delivered first. Device-based AV/VV proprietary algorithms have been developed by all manufacturers. Devicebased interval optimization by the QuickOpt®algorithm was inferior to echocardiographic optimization in the Frequent Optimization Study Using the QuickOpt Method (FREEDOM) [69]. In another study, AV optimization by the Smart-AV®algorithm did not lead to LV reverse remodeling compared with nominal settings in the Comparison of AV Optimization Methods Used in Cardiac Resynchronization Therapy (Smart-AV) study [70]. On the other hand, the Adaptive Cardiac Resynchronization Therapy study showed that an algorithm that provides automatic selection between synchronized LV or biventricular pacing, as well as AV and VV optimization, was comparable to echocardiographic optimization [71]. However, it remains to be determined whether this is attributable to AV/VV optimization or to the pacing mode.
In summary, although regarded as the gold standard, echocardiographic optimization has not been shown to improve outcomes in large multicenter randomized trials, but appears as effective as nominal device settings and at this point should still be considered superior to the current device-based algorithms for optimizing the timing of pacing stimuli [70].
Cost-effectiveness
The incremental cost-effectiveness ratio (ICER),or the additional cost of a quality-adjusted life-year(QALY) saved, is a widely accepted measure of the cost of medical interventions [72]. An acceptable ICER is less than $50,000 in the United States.
Although CRT devices are expensive, the costs are offset in part by savings from reduced rates of hospitalizations for HF as suggested by the acceptable cost-effectiveness ratios for CR, in the cost-effectiveness analyses from the COMPANION and CARE-HF trials [73, 74]. Data from the COMPANION trial were extrapolated to develop a cost-effectiveness estimate for CRT alone (CRT-P)and CRT-D. The cost-effectiveness ratio projected over 7 years was estimated to be $19,600 per QALY for CRT-P and $43,000 per QALY for CRT-D [74].Similarly, the analysis of CARE-HF patients found that over a median follow-up of 29 months, the costeffectiveness ratio was 19,319 per QALY [74]. For mild HF, a REVERSE trial analysis showed that over a 10-year time frame, CRT “on” was associated with an ICER of 14,278 per QALY saved [75].Similarly, a MADIT-CRT analysis yielded an ICER for CRT-D of $58,330 compared with ICD implantation [76]. When the ICER was calculated for a longer time period and for inclusion of a preimplantation LBBB, it decreased substantially to less than$10,000 [76]. Therefore, one must exercise caution in interpreting these numbers as the analyses are based on extrapolated results and the results vary substantially according to changes in input variables such as the magnitude of reduction in HF admission rates, cost of device implantation, and frequency of device complications. However, taken together,these analyses demonstrate that CRT-P and CRT-D are at least as cost-effective as many other medical interventions for treating HF.
Current Guideline Recommendations
Recommendations for CRT first appeared in the 2008 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines for device-based therapy of cardiac rhythm abnormalities [77], and more recently in the 2012 American College of Cardiology Foundation/American Heart Association/Heart Rhythm Society focused update incorporated into the 2008 guidelines (2012 focused update) [78] and the 2013 American College of Cardiology Foundation/American Heart Association guideline for the management of HF[9]. Importantly, the current guidelines for CRT are consistent in their recommendations (Figure 3).In addition, all guidelines recommend CRT with the expectation that appropriate medical therapy is already being provided, usually described as“guideline-directed medical therapy.”
For CRT recommendations, the 2012 focused update included data from the REVERSE trial,MADIT-CRT, and RAFT, which studied the use of CRT in patients with less severe symptoms,to make recommendations. In addition the 2012 focused update also considered the growing body of evidence suggesting that CRT is less effective in patients with narrower QRS durations (<150 ms) or non-LBBB QRS patterns. In the 2008 guidelines,the only class I recommendation for CRT was for patients with relatively severe symptoms (NYHA functional class III or IV HF) accompanied by sinus rhythm, LVEF of 35% or less, and a QRS duration of 120 ms or more with no specific consideration of QRS morphology. For the 2012 focused update(and the 2013 HF guidelines), this class I indication was expanded to patients with NYHA class II HF, sending the clear message that CRT “is indicated” for a population with milder symptoms. At the same time, the 2012 focused update ref i ned this single class I recommendation by conf i ning it only to patients with LBBB and a QRS duration of 150 ms or more (Figure 3). Patients with LBBB and a QRS duration of only 120–149 ms and those with a non-LBBB pattern and a QRS duration of 150 ms ir more, included in class I in 2008, were downgraded to class IIa, and those patients with a non-LBBB pattern with a QRS duration between 120 and 149 ms were downgraded further to class IIb. For patients with LBBB (regardless of QRS duration), patients with NYHA class II symptoms are now included as candidates for CRT (class I for a QRS duration greater than 150 ms and class IIa for a QRS duration of 120–149 ms). For patients with non-LBBB block, if the QRS duration was less than 150 ms, the indication was not expanded beyond patients with NYHA class III/ambulatory class IV symptoms, and CRT is “not recommended” (a class III recommendation) for patients with NYHA class II symptoms.The evolution in recommendations was based on the consistent results of studies demonstrating that non-LBBB conduction abnormalities are associated with failure to benef i t from CRT [55, 79–81].

Figure 3 Flowchart Showing Current Recommendations for Cardiac Resynchronization Therapy Based on the 2012 Update of the 2008 American College of Cardiology/American Heart Association/Heart Rhythm Society Guidelines for Device-Based Therapy of Cardiac Rhythm Disorders and the 2013 American College of Cardiology Foundation/American Heart Association Heart Failure Guidelines.
Conclusion and Take-Home Message
CRT remains one of the most innovative treatments of HF. It is a clinically benef i cial and cost-effective treatment for patients with mild to severe HF and a wide QRS complex. Ongoing and future research will continue to investigate ways to ref i ne implantation, optimize device function, and better elucidate the cause of nonresponse to CRT with the goal of ultimately reducing nonresponder rates and identifying other groups of patients who may benef i t from this therapy.
Conflict of Interest
The authors declare no conf l ict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or notfor-profit sectors.
1. The SOL VD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302.
2. Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction –results of the survival and ventricular enlargement trial. N Engl J Med 1992;327:669–77.
3. Cohn JN, Tognoni G. A randomized trial of the angiotensin- receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667–75.
4. Granger C B, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial.Lancet 2003;362:772–6.
5. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERITHF). Lancet 1999;353:2001–7.
6. Packer M, Coats AJ, Fowler MB,Katus HA, Krum H, Mohacsi P,et al. Effect of carvedilol on survival in severe chronic heart failure.N Engl J Med 2001;344:1651–8.
7. Pitt B, Z annad F, Remme WJ, Cody R, Castaigne A, Perez A, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–17.
8. Pitt B, R emme W, Zannad F,Neaton J, Martinez F, Roniker B,et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–21.
9. Yancy CW, Jessup M, Bozkurt B,Butler J, Casey DE Jr, Drazner MH, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239.
10. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable def i brillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–50.
11. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D,Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure.N Engl J Med 2005;352:1539–49.
12. Brutsaer t DL. Nonuniformity: a physiologic modulator of contraction and relaxation of the normal heart. J Am Coll Cardiol 1987;9:341–8.
13. Katz AM. Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N Engl J Med 1990;322:100–10.
14. Kass DA. Pathobiology of cardiac dyssynchrony and resynchronization. Heart Rhythm 2009;6:1660–5.
15. Silvet H, Amin J, Padmanabhan S,Pai RG. Prognostic implications of increased QRS duration in patients with moderate and severe left ventricular systolic dysfunction. Am J Cardiol 2001;88:182–5, A6.
16. Ypenburg C, Lancellotti P, Tops LF,et al. Mechanism of improvement in mitral regurgitation after cardiac resynchronization therapy. Eur Heart J 2008;29:757–65.
17. Hawkins NM, Petrie MC,MacDonald MR, Hogg KJ,McMurray JJ. Selecting patients for cardiac resynchronization therapy: electrical or mechanical dyssynchrony? Eur Heart J 2006;27:1270–81.
18. Befeler B, Berkovits BV, Aranda JM, Sung RJ, Moleiro F, Castellanos A. Programmed simultaneous biventricular stimulation in man, with special reference to its use in the evaluation of intraventricular reentry. Eur J Cardiol 1979;9:369–78.
19. Grines C L, Bashore TM,Boudoulas H, Olson S, Shafer P,Wooley CF. Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation 1989;79:845–53.
20. Leyva F, Nisam S, Auricchio A.20 years of cardiac resynchronization therapy. J Am Coll Cardiol 2014;64:1047–58.
21. Bakker P F, Meijburg HW, de Vries JW, Mower MM, Thomas AC, Hull ML, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Card Electrophysiol 2000;4:395–404.
22. Cazeau S, Ritter P, Bakdach S,Lazarus A, Limousin M, Henao L, et al. Four chamber pacing in dilated cardiomyopathy. Pacing Clin Electrophysiol 1994;17:1974–9.
23. Daubert JC, Ritter P, Le Breton H, Gras D, Leclercq C, Lazarus A, et al. Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. Pacing Clin Electrophysiol 1998;21:239–45.
24. Auricchi o A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P,et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002;39:2026–33.
25. Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C,et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001;344:873–80.
26. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E,et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–53.
27. Abraham WT, Young JB, León AR, Adler S, Bank AJ, Hall SA,et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverterdef i brillator, and mildly symptomatic chronic heart failure.Circulation 2004;110:2864–8.
28. Higgins S L, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol 2003;42:1454–9.
29. Linde C, Abraham WT, Gold MR,St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 2008;52:1834–43.
30. Moss AJ, Hall WJ, Cannom DS,Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–38.
31. Tang AS, Wells GA, Talajic M,Arnold MO, Sheldon R, Connolly S, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010;363:2385–95.
32. Lane RE, Chow AW, Chin D,Mayet J. Selection and optimisation of biventricular pacing: the role of echocardiography. Heart 2004;90 Suppl 6:vi10–6.
33. Jansen AH, Bracke FA, van Dantzig JM, Meijer A, van der Voort PH, Aarnoudse W, et al. Correlation of echo-Doppler optimization of atrioventricular delay in cardiac resynchronization therapy with invasive hemodynamics in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2006;97:552–7.
34. Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, et al.Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation 1999;99:2993–3001.
35. Mortensen PT, Sogaard P, Mansour H, Ponsonaille J, Gras D, Lazarus A, et al. Sequential biventricular pacing: evaluation of safety and eff i cacy. Pacing Clin Electrophysiol 2004;27:339–45.
36. Stockburg er M, Fateh-Moghadam S, Nitardy A, Langreck H,Haverkamp W, Dietz R. Optimization of cardiac resynchronization guided by Doppler echocardiography: haemodynamic improvement and intraindividual variability with different pacing conf i gurations and atrioventricular delays. Europace 2006;8:881–6.
37. Sawhney N S, Waggoner AD,Garhwal S, Chawla MK, Osborn J, Faddis MN. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm 2004;1:562–7.
38. Gras D, M abo P, Tang T, Luttikuis O, Chatoor R, Pedersen AK, et al.Multisite pacing as a supplemental treatment of congestive heart failure: preliminary results of the Medtronic Inc. InSync Study. Pacing Clin Electrophysiol 1998;21:2249–55.
39. McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C,Dryden DM, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. J Am Med Assoc 2007;297:2502–14.
40. León AR, Abraham WT, Curtis AB,Daubert JP, Fisher WG, Gurley J,et al. Safety of transvenous cardiac resynchronization system implantation in patients with chronic heart failure: combined results of over 2,000 patients from a multicenter study program. J Am Coll Cardiol 2005;46:2348–56.
41. Fish JM, B rugada J, Antzelevitch C. Potential proarrhythmic effects of biventricular pacing. J Am Coll Cardiol 2005;46:2340–7.
42. Lunati M, Gasparini M, Santini M, Landolina M, Perego GB, Pappone C, et al. Follow-up of CRTICD: implications for the use of remote follow-up systems. Data from the InSync ICD Italian Registry. Pacing Clin Electrophysiol 2008;31:38–46.
43. Crossley G H, Mead H, Kleckner K, Sheldon T, Davenport L, Harsch MR, et al. Automated left ventricular capture management. Pacing Clin Electrophysiol 2007;30:1190–200.
44. Leclercq C, Gras D, Le Helloco A, Nicol L, Mabo P, Daubert C.Hemodynamic importance of preserving the normal sequence of ventricular activation in permanent cardiac pacing. Am Heart J 1995;129:1133–41.
45. Birnie DH, Tang AS. The problem of non-response to cardiac resynchronization therapy. Curr Opin Cardiol 2006;21:20–6.
46. Yu CM, Bleeker GB, Fung JW,Schalij MJ, Zhang Q, van der Wall EE, et al. Left ventricular reverse remodeling but not clinical improvement predicts longterm survival after cardiac resynchronization therapy. Circulation 2005;112:1580–6.
47. Wu AH, Smith A, Wieczorek S,Mather JF, Duncan B, White CM,et al. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol 2003;92:628–31.
48. Thomas DE, Yousef ZR, Fraser AG.A critical comparison of echocardiographic measurements used for optimizing cardiac resynchronization therapy: stroke distance is best.Eur J Heart Fail 2009;11:779–88.
49. Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart 2003;89:54–60.
50. Achilli A, Sassara M, Ficili S,Pontillo D, Achilli P, Alessi C, et al.Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol 2003;42:2117–24.
51. Yu CM, Chan YS, Zhang Q, Yip GW, Chan CK, Kum LC, et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol 2006;48:2251–7.
52. Foley PW, Patel K, Irwin N,Sanderson JE, Frenneaux MP,Smith RE, et al. Cardiac resynchronisation therapy in patients with heart failure and a normal QRS duration: the RESPOND study.Heart 2011;97:1041–7.
53. Thibault B, Harel F, Ducharme A,White M, Ellenbogen KA, Frasure-Smith N, et al. Cardiac resynchronization therapy in patients with heart failure and a QRS complex<120 milliseconds: the Evaluation of Resynchronization Therapy for Heart Failure (LESSER-EARTH)trial. Circulation 2013;127:873–81.
54. Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J,et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395–405.
55. Sipahi I, Carrigan TP, Rowland DY, Stambler BS, Fang JC. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch Intern Med 2011;171:1454–62.
56. Zareba W, Klein H, Cygankiewicz I,Hall WJ, McNitt S, Brown M, et al.Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Def i brillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011;123:1061–72.
57. Ritter P, Padeletti L, Gillio-Meina L, Gaggini G. Determination of the optimal atrioventricular delay in DDD pacing. Comparison between echo and peak endocardial acceleration measurements. Europace 1999;1:126–30.
58. Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013;368:1585–93.
59. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP,Hsia H, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable def i brillator: the Dual Chamber and VVI Implantable Def i brillator(DAVID) trial. J Am Med Assoc 2002;288:3115–23.
60. Sweeney MO, Hellkamp AS,Ellenbogen KA, Greenspon AJ,Freedman RA, Lee KL, et al.Adverse effect of ventricular pacing on heart failure and atrial fi brillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7.
61. Singh JP, Klein HU, Huang DT,Reek S, Kuniss M, Quesada A, et al.Left ventricular lead position and clinical outcome in the Multicenter Automatic Def i brillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) trial.Circulation 2011;123:1159–66.
62. Saba S, Marek J, Schwartzman D,Jain S, Adelstein E, White P, et al.Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region trial. Circ Heart Fail 2013;6:427–34.
63. Khan FZ, Virdee MS, Palmer CR,Pugh PJ, O‘Halloran D, Elsik M, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized,controlled trial. J Am Coll Cardiol 2012;59:1509–18.
64. Leyva F, Foley PW, Chalil S,Ratib K, Smith RE, Prinzen F,et al. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2011;13:29.
65. Garrigue S, Jaïs P, Espil G, Labeque JN, Hocini M, Shah DC, et al.Comparison of chronic biventricular pacing between epicardial and endocardial left ventricular stimulation using Doppler tissue imaging in patients with heart failure. Am J Cardiol 2001;88:858–62.
66. Auricchio A, Delnoy PP, Butter C, Brachmann J, Van Erven L,Spitzer S, et al. Feasibility, safety,and short-term outcome of leadless ultrasound-based endocardial left ventricular resynchronization in heart failure patients: results of the Wireless Stimulation Endocardially for CRT (WiSE-CRT) study.Europace 2014;16:681–8.
67. Leclercq C, Gadler F, Kranig W,Ellery S, Gras D, Lazarus A, et al.A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure. J Am Coll Cardiol 2008;51:1455–62.
68. Kerlan JE, Sawhney NS, Waggoner AD, Chawla MK, Garhwal S, Osborn JL, et al. Prospective comparison of echocardiographic atrioventricular delay optimization methods for cardiac resynchronization therapy.Heart Rhythm 2006;3:148–54.
69. Kamdar R, Frain E, Warburton F,Richmond L, Mullan V, Berriman T, et al. A prospective comparison of echocardiography and device algorithms for atrioventricular and interventricular interval optimization in cardiac resynchronization therapy. Europace 2010;12:84–91.
70. Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, et al. Primary results from the SmartDelay Determined AV Optimization: a comparison to Other AV Delay Methods Used in Cardiac Resynchronization Therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation 2010;122:2660–8.
71. Martin DO, Lemke B, Birnie D,Krum H, Lee KL, Aonuma K,et al. Investigation of a novel algorithm for synchronized leftventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm 2012;9:1807–14.
72. Detsky AS, Naglie IG. A clinician’s guide to cost-effectiveness analysis. Ann Intern Med 1990;113:147–54.
73. Feldman AM, de Lissovoy G, Bristow MR, Saxon LA, De Marco T,Kass DA, et al. Cost effectiveness of cardiac resynchronization therapy in the Comparison of Medical Therapy, Pacing, and Def i brillation in Heart Failure (COMPANION) trial. J Am Coll Cardiol 2005;46:2311–21.
74. Calvert MJ, Freemantle N, Yao G,Cleland JG, Billingham L, Daubert JC, et al. Cost-effectiveness of cardiac resynchronization therapy:results from the CARE-HF trial.Eur Heart J 2005;26:2681–8.
75. Linde C, Mealing S, Hawkins N,Eaton J, Brown B, Daubert JC. Costeffectiveness of cardiac resynchronization therapy in patients with asymptomatic to mild heart failure:insights from the European cohort of the REVERSE (Resynchronization Reverses remodeling in Systolic Left Ventricular Dysfunction).Eur Heart J 2011;32:1631–9.
76. Noyes K, Veazie P, Hall WJ,Zhao H, Buttaccio A, Thevenet-Morrison K, et al. Cost-effectiveness of cardiac resynchronization therapy in the MADIT-CRT trial. J Cardiovasc Electrophysiol 2013;24:66–74.
77. Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 guidelines for devicebased therapy of cardiac rhythm abnormalities: executive summary.Heart Rhythm 2008;5:934–55.
78. Tracy CM, Epstein AE, Darbar D,DiMarco JP, Dunbar SB, Estes NA 3rd, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm 2012;9:1737–53.
79. Adelstein EC, Saba S. Usefulness of baseline electrocardiographic QRS complex pattern to predict response to cardiac resynchronization. Am J Cardiol 2009;103:238–42.
80. Bilchick KC, Kamath S, DiMarco JP,Stukenborg GJ. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients.Circulation 2010;122:2022–30.
81. Rickard J, Kumbhani DJ,Gorodeski EZ, Baranowski B,Wazni O, Martin DO, et al. Cardiac resynchronization therapy in nonleft bundle branch block morphologies. Pacing Clin Electrophysiol 2010;33:590–5.
 Cardiovascular Innovations and Applications2015年4期
Cardiovascular Innovations and Applications2015年4期
- Cardiovascular Innovations and Applications的其它文章
- Strategies to Reduce Heart Failure Hospitalizations and Readmissions:How Low Can We Go?
- Congestive Heart Failure Clinics: How to Make Them Work in a Community-Based Hospital System
- Cardiac Sarcoidosis: Sorting Fact from Fiction in This Rare Cardiomyopathy
- Unusual Cardiomyopathies: Some May Be More Usual Than Previously Thought and Simply Underdiagnosed
- Epidemiological Study of Heart Failure in China
- Noninvasive Hemodynamic Monitoring for Heart Failure: A New Era of Heart Failure Management
