离子液体
热点追踪
离子液体
·编者按·
随着环境和能源相关问题的凸显,作为一种绿色功能材料和介质,离子液体的研究正得到越来越多的重视,成为绿色化学化工等领域的研究热点,其应用研究领域也已经从最初的化工领域扩展到功能材料、能源、资源环境、生命科学等领域。
离子液体(ionic liquids)是由正、负离子组成的,在室温或室温附近呈现液体状态的有机盐。相比固态物质它是液态,相比传统有机物它是离子型的。
离子液体的发现,最早可以追溯到1914年,Walden通过浓硝酸和乙胺反应制得发明了人类史上第一种离子液体:硝基乙胺(EtNH3)NO3,也是第一代离子液体的代表。20世纪90年代,诞生了以含(CF3SO2)2N-的咪唑类离子液体为代表的第二代离子液体,相比前一代,其稳定性更高。到21世纪,根据所需要的物理性质和化学性质,在离子液体中嫁接一些功能化基团以适应需要,至此,第三代种类和功能更加丰富的离子液体诞生。
从第一代到第三代,离子液体的合成与制备始终是离子液体研究的基础与核心。其中,离子液体的常规合成方法主要沿袭了第一代离子液体的合成路线与制备方法,常见的方法为一步合成法和两步合成法。一步合成法包括由亲核试剂——叔胺(包括吡啶、咪唑等)与卤代烷烃或酯类物质(羧酸酯、磷酸酯和硫酸酯)发生亲核加成反应,或利用胺的碱性与酸发生中和反应而一步生成目标离子液体的方法。两步合成法,是在第一步的基础上,再通过离子交换、络合反应、电解法或复分解反应等方法,将卤素离子转换为目标离子液体的阴离子。其他合成方法主要是应用微波和超声波辅助合成离子液体,提高了离子液体制备的效率。
由于特殊的构成,相比传统有机溶剂和电解质,离子液体有着独特的性质。主要表现为:蒸汽压小,不会蒸发散失;稳定性高,能够在低于或接近室温到300℃之间保持液态;溶解能力强,可溶解无机化合物、有机化合物、及金属有机化合物等;热容量较大,可将离子液体用做吸热介质以促进太阳能等的转化;电导率高,可作为许多物质电化学研究的电解液。
由于具有优良的特性,离子液体在化学研究的多个领域得到了广泛的应用。在有机合成方面,由于离子液体和大量有机物质能形成两相,且具有溶剂和催化剂的双重功能,可以作为许多化学反应溶剂或催化活性载体。在萃取分离方面,因为离子液体具有较大的极性可调控性,黏度低,密度大,可以形成二相或多相体系,适合作分离溶剂。在电化学方面,离子液体以其液态范围宽、热稳定性好、无酸性质子、导电能力、“π-π环”相互作用和黏合性良好而被应用于电化学当中。在纳米材料方面,由于离子液体具有良好的分散性和稳定性,在纳米材料方面多被当作修饰剂和双功能催化剂来使用。在清洁燃料方面,离子液体具有良好的脱硫脱氮能力,因此目前已被应用于清洁燃料的制备。在环境科学方面,近年来,离子液体以其绿色环保,回收利用率高以及其对有机物有良好的溶解能力而被应用于含油污水的处理和水体中某些物质含量的测定等方面。
本专题得到了张香平研究员(中国科学院过程工程研究所)的大力支持。
·热点数据排行·
截至2015年7月28日,中国知网(CNKI)和Web of Science(WOS)的数据报告显示,以离子液体为词条检索到的期刊文献分别为4846与12602条,本专题将相关数据按照:研究机构发文数、作者发文数、期刊发文数、被引用频次进行排行,结果如下。

研究机构发文数量排名(CNKI) 研究机构发文数量排名(WOS)

作者发文数量排名(CNKI) 作者发文数量排名(WOS)

期刊发文数量排名(CNKI) 期刊发文数量排名(WOS)
根据中国知网(CNKI)数据报告,以离子液体为词条检索到的高被引论文排行结果如下。
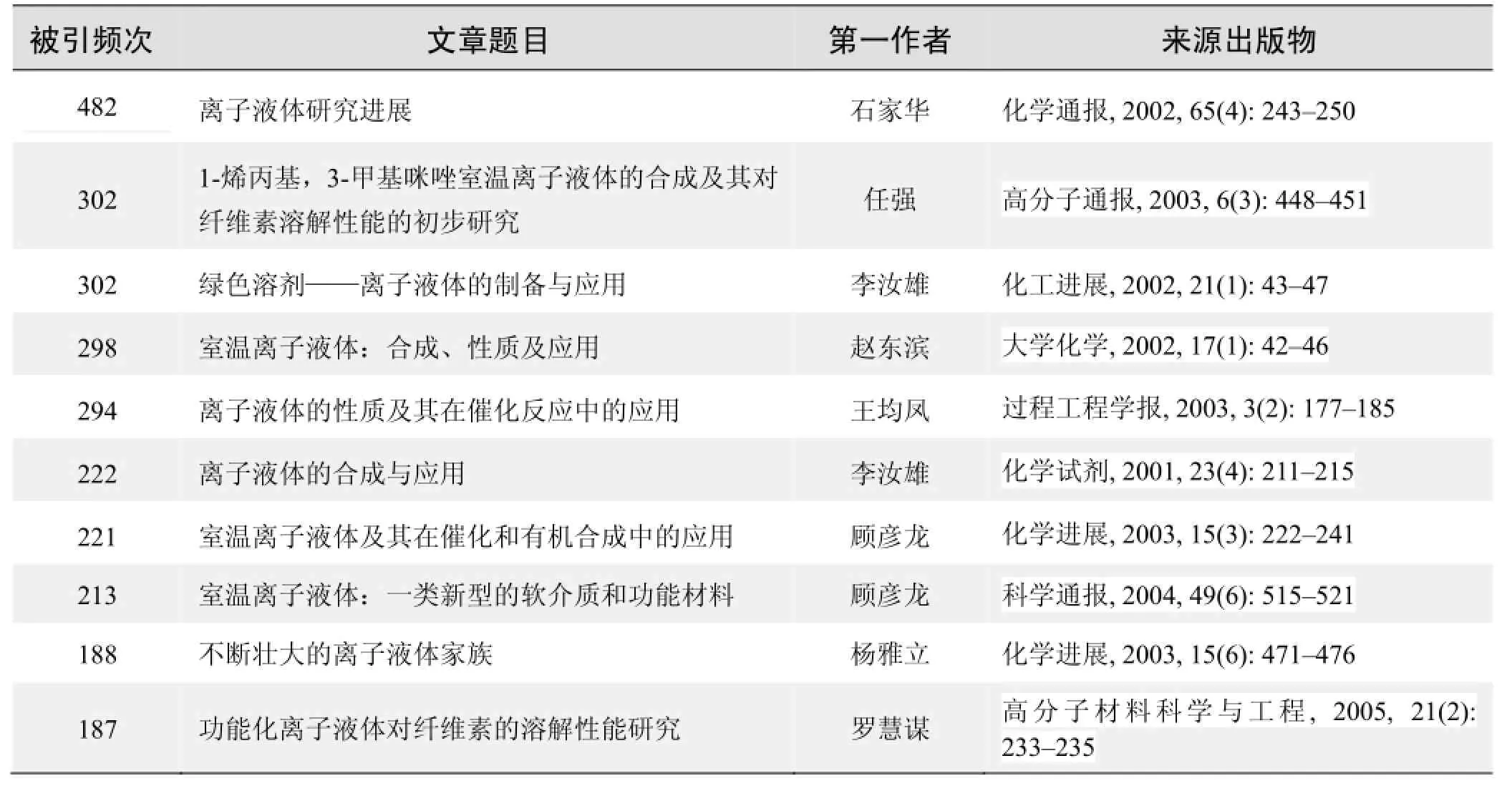
国内数据库高被引论文排行
根据Web of Science统计数据,以离子液体为词条检索到的高被引论文排行结果如下。·经典文献推荐·

国外数据库高被引论文排行
·经典文献推荐·
基于Web of Science检索结果,利用Histcite软件选取LCS(Local Citation Score,本地引用次数)TOP 30文献作为节点进行分析,得到本领域推荐的经典文献如下。

本领域经典文献
来源出版物:Journal of the Electrochemical Society, 1997, 144(11): 3881-3886
Asymmetric hydrogenation and catalyst recycling using ionic liquid and supercritical carbon dioxide
Brown, RA; Pollet, P; McKoon, E; et al.
Abstract: Asymmetric hydrogenation of tiglic acid catalyzed by Ru (O2CMe) 2 ((R)-tolBINAP) in wet ionic liquid ([bmim] PF6 with added water, bmim) 1-n-butyl-3-methylimidazolium) gave 2-methylbutanoic acid with high enantioselectivity and conversion. The product was extracted with supercritical CO2(scCO2)giving a clean separation of product and catalyst. The catalyst/ionic liquid solution was then reused repeatedly without significant loss of enantioselectivity or conversion.
Keywords: water; ACIDS
来源出版物:Journal of the American Chemical Society, 2001, 123(6): 1254-1255
Supported ionic liquid catalysis-A new concept for homogeneous hydroformylation catalysis
Mehnert, CP; Cook, RA; Dispenziere, NC; et al.
Abstract: The new concept of supported ionic liquid catalysis involves the surface of a support material that is modified with a monolayer of covalently attached ionic liquid fragments. Treatment of this surface with additional ionic liquid results in the formation of a multiple layer of free ionic liquid on the support. These layers serve as the reaction phase in which a homogeneous hydroformylation catalyst was dissolved. Supported ionic liquid catalysis combines the advantages of ionic liquid media with solid support materials which enables theapplication of fixed-bed technology and the usage of significantly reduced amounts of the ionic liquid. The concept of supported ionic liquid catalysis has successfully been used for hydroformylation reactions and can be further expanded into other areas of catalysis.
Keywords: phosphine-ligands; membranes; solvents; mixtures; rhodium
来源出版物:Journal of the American Chemical Society, 2002, 124(44): 12932-12933
CO2capture by a task-specific ionic liquid
Bates, ED; Mayton, RD; Ntai, I; et al.
Abstract: Reaction of 1-butyl imidazole with 3-bromopropylamine hydrobromide, followed by workup and anion exchange, yields a new room temperature ionic liquid incorporating a cation with an appended amine group. The new ionic liquid reacts reversibly with CO2,reversibly sequestering the gas as a carbamate salt. The new ionic liquid, which can be repeatedly recycled in this role, is comparable in efficiency for CO2capture to commercial amine sequestering reagents, and yet is nonvolatile and does not require water to function.
来源出版物:Journal of the American Chemical Society, 2002, 124(6): 926-927
Solubilities and thermodynamic properties of gases in the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate
Anthony, JL; Maginn, EJ; Brennecke, JF
Abstract: This work presents the solubility of nine different gases in 1-n-butyl-3-methylimidazolium hexafluorophosphate. The gases considered include carbon dioxide, ethylene, ethane, methane, argon, oxygen, carbon monoxide, hydrogen, and nitrogen. We also report the associated Henry's constants and enthalpies and entropies of absorption. We found carbon dioxide to have the highest solubility and strongest interactions with the ionic liquid, followed by ethylene and ethane. Argon and oxygen had very low solubilities and immeasurably weak interactions. Carbon monoxide, hydrogen, and nitrogen all had solubilities below the detection limit of our apparatus. Our results suggest that the mass transfer of gases into ionic liquids likely will be an important issue for reactions involving these gases. We also determined that ionic liquids show good potential for use as a gas-separation medium.
Keywords: equation-of-state; catalytic-hydrogenation; fluid region; temperature; pressures; solvents; water; CO2; imidazolium; argon
来源出版物:Journal of Physical Chemistry B, 2002, 106(29): 7315-7320
The room temperature ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate: Electrochemical couples and physical properties
Fuller, J; Carlin, RT; Osteryoung, RA
The room temperature ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate (EMLBF4) was demonstrated, as a versatile electrolyte by examining three representative electrochemical couples: ferrocene and tetrathiafulvalene oxidations and lithium ion reduction. Square-wave voltammetric data for ferrocene oxidation were fit to a reversible one-electron process using the COOL11 algorithm to give a half-wave potential of 0.490 V vs. Al/Al(III) and a diffusion coefficient of 5.1 × 10-7cm2/s-1. The two-electron oxidation of tetrathiafulvalene was reversible and proceeded through two consecutive one-electron steps; although data collected at lower square-wave frequencies indicated a slow precipitation of the TTF+species. Lithium ion was reduced to lithium metal at a Pt electrode following the addition of water to the EMLBF4 electrolyte, whereas Lithium ion reduction at an Al wire produced-the beta-LiAl alloy. Conductivities and kinematic viscosities of EMIBF4 were measured from 20 to 100 degrees C and had values of 14 mS cm-1and 0.275 cm2s-1, respectively, at 25 degrees C.
kinetic-parameters; molten-salts; voltammetry

