Momordica charantia Extract Protects C57BL/6j Mice from High-Fat-Diet-Induced Obesity and Insulin Resistance*
,,,
(1. National Pharmaceutical Engineering Center for Solid Preparation in Chinese Herbal Medicine, Jiangxi University of Traditional Chinese Medicine, Nanchang 330006, China; 2. Department of Pharmacy, Puyang Maternaity and Child Care Hospital, Kaizhou south road, Puyang 457000, China; 3. Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou 510632, China; 4. Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Guangzhou 510632, China)
Momordica charantia Extract Protects C57BL/6j Mice from High-Fat-Diet-Induced Obesity and Insulin Resistance*
WANGRikang1,ZHAOTingting2,SUNYongbing1,CHENHeru3,4
(1. National Pharmaceutical Engineering Center for Solid Preparation in Chinese Herbal Medicine, Jiangxi University of Traditional Chinese Medicine, Nanchang 330006, China; 2. Department of Pharmacy, Puyang Maternaity and Child Care Hospital, Kaizhou south road, Puyang 457000, China; 3. Institute of Traditional Chinese Medicine and Natural Products, College of Pharmacy, Jinan University, Guangzhou 510632, China; 4. Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, Guangzhou 510632, China)
In order to investigate the protective effects ofMomordicacharantiaextract (MCE) on obesity and insulin resistance, C57BL/6j mice fed in a high-fat diet (HFD) were used as animal model. Obese mouse model was successfully made after 2-week HFD induction. The mice were then randomly divided into normal control, model control, MCE dose of 13.5, 27.0, and 54.0 g/kg treated groups. Mice in normal and model control groups were treated with 0.5% CMC-Na, and other groups were treated with MCE (i.g.), respectively, with the same volume twice a day for ten weeks. It was shown that the body weight, epididymal and total visceral white adipose tissue weight were decreased by treatment of MCE 54.0 g/kg without the change of food intake. The increased serum TG, CHO, LDL-C, glucose, insulin concentration and insulin resistance index were significantly inhibited. The concentration of TG was also decreased by the treatment of MCE 27.0 g/kg; while the concentration of HDL-C was increased by the treatment of MCE 13.5, 27.0 and 54.0 g/kg, respectively. The adipocyte hypertrophy induced by HFD was mitigated by MCE dose-dependently.
MCE; high-fat diet; obesity; insulin resistance
Obesity results from chronic imbalance between energy intake and expenditure, and is characterized by over 30 kg/m2Body Mass Index (BMI), which is the most widely used measure for determine the prevalence of obestiy[1]. Overweight and obesity are the fifth leading cause of global deaths, accounting for at least 2.8 million adults’ deaths annually. And the alarming prevalence of childhood obesity is of particularly noteworthy since 1970s. The prevalence of obesity has been more than doubled for children and nearly 43 million children under the age of five were outweight in 2010. The obesity pandemic, if left out of control, will reach unprecedented proportions in 2050 with 165 million adults in the USA[2].
The global increase in prevalence of obesity has led to an increased need for the treatment of obese persons. It is well known that both diet and exercise are best for prevention and treatment. However, both ways require self-discipline and persistence. Another possible adjunct is drug treatment. But it may have only modest effects, accompanied by side effects and potential for drug abuse. Furthermore, the weight lowering effect lasts as long as the period when the drug is being taken. Unfortunately, as soon as the administration is stopped, the weight is regained[3-4]. Interestingly, in the Eastern world including Chian, there are some alternative therapies having been used, which herbal medicine is involved[5-6].
Momordicacharantia, also is referred to as bitter melon or bitter gourd, is a popular vegetable as well as an herb in China. It has been used as an herb for at least 600 years in South China and nowadays widely cultivated in Asia[7], Africa and South America. Bitter melon is received widespread attention in the scientific community due to its beneficial effects such as anti-diabetic, anti-cancer and anti-inflammatory[8-11]. Although various parts (roots, stems, leaves and fruits) ofMomordicacharantiaare used traditionally, studies have shown that the fruit extract ofMomordicacharantiahas potent hypoglycaemic properties[12-13]. It has been reported that juice extract of freshMomordicacharantiafruit without seeds could reduce adiposity, lower serum insulin, improve insulin sensitivity, and normalize glucose tolerance in rats fed with a high-fat-diet (HFD)[14-16]. Lyophilised power ofMomordicacharantiafruit including seeds could reduce insulin resistance and inhibit adipocyte hypertrophy in diet-induced obese (DIO) rats[17]. Recentinvitrostudies showed that juice of immatureMomordicacharantiacould inhibit primary human adipocyte differentiation[18]. Moreover, 95% ethanol extract ofMomordicacharantiaseedless fruit could depressed blood glucose in streptozotocin-diabetic rats[19]. However the effects of ethanol extract ofMomordicacharantiafruit on obesity and insulin resistance in diet-induced obese mice has not been reported yet.
Considering that C57BL/6j mice are very susceptible to diet-induced obestiy[20], the present study was designed to develop this animal model to examine the anti-obesity and anti-diabetic effects ofMomordicacharantiain C57BL/6j mice fed in a high-fat diet.
1 Materials and methods
1.1 Chemicals and Reagents
Serum triglyceride (TG), cholesterol (CHO) and glucose test kits were purchased from Roche Company (Shanghai, China); low density lipoprotein cholestrerl (LDL-C) and high density lipoprotein cholesterol (HDL-C) test kits were purchased from Leadman Company (Beijing, China); Insulin (mouse) ultrasensitive ELISA kits was purchased form ALPCO Company (America); Haematoxylin and eosin solution were purchased from Baso Company (Guangzhou, China). Other chemicals were of reagent grade.
1.2 Preparation ofMomordicacharantiaextract (MCE)
ImmatureMomordicacharantiafresh fruit was purchased from a local market, and was throughly washed with water and dried at room temperature. 45 kg of fruit was then cutted into slice and extracted with 360 L ethanol/water (70∶30,V/V) for 2 h. After filtration, the fruit was re-extrated with 270 L ethanol/water (70∶30,V/V) for 1.5 h, and filtered again, all the filtrates were combined and subjected to vacuum evaporation. The resulted gummy residues were weighted and stored at 4 ℃. Before using, it was diluted and adjusted with 0.5% CMC-Na to 1.8, 0.9 and 0.45 g (crude drug)/mL.
1.3 Animals and diets
Male C57BL/6j mouse (11~13 g) were obtained from Beijing HFK Bioscience Co., Ltd (Beijing, China). Mice were group-housed in standard individual ventilated cages with wood shavings as litter. The cages were a controlled environment an temperature (22±1) ℃ and humidity 45%~55% with a 12 h /12 h modified dark-light cycle. There were four mice in each cage. Food and water were availableadlibitum. Animal care and use were conducted with the approval of Shenyang Pharmaceutical University Institutional Laboratory Animal Care and Use Committee. All efforts were made to minimize pain and suffering and to reduce the number of animals used. After adaption for 3 days, the mice were assigned into low-fat diet group and high-fat diet group. The compositions of low-fat diet and high-fat diet were shown in Table 1. The mice in high-fat diet group were induced obestiy (diet-induced obesity, DIO) after 2 weeks (weeks -2 to -1), and then separated into high-fat diet control group (HFD-Control) and MCE 13.5, 27.0, 54.0 g/kg groups (indicated as HFD-13.5MCE, HFD-27MCE, and HFD-54MCE, respectively). The mice in low-fat diet group were separated into low-fat diet control group (LFD-Control) and MCE 54.0 g/kg groups (LFD-54MCE) as well. The mice in MCE groups were given intragastrically with MCE; and mice in control groups (LFD-Control and HFD-Control) were given 0.5% CMC-Na with the same volume (15 mL/kg) twice a day for ten weeks. The dietary was also continued.

Table 1 Composition of tested diets
The food intake of mice in each cage was recorded daily and body weight of mice were measured weekly throughout the study.
1.4 Collection of serum and adipose tissue
After 10 week of treatment, mice were fasted for 20 h. After the blood was removed, the mice were killed by decapitation. The white adipose tissues (WATs, including epididymal, mesentery, and perirenal WAT) were dissected according to the dened anatomical landmarks. The weights of tissues were measured. Visceral fat was dened as the sum of epididymal, mesentery, and perirenal WAT. They were then fixed in 4% buffered formalin until use. The collected blood was kept at room temperature for 10 min for coagulation. Then, the serum was obtained from the coagulated blood by centrifugation at 2 000 r/min for 15 min at 4 ℃. The separation of the serum wasnished within 30 min. The serum was immediately frozen at -80 ℃ until analysis.
1.5 Measurement of serum lipid, glucose and insulin levels
The serum TG, CHO, LDL-C, HDL-C and glucose concentrations were measured using commercial assay kits according to the manufacturer’s directions with MODULAR P800 automatic biochemistry analyzer (Roche, Germany). Serum insulin levels were measured by ELISA using a commercial assay kit according to manufacturer’s directions, the OD value was readed at 450 nm with Synergy HT multifunctional microplate reader (Bio-tek, America). The degree of insulin resistance was estimated by a homeostasis assessment model (HOMA-IR), which was calculated according to the formula: HOMA-IR = serum glucose (mmol/L) ( serum insulin (mU/L)/22.5[21].
1.6 Histological analysis of WAT
Epididymal adipose tissues from the mice were embedded in parafn. Standard sections of 4-μm thickness were cut with Leica RM2245 manual rotary slicer (Shanghai, China), and stained with hematoxylin and eosin. which was viewed under an inverted optical microscope (Olympus IX71,Japan) and photographed at anal magnication of 400×.
1.7 Statistical analysis
Data are expressed as the mean±SEM. The statistical signicance of differences between the mean values for the treatment groups was analyzed with one-way or two-way analysis of Variance (ANOVA) followed by Dunnett-tests using the software SPSS 13.0 (Chicago, USA).P< 0.05 was considered statistically significantly.
2 Results
2.1 Effects of the MCE on body weight and food intake
The weekly body weights and food intakes of mice were shown in Fig.1 and Fig.2. The body weight of the HFD-control group was signicantly increased compared with the LFD-control group during MCE treatment after DIO except at eighth week. The body weight of the HFD-54MCE group was signicantly decreased compared with the HFD-Control group at ninth and tenth week after MCE treatment. However, there was no signicant difference in the body weight of the HFD-27MCE and HFD-13.5MCE group compared with the HFD-Control group (Fig.1). During the MCE treatment period, the food intake did not differ signicantly among all the groups except those mice in HFD-54MCE group, which ate less than those in HFD-Control group at second week after MCE treatment (Fig.2).

Fig.1 Effect of MCE on body weight of mice after ten weeks treatmentLFD: low-fat diet; HFD: high-fat diet; MCE: Momordica charantia extract. Data are expressed as the mean±SEM (n=8~12).#-P < 0.05,##P < 0.01 vs LFD-Control group;*P < 0.05,**P < 0.01 vs HFD-Control group.
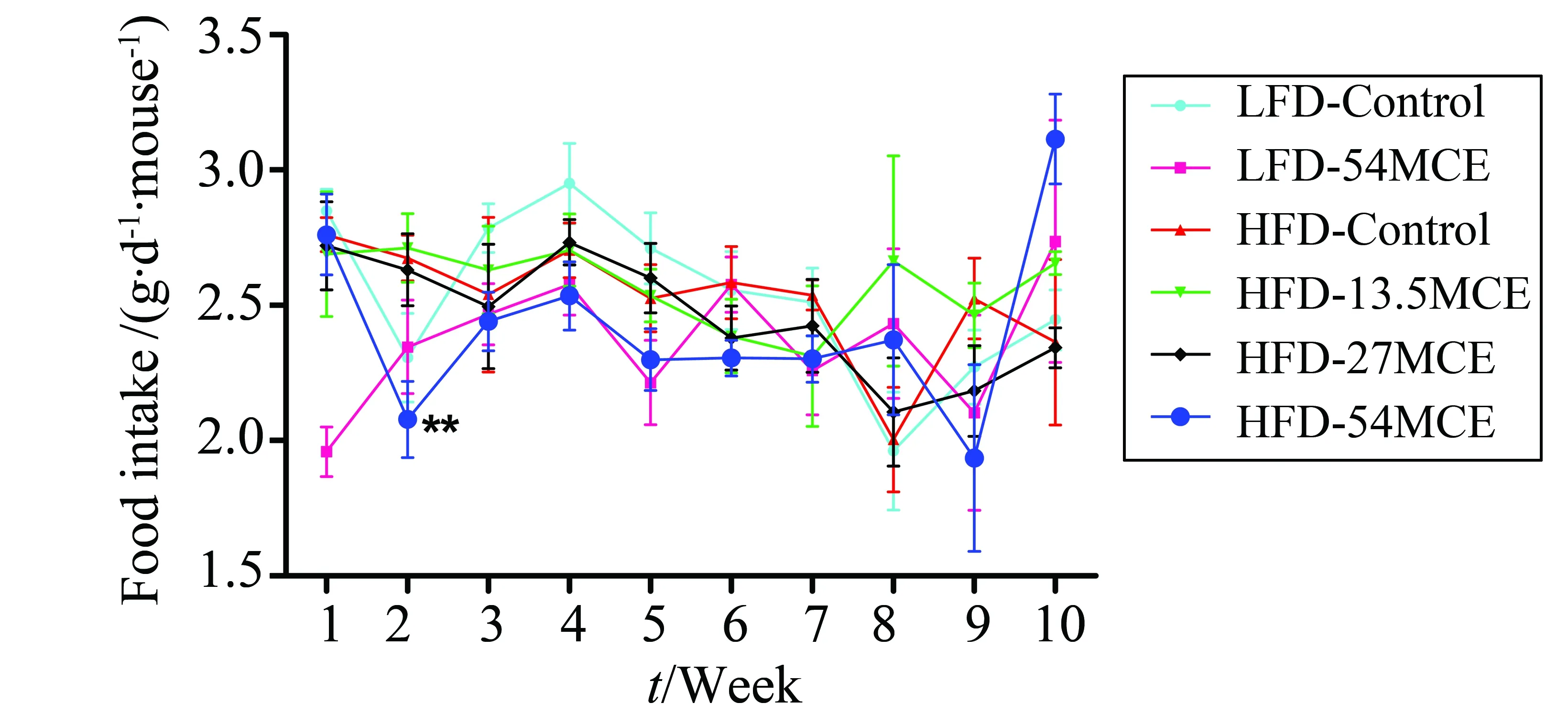
Fig.2 Effect of MCE on food intake of mice after ten weeks treatment LFD: low-fat diet; HFD: high-fat diet; MCE: Momordica charantia extract. Data are expressed as the mean±SEM (n=8~12).**P < 0.01 vs HFD-control group.
2.2 Effects of the MCE on the WAT weights
The mesentery, perirenal and epididymal WAT weights were shown in Fig.3. There were no signicant differences in mesentery WAT weight among the six groups. The perirenal, epididymal and total visceral WAT weights were signicantly increased in the HFD-Control group compared with the LFD-control group. However, the epididymal and total visceral WAT weights were decreased signifgicantly after treatement with MCE 54 g/kg for ten weeks, but treatement with MCE 13.5 and 27 g/kg had no obvious effect on WAT weights.
2.3 Effects of the MCE on the serum biochemical parameters
The serum biochemical parameters were shown in Table 2. The signicantly increased serum TG, CHO, LDL-C, glucose, and insulin levels, and decreased HDL-C were observed in the HFD-control group compared with the LFD-control group. The serum TG, CHO, LDL-C, glucose and insulin levels were signicantly reduced in the HFD-54MCE group compared with the HFD-control group, but not signicantly in the HFD-13.5MCE and HFD-27MCE group. However, HDL-C levels was restored in all the MCE-treated groups. Although there were no significant difference on HOMA-IR between HFD-control group and LFD-control group, the HOMA-IR in HFD-54MCE group was decreased markedly compared with HFD-Control group. Moreover, the concentration of LDL-C in serum was significantly decreased in LFD-54MCE group compared with LFD-Control group.

Fig.3 Effect of MCE on visceral white adipose tissue weight of mice after ten weeks treatment LFD: low-fat diet; HFD: high-fat diet; MCE: Momordica charantia extract. Data are expressed as the mean±SEM (n=8~12).#P < 0.05,##P < 0.01, compared with LFD-control group;***P < 0.001, compared with HFD-control group.
2.4 Histological analysis of the epididymal WAT
The sizes of the epididymal adipocytes in each groups were shown in Fig.4. The sizes of the adipocytes were signicantly larger in the HFD-control group than those in the LFD-control group. Interestingly, after treatment with MCE 54 g/kg for ten weeks, the hypertrophy of epididymal adipocytes were considerably
1)LFD: low-fat diet; HFD: high-fat diet; MCE:Momordicacharantiaextract. Data are expressed as the mean±SEM (n= 8~12).#P< 0.05,##P< 0.01,###P< 0.001 vs LFD-Control group;*P< 0.05,**P< 0.01,***P< 0.001 vs HFD-Control group.

Fig.4 Representative histological section of epididymal white adipose tissue, stained with haemotoxylin and eosin, from each of the six treatment groupsScale bar: 100 microns; LFD: low-fat diet; HFD: high-fat diet; MCE: Momordica charantia extract.
decreased compared with the HFD-control group, but the effects of MCE 13.5 and 27 g/kg were not as signicant as those of MCE 54 g/kg.
3 Discussion
The present study demonstrated that MCE reduced body weight and visceral white adipose tissue weight without affecting food intake and controlled hyperlipidemia, hyperglycemia and hyperinsulinemia by significantly decreasing serum lipids, glucose and insulin levels in C57BL/6j mice fed in a high-fat diet. Moreover MCE could inhibit proliferation and hypertrophy of adipocyte in epididymal adipose tissue.
C57BL/6j mice are preferred to use in many anti-obesity experiments for its susceptibility to diet-induced obesity. Some studies have shown that obesity was induced within 4 weeks after introduction of HFD to C57BL/6j mice[22-24]. However, in the present study, obesity was induced after fedding in HFD for two weeks. The inhibitory effects ofMomordicacharantiaon adiposity has been reportedinvivoandinvitro. Adiposity was reduced in rats fed with HFD when 0.75% or higher freeze-driedMomordicacharantiajuice supplemented into the diet for 15 weeks[15]. Adipocyte hypertrophy and lipogenic gene expression were inhibited in diet-induced obese rats when 5% lyophilisedMomordicacharantiapowder supplemented into the diet for 9 weeks[17]. Visceral obesity and body weight gain were inhibited in mice on high-fat diet after treatment with P and G fraction extracts ofMomordicacharantiafor 4 weeks[9]. The differentiation of primary human adipocyte was inhibited byMomordicacharantiajuice[18]. In accordance with these results, after 10 weeks treatment with 54 g/kg of ethanol extract ofMomordicacharantia, the body weight and visceral white adipose tissue weight were decreased in HFD mice.
Obesity is a chronic metabolic disorder characterized by increased fat accumulation, such as increases in the cell number and/or cell size in adipose tissue and elevated lipid concentrations in blood[25]. The increased concentrations of TG, CHO and LDL-C and hypertrophic adipocyte in epididymal adipose tissue were observed in HFD mice in our study. Interestingly, treatment with 54 g/kg ethanol extract ofMomordicacharantiacould normalize the concentrations of TG and LDL-C and the size of adipocyte in epididymal adipose tissue and significantly decreased the conecetration of CHO. In agreement with our results, decreased serum TG, CHO and LDL-C and increased serum HDL-C were reported after administration ofMomordicacharantiaextrat in diabetic rats[26-28]. Furthermore, our results shown that the concentration of HDL-C was decreased in HFD-Control group, and was restored by the treatments of MCE 13.5, 27.0 and 54.0 g/kg, respectively.
Recent advances have shown that adipose tissue not only stores excess energy in the form of fat but also is a critical endocrine organ that is innately involved in regulating obesity and other metabolic processes. For example, resistin was considered to be a mechanistic mediator form adipocytes to insulin resistance[18, 29]. Insulin resistance is the salient feature of type 2 diabetes mellitus. Insulin resistance occurs when normal circulating concentrations of the hormone fail to regulate body glucose homeostasis[30]. Since insulin resistance is a major metabolic abnormality of T2D, there has been considerable interest in insulin-sensitizing agents to counteract insulin resistance for the treatment of this disease[31]. The present study proved thatMomordicacharantiawas effective to decrease the serum concentration of glucose and insulin and to improve insulin resistance in a diet-induced obese mice model. A substantial number of reports indicated thatMomordicacharantiawas able to exert a hypoglycemic effects in a variety of animal models, such as STZ-induced diabetic mice, KK-Ay mice and alloxan-induced rats, the related mechanisms included protection of β-cells, increase of glycogen content, enhancement of GLUT4 translocation and inhibition of gluconeogenesis[19, 32-34]. The decreased visceral white adipose weight might be another mechanism responsible for amelioration of insulin resistance, for some substance secreted from adipocytes might be reduced, such as leptin or resistin, but this was needed further investigation.
InMomordicacharantia, there exist many active chemicals including saponins, alkaloids, fixed oils, triterpenoids, steriods, polypeptides, glycosides, carotenoids, flavanoids and polyphenols[35-39]. Quercetin and gallic acid, two kinds of polyphenols contained inMomordicacharantia, have been demonstrated to inhibit adipogenesis and induce apoptosis in 3T3-L1 mouse adipocytes[40-41]. The hypoglycemic effects ofMomordicacharantiamight result from saponins known as charantin, alkaloids known as glycoalkaloid, and triterpenoids known as momordicoside[39,42]. The polyphenols and flavanoids inMomordicacharantiabeside quercetin and gallic acid have been displayed to inhibit obesity and metalism disorderinvivoorvitro[43-46]. Which components are responsible for the anti-obesity and anti-diabetic effects in the present investigation remains unknown.
Some clinical trials about hypoglycemic effects ofMomordicacharantiaextract have been carried out in type 2 diabetes[47-51], but the controversial results are reported too[52]. Despite the need for more information in randomised controlled trails, but no serious adverse effects on humans have been reported until now. However, long term studies testing the effects ofMomordicacharantiaextract on body weight, glucose and lipid metabolism as well as identifying the pharmacokinetics and effective dose ofMomordicacharantiaextract is warranted, before it can be recommended as an effective alternative and/or complementary therapy.
In conclusion, MCE signifcantly decreased body weight and serum lipids, improved insulin resistance and inhibited visceral adipose tissue accumulation and hypertrophy of epididymal adipocyte in HFD mice. All these results suggested that MCE may serve as an alternative therapy for obesity and diabetes.
[1] ROMERO-CORRAL A, SOMERS V K, SIERRA-JOHNSON J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population[J]. Int J Obes, 2008, 32: 959-966.
[2] SECORD A A, GEHRIG P A. Obesity: Too big a problem to ignore[J]. Gynecol Oncol, 2012, 126(2): 274-276.
[4] FUJIOKA K. Management of obesity as a chronic disease: Nonpharmacologic, pharmacologic, and surgical options[J]. Obes Res, 2002, 10(2): 116-123.
[5] HASANI-RANJBAR S, LARIJANI B, ABDOLLAHI M. A systematic review of Iranian medicinal plants useful in diabetes mellitus[J]. Arch Med Sci, 2008, 4(3): 285-292.
[6] LIU J P, ZHANG M, WANG W, et al. Chinese herbal medicines for type 2 diabetes mellitus[J]. Cochrane Database Syst Rev, 2002: CD003642.
[7] YIN J, ZHANG H, YE J. Traditional chinese medicine in treatment of metabolic syndrome[J]. Endocr Metab Immune Disord Drug Targets, 2008, 8(2): 99-111.
[8] ABASCAL K, YARNELL E. Using Bitter Melon to treat diabetes[J]. Altern Complement Ther, 2005, 11(4): 179-184.
[9] SHIH C C, LIN C H, LIN W L. Effects ofMomordicacharantiaon insulin resistance and visceral obesity in mice on high-fat diet[J]. Diabetes Res Clin Pract, 2008, 81(2): 134-143.
[10] NERURKAR P V, JOHNS L M, BUESA L M, et al.Momordicacharantia(bitter melon) attenuates high-fat diet-associated oxidative stress and neuroinflammation[J]. J Neuroinflammation, 2011, 8(1): 64-83.
[11] AKIHISA T, HIGO N, TOKUDA H, et al. Cucurbitane-type triterpenoids from the fruits ofMomordicacharantiaand their cancer chemopreventive effects[J]. J Nat Prod, 2007, 70(8): 1233-1239.
[12] YIBCHOK-ANUN S, ADISAKWATTANA S, YAO C Y, et al. Slow acting protein extract from fruit pulp ofMomordicacharantiawith insulin secretagogue and insulinomimetic activities[J]. Biol Pharm Bull, 2006, 29(6): 1126-1131.
[13] MIURA T, ITOH Y, IWAMOTO N, et al. Suppressive activity of the fruit ofMomordicacharantiawith exercise on blood glucose in type 2 diabetic mice[J]. Biol Pharm Bull, 2004, 27(2): 248-250.
[14] SRIDHAR M G, VINAYAGAMOORTHI R, SUYAMBUNATHAN V A, et al. Bitter gourd (Momordicacharantia) improves insulin sensitivity by increasing skeletal muscle insulin-stimulated IRS-1 tyrosine phosphorylation in high-fat-fed rats[J]. Br J Nutr, 2008, 99(4): 806-812.
[15] CHEN Q X, CHAN L L Y, LI E T S. Bitter Melon (Momordicacharantia) reduces adiposity, lowers serum insulin and normalizes glucose tolerance in rats fed a high fat diet[J]. J Nutr, 2003, 133(4): 1088-1093.
[16] CHEN Q X, LI E T S. Reduced adiposity in bitter melon (Momordicacharantia) fed rats is associated with lower tissue triglyceride and higher plasma catecholamines[J]. Br J Nutr, 2005, 93(5): 747-754.
[17] HUANG H L, HONG Y W, WONG Y H, et al. Bitter melon (MomordicacharantiaL.) inhibits adipocyte hypertrophy and down regulates lipogenic gene expression in adipose tissue of diet-induced obese rats[J]. Br J Nutr, 2008, 99(2): 230-239.
[18] NERURKAR P V, LEE Y K, NERURKAR V R.Momordicacharantia(bitter melon) inhibits primary human adipocyte differentiation by modulating adipogenic genes[J]. BMC Complementary Altern Med, 2010, 10(34): 1-10.
[19] SHIBIB B A, KHAN L A, RAHMAN R. Hypoglycaemic activity ofCocciniaindicaandMomordicacharantiain diabetic rats: depression of the hepatic gluconeogenic enzymes glucose-6-phosphatase and fructose-1,6-bisphosphatase and elevation of both liver and red-cell shunt enzyme glucose-6-phosphate dehydrogenase[J]. Biochem J, 1993, 292: 267-270.
[20] LIN S, THOMAS T C, STORLIEN L H, et al. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice[J]. International Journal of Obesity, 2000, 24(5): 639-646.
[21] PRIOR R L, WILKES S, ROGERS T, et al. Dietary black raspberry anthocyanins do not alter development of obesity in mice fed an obesogenic high-fat diet[J]. J Agric Food Chem, 2010, 58: 3977-3983.
[22] YAO Y, LI X B, ZHAO W, et al. Anti-obesity effect of an isoflavone fatty acid ester on obese mice induced by high fat diet and its potential mechanism[J]. Lipids in Health and Disease, 2010, 9(49): 1-12.
[23] SHIMIZU K, IDA T, TSUTSUI H, et al. Anti-obesity effect of phosphatidylinositol on diet-induced obesity in mice[J]. J Agric Food Chem, 2010, 58: 11218-11225.
[24] JAYAPRAKASAM B, OLSON L K, SCHUTZKI R E, et al. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in cornelian cherry (Cornusmas)[J]. J Agric Food Chem, 2006, 54: 243-248.
[25] LEE Y S, CHA B Y, SAITO K, et al. Effects of aCitrusdepressaHayata (Shiikuwasa) extract on obesity in high-fat diet-induced obese mice[J]. Phytomedicine, 2011, 18(8/9): 648-654.
[26] CHATURVEDI P, GEORGE S, MILINGANYO M, et al. Effect ofMomordicacharantiaon lipid prole and oral glucose tolerance in diabetic rats[J]. Phytother Res, 2004, 18(11): 954-956.
[27] FERNANDES N P, LAGISHETTY C V, PANDA V S, et al. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardizedMomordicacharantiafruit extract[J]. BMC Complement Altern Med, 2007, 7: 29-37.
[28] AHMED I, LAKHANI M S, GILLETT M, et al. Hypotriglyceridemic and hypocholesterolemic effects of anti-diabeticMomordicacharantia(karela) fruit extract in streptozotocin-induced diabetic rats[J]. Diabetes Res Clin Pract, 2001, 51(3): 155-161.
[29] STEPPAN C M, BAILEY S T, BHAT S, et al. The hormone resistin links obesity to diabetes[J]. Nature, 2001, 409(18): 307-312.
[30] SHULMAN G I. Cellular mechanisms of insulin resistance[J]. J Clin Invest, 2000, 106(2): 171-176.
[31] MOLLER D E. New drug targets for type 2 diabetes and the metabolic syndrome[J]. Nature, 2001, 414(13): 821-827.
[32] RATHI S S, GROVER J K, VIKRANT V, et al. Prevention of experimental diabetic cataract by Indian Ayurvedic plant extracts[J]. Phytother Res, 2002, 16(8): 774-777.
[33] MIURA T, ITOH C, IWAMOTO N, et al. Hypoglycemic activity of the fruit of theMomordicacharantiain type 2 diabetic mice[J]. J Nutr Sci Vitaminol (Tokyo), 2001, 47(5): 340-344.
[34] SINGH N, TYAGI S D, AGARWAL S C. Effects of long term feeding of acetone extract ofMomordicacharantia(whole fruit powder) on alloxan diabetic albino rats[J]. Indian J Physiol Pharmacol, 1989, 33(2): 97-100.
[35] KHANNA P, JAIN S C, PANAGARIYA A, et al. Hypoglycemic activity of polypeptide-p from a plant source[J]. J Nat Prod, 1981, 44(6): 648-655.
[36] JANTAN I, RAFI I A, JALIL J. Platelet-activating factor (PAF) receptor-binding antagonist activity of Malaysian medicinal plants[J]. Phytomedicine, 2005, 12: 88-92.
[37] ANILA L, VIJAYALAKSHMI N R. Beneficial effects of flavonoids fromSesamumindicum,EmblicaofficinalisandMomordicacharantia[J]. Phytother Res, 2000, 14(8): 592-595.
[38] GROVER J K, YADAV S P. Pharmacological actions and potential uses ofMomordicacharantia: a review[J]. J Ethnopharmacol, 2004, 93(1): 123-132.
[39] RAMAN A, LAU C. Anti-diabetic properties and phytochemistry ofMomordicacharantiaL. (Cucurbitaceae)[J]. Phytomedicine, 1996, 2(4): 349-362.
[40] PARK H J, YANG J Y, AMBATI S, et al. Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes [J]. J Med Food, 2008, 11(4): 773-783.
[41] YANG J Y, DELLA-FERA M A, RAYALAM S, et al. Enhancedinhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin[J]. Life Sci, 2008, 82: 1032-1039.
[42] TAN M J, YE J M, TURNER N, et al. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway[J]. Chemistry & Biology, 2008, 15: 263-273.
[43] DUCHNOWICZ P, BRONCEL M, PODSDEK A, et al. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (invitrostudy)[J]. Eur J Nutr, 2012, 51: 435-443.
[44] BASARKAR P W, NATH N. Hypocholesterolemic and hypolipidemic activity of quercetin-a vitamin P-like compound in rats[J]. Indian J Med Res, 1983, 77: 122-126.
[45] MAKIHARA H, SHIMADA T, MACHIDA E, et al. Preventive effect ofTerminaliabelliricaon obesity and metabolic disorders in spontaneously obese type 2 diabetic model mice[J]. J Nat Med, 2012, 66(3): 459-467.
[46] WU C H, YANG M Y, CHAN K C, et al. Improvement in high-fat diet-induced obesity and body fat accumulation by aNelumbonuciferaleaf flavonoid-rich extract in mice[J]. J Agric Food Chem, 2010, 58:7075-7081.
[47] WELIHINDA J, KARUNANAYAKE E H, SHERIFF M H H, et al. Effect ofMomordicacharantiaon the glucosetolerance in maturityonset diabetes[J]. J Ethnopharmacol, 1986, 17(3): 277-282.
[48] LEATHERDALE B A, PANESAR R K, SINGH G, et al. Improvement in glucose tolerance due toMomordicacharantia(karela)[J]. Br Med J (Clin Res Ed), 1981, 282: 1823-1824.
[49] SRIVASTAVA Y, VENKATAKRISHNA-BHATT H, VERMA Y, et al. Antidiabetic and adaptogenic properties ofMomordicacharantiaextract: An experimental and clinical evaluation[J]. Phytother Res, 1993, 7(4): 285-289.
[50] BASCH E, GABARDI S, ULBRICHT C. Bitter melon (Momordicacharantia):A review of efficacy and safety[J]. Am J Health Syst Pharm, 2003, 60: 356-359.
[51] AHMAD N, HASSAN M R, HALDER H, et al. Effect ofMomordicacharantia(Karolla) extracts on fasting and postprandial serum glucose levels in NIDDM patients[J]. Bangladesh Med Res Counc Bull, 1999, 25(1): 11-13.
[52] DANS A M L, VILLARRUZ M V C, JIMENO C A, et al. The effect ofMomordicacharantiacapsule preparation on glycemic control in Type2 Diabetes Mellitus needs further studies[J]. J Clin Epidemiol, 2007, 60: 554-559.
2015-05-18 基金项目: 国家自然科学基金资助项目(81172982); 江西省自然科学基金资助项目(20151BAB215030)
王日康(1986年生), 男;研究方向:中药药理学; 通讯作者: 孙勇兵,陈河如;E-mail:1416747812@qq.com,thrchen@jnu.edu.cn
R961
A
0529-6579(2015)06-0006-08
苦瓜提取物对高脂肪喂食诱导的肥胖和胰岛抵抗C57BL/6j 小鼠保护作用研究
王日康1,赵婷婷2, 孙勇兵1, 陈河如3,4
(1. 江西中医药大学中药固体制剂制造技术国家工程研究中心, 江西 南昌 330006; 2. 河南省濮阳市妇幼保健院药学部, 河南 濮阳 457000; 3. 暨南大学药学院中药及天然药物研究所, 广东 广州 510632; 4. 广东省中药药效物质基础及创新药物研究重点实验室, 广东 广州510632)
通过高脂肪喂食诱导的C57BL/6j 小鼠动物模型研究苦瓜提取物对肥胖和胰岛抵抗的保护作用。小鼠喂养2周后成功建立肥胖小鼠模型, 随机分为正常对照组,肥胖小鼠模型组和不同浓度的苦瓜提取物灌胃组, 灌胃质量分数分别为13.5、27和54 g/kg。以10周为期,每天以同等体积流质灌胃两次,其中,正常对照组和模型组用w=0.5%羧甲基纤维素钠灌胃, 药物组用不同浓度的苦瓜提取物灌胃。记录小鼠每日食物摄入量和每周体质量。同时测定内脏白色脂肪组织质量、血清甘油三酯(TG)、胆固醇(CHO)、低密度脂蛋白胆固醇(LDL-C)、高密度脂蛋白胆固醇(HDL-C)、血糖和胰岛素水平,最后根据HOMA法计算胰岛素抵抗指数,HE染色确定附睾白色脂肪组织的脂肪细胞是否肥大。结果显示,54 g/kg 苦瓜提取物组对小鼠摄食无影响,但小鼠体质量、附睾和内脏白色脂肪组织总质量减少,该剂量的苦瓜提取物显著抑制被提升的血清TG、LDL-C、葡萄糖、胰岛素浓度和胰岛素抵抗指数;而27 g/kg苦瓜提取物则显著降低TG浓度;13.5、27和54 g/kg 苦瓜提取物分别显著升高HDL-C水平;苦瓜提取物能剂量依赖性地减轻高脂饮食诱导的脂肪细胞肥大。
苦瓜; 高脂喂食; 糖尿病; 胰岛素抵抗; 肥胖
10.13471/j.cnki.acta.snus.2015.06.002

