燃料电池
A Mathematical-Model of the Solid-Polymer-Electrolyte Fuel-Cell
Bernardi, DM; Verbrugge, MW
Two-phase flow and transport in the air cathode of proton exchange membrane fuel cells
Wang, ZH; Wang, CY; Chen, KS
A Water and Heat Management Model for Proton-Exchange-Membrane Fuel-Cells
Nguyen, TV; White, RE
Two-dimensional model for proton exchange membrane fuel cells
Gurau, V; Liu, HT; Kakac, S
燃料电池技术发展现状与展望*
侯明,衣宝廉
(中国科学院大连化学物理研究所,辽宁大连116023)
燃料电池现状与未来
衣宝廉
燃料电池
·编者按·
能源是社会发展和科技进步的重要物质基础,是国民经济发展的动力,也是衡量一国综合国力、国家文明发达程度和人民生活水平的重要指标.目前我们所用的能源仍然是以化石燃料为主的传统能源,这些传统能源的能量转化率低、污染严重、储量有限且不可再生.燃料电池(Fuel Cell,FC)技术清洁、高效、无污染,被视为21世纪最具发展潜力的清洁能源技术,也是近年来各国争相占领的新能源技术制高点之一.
1839年,英国科学家格罗夫(W. R. Grove)首次提出了燃料电池技术,20世纪60年代美国国家航空航天局(NASA)率先开始燃料电池技术和产业化的研究,并将其作为辅助电源应用到Gemini航天领域,为探测器、人造卫星和太空舱提供电力.从此以后,该技术引起了世界各国政府、科研机构和企业的高度重视,就开始被广泛使用在工业、住屋、交通等方面,作为基本或后备供电装置,逐步走向了实用化.燃料电池是把燃料中的化学能通过电化学反应直接转换为电能的发电装置.按电解质分类,燃料电池一般包括质子交换膜燃料电池(Proton Exchange Membrane Fuel Cell,PEMFC)、磷酸燃料电池(Phosphoric Acid Fuel Cell,PAFC)、碱性燃料电池(Alkaline Fuel Cell,AFC)、固体氧化物燃料电池(Solid Oxide Fuel Cell,SOFC)及熔融碳酸盐燃料电池(Molten Carbonate Fuel Cell,MCFC)等.与火电厂,内燃机和燃气轮机等发电装置相比,燃料电池具有能量转化效率高、稳定性高、零排放无环境污染等诸多优点.同时,由于燃料电池设备可集中也可分散性配置,在特殊的场合下,燃料电池模块化的设置可提供极高的稳定性,这也降低了供电系统不安全的风险.在所有燃料电池中,碱性燃料电池(AFC)发展速度最快,主要应用于航空航天任务;质子交换膜燃料电池(PEMFC)已广泛应用于交通动力和小型电源装置;磷酸燃料电池(PAFC)作为中型电源应用进入了商业化阶段;熔融碳酸盐型燃料电池(MCFC)也已完成工业试验阶段;起步较晚的固态氧化物燃料电池(SOFC)是发电领域最有应用前景的燃料电池.
近年来,有关燃料电池技术的新进展层出不穷.日本、德国等国家对燃料电池的研发投入很大,仅汽车制造巨头用于氢能车的研发就达数亿美元,其研发成果很多都已经到了产业化水平.美国犹他大学的工程师最近研制出可在室温下工作的燃料电池,用酶就能使喷气发动机燃料产生电能,这种新型燃料电池可以给手持电子设备、离网型发电机和传感器供电.目前,由于燃料电池中的核心部件“质子交换膜”存在燃料渗透等难题,极大限制了醇类燃料电池的大规模应用.中德荷科学家的研究表明:如果采用石墨烯和氮化硼等单原子层二维材料作为质子交换膜,可使现代燃料电池更高效、更安全、更环保、更轻薄.麻省理工学院研究人员在评论中指出,本项研究取得的突破性进展在理论上已经达到美国能源部设定的2020年质子交换膜输运性能目标.
本专题得到了衣宝廉院士(中国科学院大连化学物理研究所燃料电池工程中心)的大力支持.
·热点数据排行·
截至2015年5月4日,中国知网(CNKI)和Web of Science(WOS)的数据报告显示,以燃料电池(Fuel Cell)为词条可以检索到的期刊文献分别为2757与22018条,本专题将相关数据按照:研究机构发文数、作者发文数、期刊发文数、被引用频次进行排行,结果如下.
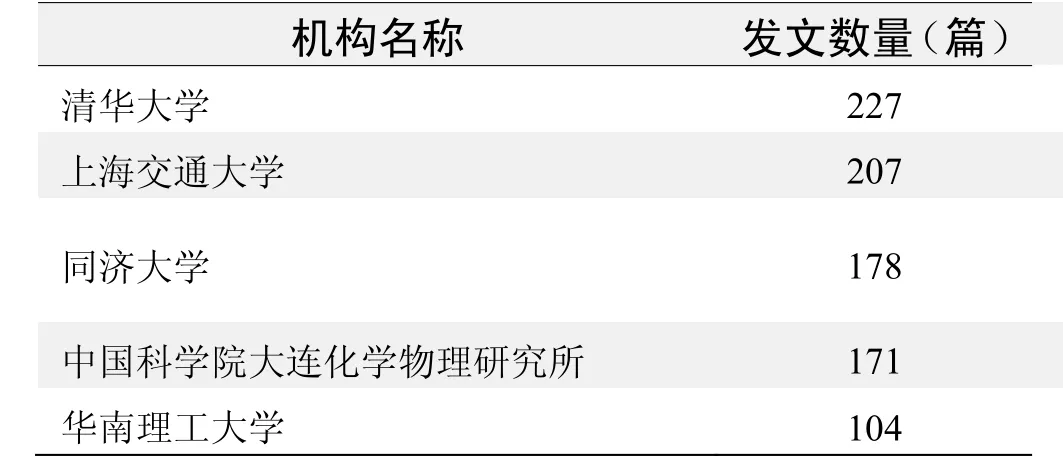
研究机构发文数量排名(CNKI)

研究机构发文数量排名(WOS)

作者发文数量排名(CNKI)

作者发文数量排名(CNKI)
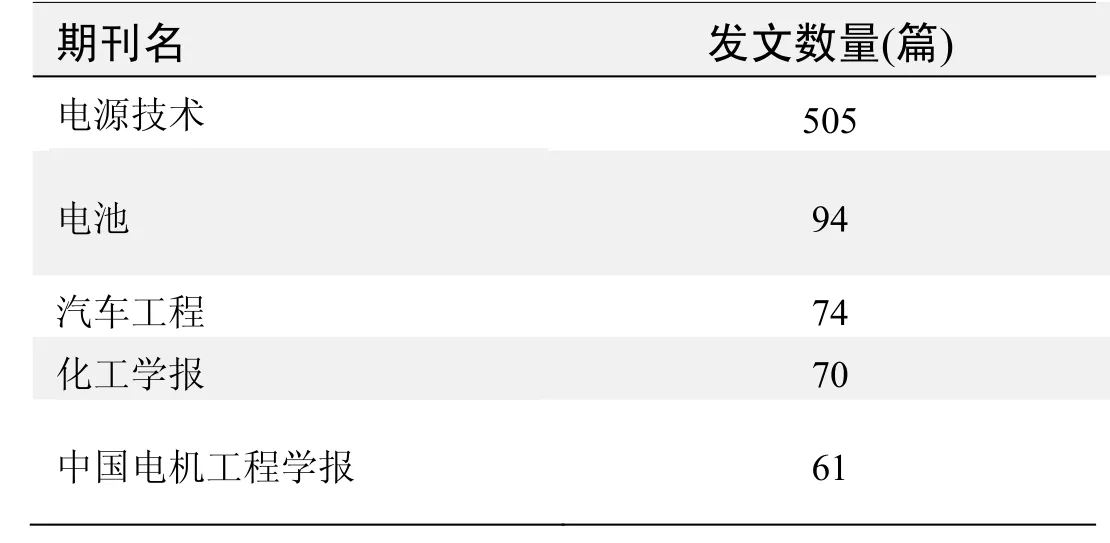
期刊发文数量排名(CNKI)

期刊发文数量排名(WOS)
根据中国知网(CNKI)数据报告,以燃料电池(Fuel Cell)为词条可以检索到的高被引论文排行结果如下.

国内数据库高被引论文排行
根据Web of Science统计数据,以燃料电池(Fuel Cell)为词条可以检索到的高被引论文排行结果如下.

国外数据库高被引论文排行
·经典文献推荐·
基于Web of Science检索结果,利用Histcite软件选取LCS(Local Citation Score,本地引用次数)TOP 30文献作为节点进行分析,得到本领域推荐的经典文献如下.
来源出版物:Journal of the Electrochemical Society, 1991, 138(8): 2334-2342
A Mathematical-Model of the Solid-Polymer-Electrolyte Fuel-Cell
Bernardi, DM; Verbrugge, MW
Abstract: We present a mathematical model of the solid-polymer-electrolyte fuel cell and apply it to (i)investigate factors that limit cell performance and (ii)elucidate the mechanism of species transport in the complex network of gas, liquid, and solid phases of the cell. Calculations of cell polarization behavior compare favorably with existing experimental data. For most practical electrode thicknesses,model results indicate that the volume fraction of the cathode available for gas transport must exceed 20% in order to avoid unacceptably low cell-limiting current densities. It is shown that membrane dehydration can also pose limitations on operating current density;circumvention of this problem by appropriate membrane and electrode design and efficient water-management schemes is discussed. Our model results indicate that for a broad range of practical current densities there are no external water requirements because the water produced at the cathode is enough to satisfy the water requirement of the membrane. Inefficiencies due to the transport of unreacted hydrogen or oxygen through the membrane are shown to be insignificant at practical operating current densities. The transport of gases dissolved in the membrane phase, however, limits the utilization of catalyst. Predictions of cell performance with different types of membranes are also examined, and the model results compare favorably with experimental data.
来源出版物:Journal of the Electrochemical Society, 1992, 139(9): 2477-2491
Two-phase flow and transport in the air cathode of proton exchange membrane fuel cells
Wang, ZH; Wang, CY; Chen, KS
Abstract: Two-phase flow and transport of reactants and products in the air cathode of proton exchange membrane (PEM)fuel cells is studied analytically and numerically. Single- and two-phase regimes of water distribution and transport are classified by a threshold current density corresponding to first appearance of liquid water at the membrane/cathode interface. When the cell operates above the threshold current density, liquid water appears and a two-phase zone forms within the porous cathode. A two-phase, multicomponent mixture model in conjunction with a finite-volume-based computational fluid dynamics (CFD)technique is applied to simulate the cathode operation in this regime. The model is able to handle the situation where a single-phase region co-exists with a two-phase zone in the air cathode. For the first time, the polarization curve as well as water and oxygen concentration distributions encompassing both single- and two-phaseregimes of the air cathode are presented. Capillary action is found to be the dominant mechanism for water transport inside the two-phase zone of the hydrophilic structure. The liquid water saturation within the cathode is predicted to reach 6.3% at 1.4 A cm-2for dry inlet air.
Keywords: two-phase transport; PEM fuel cells; analytical modeling; numerical simulation; water management
来源出版物:Journal of Power Sources, 2001, 94(1): 40-50
A Water and Heat Management Model for Proton-Exchange-Membrane Fuel-Cells
Nguyen, TV; White, RE
Abstract: Proper water and heat management are essential for obtaining high-power-density performance at high energy efficiency for proton-exchange-membrane fuel cells. A water and heat management model was developed and used to investigate the effectiveness of various humidification designs. The model accounts for water transport across the membrane by electro-osmosis and diffusion, heat transfer from the solid phase to the gas phase and latent heat associated with water evaporation and condensation in the flow channels. Results from the model showed that at high current densities (>1 A/cm2)ohmic loss in the membrane accounts for a large fraction of the voltage loss in the cell and back diffusion of water from the cathode side of the membrane is insufficient to keep the membrane hydrated (i.e. conductive). Consequently, to minimize this ohmic loss the anode stream must be humidified, and when air is used instead of pure oxygen the cathode stream must also be humidified.
Keywords: mathematical-model; acid membranes; transport
来源出版物:Journal of the Electrochemical Society, 1993, 140(8): 2178-2186
Two-dimensional model for proton exchange membrane fuel cells
Gurau, V; Liu, HT; Kakac, S
Abstract: A 2-D mathematical model for the entire sandwich of a proton-exchange membrane fuel cell including the gas channels was developed. The self-consistent model for porous media was used for the equations describing transport phenomena in the membrane,catalyst layers, and gas diffusers, while standard equations of Navier-Stokes, energy transport, continuity, and species concentrations are solved in the gas channels. A special handling of the transport equations enabled us to use the same numerical method in the unified domain consisting of the gas channels, gas diffusers, catalyst layers and membrane. It also eliminated the need to prescribe arbitrary or approximate boundary conditions at the interfaces between different parts of the fuel cell sandwich. By solving transport equations, as well as the equations for electrochemical reactions and current density with the membrane phase potential, polarization curves under various operating conditions were obtained. Modeling results compare very well with experimental results from the literature. Oxygen and water vapor mole fraction distributions in the coupled cathode gas channel-gas diffuser were studied for various operating current densities. Liquid water velocity distributions in the membrane and influences of various parameters on the cell performance were also obtained.
Keywords: polymer-electrolyte; mathematical-model; oxygen reduction; platinum
来源出版物:AIChE Journal, 1998, 44(11): 2410-2422
·推荐综述·
燃料电池技术发展现状与展望*
侯明,衣宝廉
(中国科学院大连化学物理研究所,辽宁大连116023)
1燃料电池工作原理与分类
燃料电池(Fuel Cell,FC)是把燃料中的化学能通过电化学反应直接转换为电能的发电装置.按电解质分类,燃料电池一般包括质子交换膜燃料电池(Proton Exchange Membrane Fuel Cell,PEMFC)、磷酸燃料电池(Phosphoric Acid Fuel Cell,PAFC)、碱性燃料电池(Alkaline Fuel Cell,AFC)、固体氧化物燃料电池(Solid Oxide Fuel Cell,SOFC)及熔融碳酸盐燃料电池(Molten Carbonate Fuel Cell,MCFC)等.以质子交换膜燃料电池为例,主要部件包括:膜电极组件(Membrane Electrode Assembly,MEA)、双极板及密封元件等.膜电极组件是电化学反应的核心部件,由阴阳极多孔气体扩散电极和电解质隔膜组成.电解质隔膜两侧分别发生氢氧化反应与氧还原反应,电子通过外电路做功,反应产物为水.额定工作条件下,一节单电池工作电压仅为0.7 V左右.为了满足一定应用背景的功率需求,燃料电池通常由数百个单电池串联形成燃料电池堆或模块.因此,与其它化学电源一样,燃料电池的均一性非常重要.燃料电池发电原理与原电池类似,但与原电池和二次电池比较,需要具备一相对复杂的系统,通常包括燃料供应、氧化剂供应、水热管理及电控等子系统,其工作方式与内燃机类似.理论上只要外部不断供给燃料与氧化剂,燃料电池就可以持续发电.
燃料电池从发明至今已经经历了100多年的历程.由于能源与环境已成为人类社会赖以生存的重点问题,近20年以来,燃料电池这种高效、洁净的能量转化装置得到了各国政府、开发商及研究机构的普遍重视.燃料电池在交通运输、便携式电源、分散电站、航空/天及水下潜器等民用与军用领域展现出广阔的应用前景.目前,燃料电池汽车、电站及便携式电源等均处于示范阶段,在商业化道路上还需要解决成本、寿命等一些瓶颈问题.成本和寿命是相互联系的,同时满足两者需求是实现民用燃料电池应用所面临的主要挑战.航天飞机、潜艇动力用燃料电池目前国际上均已应用,在只侧重寿命、可靠性的特殊领域,现有燃料电池技术是可以满足应用需求的.因此,根据不同的应用背景采用不同的技术路线,是制定燃料电池技术发展战略的重要基础.
2燃料电池的应用
2.1航天领域
早在上个世纪60年代,燃料电池就成功地应用于航天技术,这种轻质、高效的动力源一直是美国航天技术的首选.以燃料电池为动力的Gemini宇宙飞船1965年研制成功,采用的是聚苯乙烯磺酸膜,完成了8天的飞行.由于这种聚苯乙烯磺酸膜稳定性较差,后来在Apollo宇宙飞船采用了碱性电解质燃料电池,从此开启了燃料电池航天应用的新纪元.在Apollo宇宙飞船1966年至1978年服役期间,总计完成了18次飞行任务,累积运行超过了10000 h,表现出良好的可靠性与安全性.除了宇宙飞船外,燃料电池在航天飞机上的应用是航天史上又一成功的范例.美国航天飞机载有3个额定功率为12 kW的碱性燃料电池,每个电堆包含96节单电池,输出电压为28 V,效率超过70%.单个电堆可以独立工作,确保航天飞机安全返航,采用的是液氢、液氧系统,燃料电池产生的水可以供航天员饮用.从1981年首次飞行直至2011年航天飞机宣布退役,在30年期间里燃料电池累积运行了101000 h,可靠性达到99%以上.
中国科学院大连化学物理研究所早在70年代就成功研制了以航天应用为背景的碱性燃料电池系统,A型额定功率为500 W,B型额定功率为300 W,燃料分别采用氢气和肼在线分解氢,整个系统均经过环境模拟实验,接近实际应用.这一航天用燃料电池研制成果,为我国此后燃料电池在航天领域应用奠定了一定的技术基础.
2.2潜艇方面
燃料电池作为潜艇AIP(Air-Independent Propulsion, AIP)动力源,从2002年第一艘燃料电池AIP潜艇下水至今已经有6艘在役,还有一些FC-AIP潜艇在建造中.2009年10月意大利军方订购的2艘改进型FC-AIP潜艇开始建造,潜艇水面排水量为1450吨,总长为56 m,最大直径为7 m,额定船员24名,水下最大航速为20节,计划在2015—2016年开始服役.FC-AIP潜艇具有续航时间长、安静、隐蔽性好等优点,通常柴油机驱动的潜艇水下一次潜航时间仅为2天,而FC-AIP潜艇一次潜航时间可达3周.这种潜艇用燃料电池是由西门子公司制造,采用镀金金属双极板.212型艇装载了额定功率为34 kW的燃料电池模块,214型艇装载了120 kW燃料电池模块,2种型号的燃料电池模块参数,额定工况下效率接近60%.
3燃料电池示范
除了上述实际应用外,燃料电池还在多个领域展现了不同规模的示范,包括电动汽车、电站、应急不间断电源、便携式电源及充电器等.在这些领域,燃料电池展示了一定的应用前景.示范的目的是发现问题并解决问题,不断完善技术,使之逐步接近商业化目标.
3.1电动汽车
随着汽车保有量的增加,传统燃油内燃机汽车造成的环境污染日益加剧,同时,也面临着对石油的依存度日益增加的严重问题.燃料电池作为汽车动力源是解决因汽车而产生的环境、能源问题的可行方案之一,近20年来得到各国政府、汽车企业、研究机构的普遍重视.燃料电池汽车示范在国内外不断兴起,较著名的是欧洲城市清洁交通示范项目(Clean Urban Transport for Europe,CUTE),第1期共有27辆车在9个欧洲城市运行2年;并于2006—2009年进行第2期示范(Hy-Fleet: CUTE),33辆燃料电池客车在包括北京的10个城市运行;整个项目累计运行140000 h,行驶约2100000 km,承载乘客约850万;目前,正在着手进行第3期(Clean Hydrogen in European cities project,CHIC)示范.代表性的车型是由Daimler公司制造的燃料电池客车Citaro,分别采用纯燃料电池、燃料电池与蓄电池混合动力,加拿大Ballard公司提供燃料电池模块,电堆采用模压石墨双极板,具有较好的操作弹性.
通过示范,车用燃料电池技术取得了长足的进展.近年来,燃料电池汽车在性能、寿命与成本方面均取得一定的突破.在性能方面,美国GM公司的燃料电池发动机体积比功率已与传统的四缸内燃机相当,德国Daimler公司通过3辆B型Mercedes-Benz燃料电池轿车F-Cell的环球旅行向世人展示了燃料电池汽车的可使用性,其续驶里程、最高时速、加速性能等已与传统汽油车相当,计划2014年开始实施批量生产;在寿命方面,美国UTC Power公司的燃料电池客车至2011年8月已经累积运行了10000 h,寿命指标已达到商业化目标;在成本方面,各大汽车公司都致力于降低燃料电池Pt用量,经过不断地技术改进,美国GM公司一台94 kW的发动机,Pt用量从上一代的80 g降低到30 g,并计划2015年Pt用量再降低至1/3,达到每辆车Pt用量10 g.日本Toyota公司也宣布燃料电池发动机催化剂Pt用量降低到原来的1/3,预计2015年单车成本降低至50000美元,并计划于2015年实现燃料电池汽车商业化.
我国燃料电池汽车,自“九五”末期第一台燃料电池中巴车的问世,到“十一五”2008年北京奥运会和2010年上海世博会燃料电池汽车的示范运行,十几年的发展,燃料电池电动汽车技术取得了可喜的进步.在北京奥运会上,燃料电池轿车成为“绿色车队”中的重要成员.20辆帕萨特“领驭”燃料电池轿车为北京奥运会提供了交通服务,单车无故障行驶里程达到了5200 km;在上海世博会上,包括100辆观光车、90辆轿车和6辆大巴车,总计196辆燃料电池汽车完成了历时6个月的示范运行.其中,100辆观光车是由国内研制,装有5 kW燃料电池系统.70辆轿车装载的是国内研发的燃料电池系统,分别采用55 kW和33 kW两种类型的燃料电池发动机,前者是常规电-电混合模式,后者是Plug-in模式,平均单车运行里程4500~5000 km,最长的单车运行累积里程达到10191 km.3辆大巴车装载的是863“节能与新能源汽车重大项目”资助的80 kW燃料电池发动机,累积运行了15674 km,最长单车里程为6600 km.此外,还参加了北京公交车示范运行以及国际一些示范或赛事,包括国际清洁能源Bibendum大赛、美国加州示范及新加坡世青赛等,展示了中国燃料电池技术的进步.目前,燃料电池发动机技术明显提升,在中国科技部支持下,国产PEMFC关键材料和部件的开发取得了重大进展,研制成功了高导电性及优化孔结构的碳纸、增强型复合质子交换膜、高稳定性/高活性Pt-Pd复合电催化剂及薄型全金属双极板等.经过膜电极技术的优化,电催化剂利用率得到大幅提高,流场优化提高了高电流密度下水管理能力,使额定工作点由0.66 V@0.5 A·cm-2提升至0.66 V@1.0 A·cm-2,比功率达到1300 W·L-1(新源动力提供),在同样功率输出情况下,体积和质量分别减小了一半.
3.2燃料电池固定式分散电站
污染重、能效低一直是困扰火力发电的核心问题,燃料电池作为低碳、减排的清洁发电技术,受到国内外的普遍重视.燃料电池电站不同于燃料电池汽车,没有频繁启动问题,因此可以采用以下4种燃料电池技术,分别是磷酸燃料电池、质子交换膜燃料电池、固体氧化物燃料电池和熔融碳酸盐燃料电池.
PAFC电站代表性的开发商是UTC Power公司,其开发的PureCell○RModel系列200 kW和400 kW磷酸燃料电池发电系统,20年多年里已经在19个国家安装运行近300台,部分电站运行已经超过40000 h的设计寿命.发电系统以天然气为原料,由燃料处理、燃料电池模块及电调节与控制3个部分组成,电效率接近40%(LHV).若计入热回收,总效率可以接近80%~90%(LHV).磷酸燃料电池电站在技术上发展比较成熟,但由于使用贵金属催化剂,大规模商业化还面临成本高的瓶颈问题.
PEMFC电站的代表性开发商是Ballard公司,主要开发250 kW~1 MW的示范电站,目前示范数量还不多,国内华南理工大学也进行了300 kW PEMFC电站的示范.质子交换膜燃料电池用于固定电站与用于燃料电池汽车相比,由于工况相对缓和,不需要像燃料电池汽车那样频繁变载,避免了动态工况引起的燃料电池材料衰减,相对延长了寿命.但是,成本问题还是PEMFC电站商业化面临的主要问题.另外,由于PEMFC的操作温度在80~90℃之间,故其热品质比较低,热量回收效率不高,影响整体燃料利用率.再有,为了防止PEMFC燃料电池中毒,燃料需要净化,会增加一部分成本.高温质子交换膜燃料电池(HTPEMFC)操作温度可以达到150~200℃,一定程度上可以缓解上述问题,目前HT-PEMFC技术还处于研发中.
Siemens Westinghouse公司开发了固体氧化物燃料电池电站,以阴极作支撑的管式SOFC 机械强度高,热循环性能好,易于组装与管理.自2000年以来,西门子-西屋公司已建成多台大型100~250 kW分散电站进行试验运行,其中以天然气为燃料的100 kW SOFC 系统总计运行20000 h,220 kW SOFC与燃气轮机联合发电系统效率可达到60%~70%.但现有的技术如电化学气相沉积和多次高温烧结等导致阴极支撑型SOFC电池成本过高、难以推广.借助廉价的湿化学法、等离子喷涂等技术替代电化学气相沉积制备电解质薄膜,并运用改进烧结工艺、减少烧结次数等手段,有望达到大幅度降低阴极支撑管型SOFC成本的目的.
MCFC电站,美国Fuel Cell Energy公司处于国际领先地位,其开发的MCFC电站已在全球装机60余台,主要用于医院、宾馆、大学及废水处理厂等场所示范发电.MCFC操作温度较高(650~700℃),可以实现热电联供及与气轮机联合循环发电,以进一步提高燃料的能量转化效率.由于熔盐的强腐蚀性以及高温对材料是一个挑战,寿命是MCFC要解决的关键问题.
3.3备用电源与家庭电源
与现有的柴油发电机比较,燃料电池作为不间断备用电源,具有高密度、高效率、长待时及环境友好等特点,可以为电信、银行等重要部门或偏远地区提供环保型电源.家庭与一些公共场所大多采用1~5 kW小型热电联供装置,家庭电源通常以天然气为燃料,这样可以兼容现有的公共设施,提供电网以外的电,废热可以以热水的形式利用,备用电源也可采用甲醇液体燃料.在燃料电池电源产品研发方面,日本的Ebara-Ballard公司1 kW家庭型燃料电池电源,其产品已经在700多个场所试验,并建立了年产4000台的生产基地;美国Idatech公司研制的5 kW UPS已于2008年拿到印度ACME集团30000台的订单;美国Plug Power公司已实现近千台的5 kW电源的销售,主要用于通讯、军事等方面;此外,Relion与Altergy公司也开拓了燃料电池备用电源市场(图9B).我国也已研制了10 kW的供电系统,以家庭用电为示范,已经运行了2500 h.
3.4燃料电池可移动电源、充电器
燃料电池作为小型可移动电源或二次电池的充电器,也是目前研发的热点.主要技术基础是采用直接甲醇燃料电池,即以甲醇为燃料,这种液体燃料具有携带方便、比能量高等特点.直接甲醇燃料电池初期是瞄准手机、笔记本电脑电源市场,旨在提供长待时电池,但由于在系统管理、小型化等技术方面还有待突破,近期人们又把目光集中到了充电器市场.东芝公司2009年发布了甲醇燃料电池充电器产品DynarioTM,可为手机等电子器件充电,以满足手机日益增加的多功能化需求.经由USB接口在20 s内可为一部手机充电,燃料罐14 mL储存高浓度甲醇,可以充2部常规手机.该产品已经通过了国际电工协会(International Electrotechnical Commission, IEC)的安全标准,首次试售3000部,收集用户反馈意见与市场反应以便进行改进.国内也研制成功了多功能直接甲醇燃料电池充电器,为野外移动通讯设备等供电,其工作时间可从原来的几个小时提高到1~3天.经过环境模拟试验,表现出良好的环境适应性和可使用性.此外,直接甲醇燃料电池在军民微小型可移动电源领域也展示了广阔的应用前景.国内研制的额定输出功率为25~50 W的DMFC移动电源系统,经同行专家现场测试表明,能量密度达502 Wh·kg-1,约为锂离子电池的3倍.随着现代化战争装备的日益先进,单兵作战需要更多电子装备,直接甲醇燃料电池可在单兵作战电源发挥优势.美国陆军开发了型号为M-25燃料电池单兵电源,用于数字通讯、GPS等电子装备.经过实际测试表明,这种电池可以在平均20W功率下使用72 h,而质量比传统电池降低了80%.该项目得到美国陆军采办挑战项目总计约3亿美元的资助.此外,供陆军指挥系统的无线电卫星通讯、远程监控装置等微小型移动电源也引起各国的普遍关注.目前,直接甲醇燃料电池在技术方面还需要进一步解决寿命、稳定性等关键问题,性能有待进一步提升.重点是通过研制新型的阻醇膜、多元合金催化剂以及调变膜电极组件结构等,解决材料在运行过程中的稳定性与耐久性、系统水热管理等问题,并同时解决工程化实际问题,使DMFC在充电器与可移动电源等领域尽早实现商业化.
4燃料电池技术发展思路
如上所述,燃料电池应用主要集中在潜艇、航天等特殊领域,且技术已相对成熟.而民用领域如燃料电池电动汽车、电站等尚处于示范阶段,相关技术距离商业化还有一定的差距,存在着成本、寿命等瓶颈问题.其原因可以归结为民用产品与特殊应用产品对成本承受力的差异.特殊领域由于面对的是特殊应用,对成本目标没有苛刻的要求,而民用产品面对的是广大消费群体,低成本是应用的前提条件.民用产品在追求低成本的同时,寿命也面临着挑战,如减少Pt用量虽可降低燃料电池成本,但低Pt催化剂电池的耐久性却更加严峻.因此,兼顾低成本与长寿命是实现燃料电池民用产品商业化要解决的关键问题.以催化剂为例,特殊领域贵金属催化剂担载量是民用产品的1~2个数量级,因而它的抗衰减能力比民用产品大大提高,燃料电池所面临的寿命问题也会迎刃而解.因此,现阶段我国一方面要大力推进燃料电池在特殊领域的应用,力争占领未来此领域动力源的制高点;另一方面要促进燃料电池在民用领域的技术进步,加快燃料电池民用产品的商业化步伐.
4.1提高性能与可靠性,加快我国燃料电池技术在特殊领域的应用
1)航空航天
燃料电池在航天领域的应用,除了前面叙述的在Apollo航天飞机等的成功应用外,以燃料电池为动力的平流层飞艇、无人机等也成为国际研发热点,燃料电池在航天领域已展示了广阔的应用前景.
在航天技术中,高比能量是追求的重要指标之一.目前,有2条技术路线,一是采用氢/氧或氢/空燃料电池技术,即利用携带的氢气与氧气或空压机,提供一定航程所需的燃料与氧化剂;另一种是依据可再生燃料电池技术(Regenerative Fuel Cell, RFC),即飞行器向日时由太阳能电池提供动力同时电解水生成氢气与氧气,背日时电解产物氢和氧使燃料电池发电作为飞行器动力源.氢/氧燃料电池重点解决的是燃料和氧化剂的携带与燃料电池耦合技术;氢/空燃料电池的瓶颈技术是高效空压机,目前国内这方面技术还处于研发过程中.相比之下,可再生燃料电池在航天技术方面的应用引起人们更多的重视,尤其是一体化RFC技术,使系统集成更加紧凑,有利于提高系统比能量.
再生燃料电池由于电解过程需要较高的电位(1.5~1.8 V),对燃料电池材料是一个挑战.其中导电、耐腐蚀兼容的双极板与扩散层材料是研发的重点,如轻质的Ti双极板与多孔Ti扩散层材料在高电位下具有较高的耐腐蚀性,但是原材料表面接触电阻较大,需要经过表面处理.使用贵金属Pt、Au等可增加导电和耐腐蚀性,然而成本较高.其它的替代方案目前正在研究中.为了提高系统比能量,需要燃料与氧化剂5~10 MPa高压储存,因此RFC的高压水电解技术更应重点关注.除了高压要求的电解池硬件强度及密封问题外,高压下气液两相流动的传递过程对电化学反应的影响也需进行研究.为了提高系统比能量,RFC系统水管理和热管理也应当进一步改进,如采用无泵水循环技术可减少系统部件、减轻质量,采用热解石墨或热泵技术可以实现更加高效排热.
目前RFC可分为燃料电池和电解池分体式和一体式2种.分体式技术比较成熟,一体式技术还处于研究阶段,关键是双效氧电极技术,一体式RFC研制成功可极大地提高系统比功率与比能量.此外,为了适应空间环境,动力系统的环境适应性也要特别考虑.可根据环境实验项目,作RFC电池与系统的结构设计,以满足空间应用的需求.
2)水下潜器
水下应用燃料电池除了能给潜艇提供安静、长航时的动力源系统外,还可以用于水下机器人、水下蛙人等动力源.
水下燃料电池均以氢氧燃料电池技术为基础,其中排水与零排放技术是目前研究的热点.燃料电池生成水在阴极侧,在氢-空燃料电池中可以通过气体吹扫与夹带把生成水排出燃料电池,以保证电池安全可靠运行.但在以氧气为氧化剂的情况下,按给定的反应计量比,氧气的体积仅为空气的1/5,如此必将减弱生成水的排出能力,导致电池发生“水淹”,不能正常运行.采用氢氧尾气循环和内部排水技术等可实现氢氧燃料电池的有效排水.
水下操作更苛刻的要求是零排放.目前,零排放技术有2种,一种是氢氧吸收技术,另一种是氢氧复合技术.氢氧吸收是利用储氢、储氧材料,把尾排的氢氧储存起来,但其储存量受储罐容积限制;氢氧复合是利用催化作用把排出的少量氢、氧复合生成水,达到零排放目的.在氢氧复合技术中,控制好氢氧化学反应计量比是关键.另外氢氧复合催化剂在有水生成情况下的稳定性也是要关注的问题.
在水下用燃料电池系统中,氢气供给有多种方式,如金属固态储氢、高压气态储氢、低温液态储氢以及甲醇重整制氢等.可根据不同的应用背景选择不同的储氢技术,如水下机器人通常优先选择高压气态高纯氢以满足一定的续航里程;而潜艇比较成熟的技术还局限于使用金属固态储氢,其中储放氢过程的能量须与燃料电池耦合;对甲醇重整制氢过程,也要考虑制氢过程与燃料电池发电过程的热平衡,以提高整个系统的工作效率.
水下燃料电池系统部件模块化是提高可靠性的重要措施,一个动力系统可以由多个模块并、串联而成,当一组模块出现故障,可以瞬间切断,在线更换,以提供系统长航时运行能力.
4.2解决寿命与成本问题,促进民用燃料电池产品的商业化
燃料电池汽车、电站等民用产品面临着的低成本与长寿命兼顾问题,是制约商业化的瓶颈问题.需要从燃料电池材料、部件与系统3方面进行改进与创新,以促进燃料电池尽早走向应用.车用燃料电池的问题尤为突出,下面以车用质子交换膜燃料电池为例,探讨成本与耐久性兼容的解决方案.
1)燃料电池核心材料的创新
① 发展贵金属部分替代或完全替代的催化剂改进目前使用的Pt/C催化剂是降低成本与提高寿命的关键.研究显示,由于燃料电池动态工况或高电位会引起催化剂的团聚、流失,从而引起催化剂活性比表面积下降,造成燃料电池性能严重衰减.此外,由于Pt成本较高且资源有限,降低Pt催化剂用量势在必行.然而在低Pt催化剂研发的同时,也必须解决相关的稳定性,以期建立低成本、长寿命的最优解决方案.
优化制备方法,利用形貌控制,可有效地提高催化剂活性与稳定性.孙世刚等利用高指数晶面Pt具有的开放表面结构、高密度的台阶原子以及其所处的短程有序环境等特点,使催化剂的活性和稳定性方面均得到显著提高.
Pt合金催化剂目前显示出较好的发展前景,借助加入第2或第3种非Pt金属,利用电子或几何效应,在达到低Pt、高活性的同时,稳定性也相应提高.其中核壳型催化剂是研究热点之一,利用非贵金属为支撑核,表面贵金属为壳的结构,可降低Pt用量,提高质量比活性.如由欠电位沉积方法制备的Pt-Pd-Co/C单层核壳催化剂总质量比活性是商业催化剂Pt/C的3倍;利用脱合金方法制备的Pt-Cu-Co/C核壳电催化剂,质量比活性可达Pt/C的4倍.此外,Pt催化剂表面的修饰也可以起到提高稳定性作用,如以金簇修饰Pt纳米粒子,提高了Pt的氧化电势,起到了抗Pt溶解的作用,经过30000次循环后金修饰的铂催化剂的活性表面积与初始状态相比并没有明显降低.
Pt3Pd/C比Pt/C抗衰减能力之所以有较大提高,原因在于加入Pd提高了Pt的氧还原活性,改善了其抗氧化能力.中国科学院大连化学物理研究所包信和研究组借助贵金属Pt表面与单层氧化亚铁薄膜中铁原子的强相互作用产生的界面限域效应,成功构建了表面配位不饱和亚铁结构催化剂,在一氧化碳低温活化过程中显示出非常独特的催化活性,可高效去除CO毒物,该催化剂在PEMFC实际工况条件下稳定运行超过1500 h;最近,又将界面限域的概念扩展到PtNi、PtSn、PtCo等催化体系,发现了界面限域的配位不饱和Ni物种及其在低温氧化反应中的重要作用.
催化剂载体的改进,也是提高催化剂稳定性的有效途径之一.由于目前Pt催化剂载体大多采用XC-72碳黑,在高电位及电位循环下会发生载体腐蚀,是造成催化剂团聚与流失的主要原因.改进催化剂载体可以从2个方面着手,一是改进目前的碳载体材料,如高温石墨化处理、添加官能团等方法,可以提高高电位下载体的耐腐蚀性;二是采用新的载体材料,碳纳米管或氮掺杂的碳纳米管、纳米碳须、WxCy、氧化铟锡等碳与非碳载体.这些新型载体材料在一定程度上提高了耐腐蚀性,但是比表面积均远低于现有的载体材料.目前具有高导电、高比表面积与高耐蚀性兼顾的载体材料还是研究的难点.
在探求低Pt催化剂的同时,非Pt催化剂的研究也一直在进行中.如金属硫族化合物、金属大环配合物等展示了较好的初活性,但稳定性还远满足不了要求.近期,Lefèvre等在非Pt催化剂的研究方面取得了进展,以载量为5.3 mg·cm-2的非贵金属Fe/N/C电催化剂制备的电极,低电流密度下与Pt载量为0.4 mg·cm-2的Gore电极性能相当.但因前者担载量比Pt催化剂高出几倍甚至十几倍,电极厚度随之增加,从而导致电极反应的传质阻力大幅度增加,而且其稳定性还需进一步改善.此外,近期研究的氮掺杂碳基非Pt催化剂也表现出较高的氧还原反应催化活性与稳定性.
至今,酸性体系下能使用的非Pt催化剂还没有突破性进展.目前,人们把目光聚集到碱性体系的聚合物燃料电池.由于碱性环境中的氧还原动力学快于酸性条件,催化剂可实现贵金属替代,使燃料电池成本得到根本性的降低.武汉大学庄林研究组结合实验与计算提出了利用非化学计量比金属氧化物修饰调控Ni表面电子结构,所得Ni基HOR催化剂表面的反应选择性、抗氧化性均大幅度提高,藉此组装的碱性聚合物电解质燃料电池可以完全摆脱对贵金属催化剂的依赖.目前技术难点是研究高离子传导性、高稳定性的碱性离子交换膜.一些学者进行了季胺或季膦型聚合物膜的研究,通过对电解质可溶性溶剂的选择,制备出带有立体化三相界面的非贵金属催化剂膜电极,但聚合物膜的离子传导性与稳定性还有待于进一步提高.
② 进一步促进高性能、廉价国产材料的批量供应
除了催化剂外,其它材料如质子交换膜、碳纸等也是制约成本与寿命的重要因素.其中Nafion系列的均质全氟磺酸膜在燃料电池环境中由于反应过程中氢氧自由基的攻击,发生衰减,影响电池寿命.此外,该种膜大部分依赖于进口,成本较高.因此需要研制新型高稳定的国产化质子交换膜替代Nafion膜,主要是从提高机械性能与化学稳定性出发进行改进.例如采用多孔材料、碳纳米管、TiO2纳米管等与全氟磺酸树脂复合的增强膜,可有效地增强膜的机械性能,使之在动态工况下,稳定性显著提高.在膜中加入自由基淬灭剂,也可抵抗发电过程氢氧自由基的攻击,从而提高化学稳定性.再者,短侧链膜因其具有较好的质子传导率及高的稳定性也引起了关注,制备带自由基淬灭剂的短侧链复合膜是一个比较有前景的发展方向.
碳纸目前也是采用进口材料,在科技部“863课题”支持下,国产化的替代产品已经基本研制成功,其性能已接近国际先进水平,有待更进一步优化及研制批量化生产工艺与设备,以满足燃料电池商业化的需求.高稳定性、低成本的国产化材料,是发展国内燃料电池技术的必由之路.
2)燃料电池关键部件的改进
① 高催化剂利用率、性能稳定的膜电极技术除了催化剂本身以外,改进、优化燃料电池膜电极组件制备方法是有效提高Pt利用率、降低成本的重要手段.国际上已经发展了3代MEA技术路线:一是把催化层制备到扩散层上,通常采用丝网印刷方法,该技术已经成熟;二是把催化层制备到膜上,与第一种方法比较,在一定程度上提高了催化剂的利用率与耐久性;三是有序化的MEA,把催化剂如Pt制备到有序化的纳米结构上,使电极呈有序化结构,有利于降低大电流密度下的传质阻力,进一步提高燃料电池性能,降低催化剂用量.利用有序化MEA制备技术,3M公司研制的纳米结构薄膜MEA,其Pt担载量可降至0.15~0.25 mg·cm-2,并显示出较好的性能.
② 高均一性电堆技术
提高电堆的一致性,提升额定工作点电流密度,也是降低燃料电池Pt用量以及其它硬件成本的重要环节.车用燃料电池为了满足一定功率需求,电堆通常都是由数百节单电池组成,电堆内单电池间的一致性是保证燃料电池能够高功率运行的关键.一致性除了与燃料电池材料、部件加工的均一性有关外,还与电堆的水、气、热分配密切相关.从设计、制备及操作3方面出发进行调控,通过模拟仿真手段研究流场结构、阻力分配对流体分布的影响,找出关键影响因素,重点研究水的传递、分配与水生成速率、水传递系数、电极/流场界面能之间的关系,研究稳态与动态载荷条件对电堆阻力的影响,保证电堆在运行过程中保持均一性,从而可以大幅提升额定点工作电流密度以及电堆的功率密度,降低成本.
3)燃料电池系统技术的完善
① 缓解燃料电池衰减的控制策略
研究发现,动态循环工况、启动/停车过程、连续低载或怠速运行等都是引起燃料电池衰减的主要原因.针对这些,提出车用燃料电池的合理控制策略,规避可能引起衰减因素的出现,起到保护材料遭到侵害的作用.燃料电池关键材料的研究需要相对长的时间,就目前看,可以在现有材料的基础上通过改变控制策略,以提高其耐久性.
采用二次电池、超级电容器等储能装置与燃料电池构建电-电混合动力,既可减缓燃料电池输出功率变化速率,又可避免燃料电池载荷的大幅度波动,以使燃料电池能在相对稳定的情况下工作,避免加载瞬间由于空气饥饿引起的电压波动,减缓运行过程中因频繁变载而引起的电位扫描最终导致催化剂的加速衰减.还可采用“前馈”控制策略,即在加载前预置一定量的反应气,以减轻反应气的饥饿现象.利用辅助负载限电位法,亦可有效地抑制启动、停车瞬间由于阳极侧易形成氢空界面而产生的高电位.此外,碳腐蚀速率与进气速率密切相关.在启动过程中快速进气可以降低高电位停留时间,达到减少碳载体损失的目的.利用混合动力控制策略,在低载时给二次电池充电,提高电池的总功率输出,也可起到降低电位的目的.此外,美国UTC公司在一个专利中提出了怠速限电位的方法.通过调小空气量、同时循环尾排空气以降低氧浓度的办法,达到抑制电位过高的目的.合理的控制策略可实现燃料电池内部有效水管理,保持燃料电池内水处在一定的合适范围,尤其在动态工况下,使水能够跟踪动态操作变化,保证燃料电池正常稳定工作,避免由于干湿度频繁变化导致的失效或性能衰减.
② 高比功率、高可靠性的系统集成技术
目前,国内车用燃料电池系统质量比功率仅为300 W·kg-1,而国际先进水平的系统质量比功率已经达到650 W·kg-1.造成差距的主要原因是国际上大多都是汽车制造商在从事燃料电池发动机的制造,他们利用传统汽车工业技术基础,研制出高集成度的产品.有鉴于此,国内燃料电池开发单位需要与汽车厂开展合作,移植传统汽车工业的成熟技术,推进燃料电池系统技术的进步,并进一步提高部件可靠性,延长无故障间隔时间,促进燃料电池商业化.
5结语
燃料电池经过近半个多世纪的发展,已经实现了在航天飞机、宇宙飞船及潜艇等特殊领域的应用,而民用方面由于受寿命与成本的制约,至今在电动汽车、电站、便携式电源或充电器等各行业还处于示范阶段.未来我国应大力推进燃料电池在特殊领域的应用,增强我国的国防军事实力;同时,要集中解决寿命与成本兼顾问题,从材料、部件、系统等3个层次进行技术改进与创新,加快燃料电池民用商业化步伐,提供高能效、环境友好的燃料电池发电技术,为建立低碳、减排、不依赖于化石能源的能量转化技术新体系做贡献.
·高被引论文摘要·
被引频次:243
燃料电池现状与未来
衣宝廉
简述了国外燃料电池发展状态,近年来取得的重要进展;尤其是在提高质子交换膜型燃料电池的性能、电池组与电池系统的比功率比能量方面的技术突破,这种电池作为电动汽车动力源和潜艇AIP推进动力源应用前景和必须解决的主要技术、经济问题.熔融碳酸盐和固体氧化物燃料电池作为区域性分散电站的可能性和必须解决的技术问题.简述了国内在燃料电池研究中取得的主要成果和目前发展状态.简介了国内千瓦级碱性燃料电池和质子交换膜燃料电池主要性能,并对国内燃料电池发展提出了参考意见.
燃料电池;碱性氢氧燃料电池;磷酸燃料电池;质子交换膜燃料电池;熔融碳酸盐燃料电池;固体氧化物燃料电池
来源出版物:电源技术,1998, 22(5): 216-221
被引频次:145
聚合物电解质燃料电池的研究进展
陈延禧
摘要:综述了聚合物电解质燃料电池(PEMFC)最新的研究进展,包括离子交换膜、膜电极结构及工艺、电催化剂、水和热管理.最后,对我国的PEMFC研究提出了意见.
关键词:燃料电池;聚合物电解质燃料电池;离子交换膜;电催化剂
来源出版物:电源技术,1996, 20(1): 21-27
被引频次:132
燃料电池概述
刘建国,孙公权
摘要:燃料电池在固定与分散电站、交通运输、移动电源等方面广阔的应用前景现已受到许多研究单位和公司的广泛关注.文章简要介绍了几种主要类型燃料电池(碱性燃料电池alkaline fuel cell, AFC)、磷酸燃料电池(phosphoric acid fuel cell, PAFC)、熔融碳酸盐燃料电池(molten carbonate fuel cell, MCFC)、固体氧化物燃料电池(solid oxide fuel cell, SOFC)、质子交换膜燃料电池(protonex change membrane fuel cell, PEMFC)、直接醇类燃料电池(direct alcohol fuel cell, DAFC)的特点、研究状况、市场需求和技术挑战.初步探讨了我国燃料电池研究开发的前景.
关键词:燃料电池;能源;能量转换效率
来源出版物:物理,2004, 33(2): 79-84
被引频次:129
中国燃料电池的发展
毕道治
摘要:中国燃料电池的研究始于1958年.回顾了中国燃料电池40年的发展历程并简介了近期(1998年1月—2000年12月)燃料电池发展计划.概述了70年代中国燃料电池开发高潮时期在空间用碱性氢氧燃料电池开发中所取得的主要成果及各种地面用碱性燃料电池包括氨空气电池;肼空气电池及乙二醇空气电池等的研究开发情况.介绍了进入90年代以来中国在质子交换膜燃料电池、熔融碳酸盐燃料电池及固体氧化物燃料电池等领域的最新研究开发状况,并结合中国的能源资源状况及城市大气污染等问题对中国燃料电池的开发应用前景进行了讨论.
关键词:燃料电池;碱性氢氧燃料电池;质子交换膜燃料电池;熔融碳酸盐燃料电池;固体氧化物燃料电池
来源出版物:电源技术,2000, 24(2): 103-107
被引频次:107
我国燃料电池发展概况
陆天虹,孙公权
摘要:燃料电池研究与开发的原因主要在于其质量轻、体积小、能量转换效率高等.本文综述了我国燃料电池的发展概况.依燃料电池所用电解质的类型,分别讨论了碱性燃料电池、质子交换膜燃料电池、熔融碳酸盐燃料电池和固体氧化物燃料电池在我国的研究、开发与进展状况,并与国外燃料电池近年来的研究水平作了简单的比较.
关键词:碱性燃料电池;质子交换膜燃料电池;熔融碳酸盐燃料电池;固体氧化物燃料电池
来源出版物:电源技术,1998, 22(4): 182-184
被引频次:102
燃料电池技术的发展与我国应有的对策
查全性
摘要:本文介绍了几种主要类型燃料电池的最新进展,基于世界发展趋势和我国具体情况,作者在碱性燃料电池、磷酸型燃料电池、熔融碳酸盐电池和聚合物电解质燃料电池方面提出了相应对策,以促进我国燃料电池的研究与开发.
关键词:燃料电池;碱性燃料电池;磷酸燃料电池;熔融碳酸盐燃料电池;聚合物电解质燃料电池
来源出版物:应用化学,1993, 10(5): 38-42
被引频次:95
燃料电池——有前途的分布式发电技术
张颖颖,曹广益,朱新坚
摘要:在现代发电系统中,分布式发电技术日益成为传统大电网的有力补充.文章简要介绍了分布式发电的优势、种类及其各自的特点,以及各种分布式发电技术的市场发展趋势,详细分析了各种燃料电池在分布式发电市场中的应用现状,对燃料电池在分布式发电市场中的应用前景进行了展望,并结合中国国情指出了我国有条件并且应该加快发展燃料电池分布式发电技术.
关键词:大电网;分布式发电;燃料电池;商业化;电力体制改革;可持续发展
来源出版物:电网技术,2005, 29(2): 57-61
被引频次:91
固体氧化物燃料电池
彭苏萍,韩敏芳,杨翠柏,等
摘要:高效、洁净、全固态结构、高温运行的固体氧化物燃料电池(SOFC)是把反应物的化学能直接转化为电能的电化学装置,这种新型发电技术是目前发展最快的能源技术之一,有望在近年内走向商业化应用.SOFC单体电池由致密的电解质和多孔的阳极、阴极组成,现在主要发展了管状结构和平板式结构两种形式.单体电池通过致密的连接体材料以各种方式组装成电池组,广泛应用于大型发电厂、热电耦合设备、小型供能系统和交通工具等,市场前景广阔.
关键词:固体氧化物燃料电池(SOFC);新型能源
来源出版物:物理,2004, 33(2): 90-94
被引频次:91
中温固体氧化物燃料电池电解质材料的研究进展
魏丽,陈诵英,王琴
摘要:评述了中温固体氧化物燃料电池(中温SOFC)中固体电解质的研究进展,对ZrO2基、CeO2基、Bi2O3基和ABO3型的钙钛矿类4种电解质材料的最新进展和今后的发展趋势作了评述.对几种电解质材料的优缺点进行了分析,同时对高温电解质YSZ薄膜化技术也作了简要介绍,因此不难得出,寻求新的、优良的中温SOFC的电解质材料仍然是新世纪推动中温SOFC实用化的关键任务之一,而YSZ薄膜化技术的研究则是研究的另一个重点,且最有可能取得突破.
关键词:材料科学;中温SOFC;电解质材料;电导率;YSZ薄膜
来源出版物:稀有金属,2003, 27(2): 286-292
被引频次:85
燃料电池电动汽车的技术难关和发展前景
陈全世,齐占宁
摘要:本文在阐述了质子交换膜燃料电池上作原理的基础上,首先介绍了其质子交换膜与催化剂的研究现状.然后针对汽车领域的需要,给出了燃料电池发动机的概念,并对其燃料和氧化剂供给、水/热管理和控制等各子系统所要解决的技术难关进行了系统分析.同时对燃料电池车商业化所必然要涉及的氢燃料供给和价格等问题进行了较客观的论述.最后对燃料电池车的发展前景进行了预测,提出了相应的发展措施.
关键词:质子交换膜燃料电池;电动汽车;技术难关;前景
来源出版物:汽车工程,2001, 23(6): 361-364
被引频次:2406
Materials for fuel-cell technologies
Steele, BCH; Heinzel, A
Abstract: Fuel cells convert chemical energy directly into electrical energy with high efficiency and low emission of pollutants. However,before fuel-cell technology can gain a significant share of the electrical power market, important issues have to be addressed. These issues include optimal choice of fuel, and the development of alternative materials in the fuel-cell stack. Present fuel-cell prototypes often use materials selected more than 25 years ago. Commercialization aspects, including cost and durability, have revealed inadequacies in some of these materials. Here we summarize recent progress in the search and development of innovative alternative materials.
Keywords: polymer electrolyte; stainless-steel; bipolar plates; 500-degrees-c; operation
来源出版物:Nature, 2001, 414(6861): 345-352
被引频次:1674
On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells
Kreuer, KD
Abstract: The transport properties and the swelling behaviour of NAFION and different sulfonated polyetherketones are explained in terms of distinct differences on the microstructures and in the pK(a)of the acidic functional groups. The less pronounced hydrophobic/hydrophilic separation of sulfonated polyetherketones compared to NAFION corresponds to narrower, less connected hydrophilic channels and to larger separations between less acidic sulfonic acid functional groups. At high water contents, this is shown to significantly reduce electroosmotic drag and water permeation whilst maintaining high proton conductivity. Blending of sulfonated polyetherketones with other polyaryls even further reduces the solvent permeation (a factor of 20 compared to NAFION), increases the membrane flexibility in the dry state and leads to an improved swelling behaviour. Therefore, polymers based on sulfonated polyetherketones are not only interesting low-cost alternative membrane material for hydrogen fuel cell applications, they may also help to reduce the problems associated with high water drag and high methanol cross-over in direct liquid methanol fuel cells (DMFC). The relatively high conductivities observed for oligomers containing imidazole as functional groups may be exploited in fully polymeric proton conducting systems with no volatile proton solvent operating at temperatures significantly beyond 100 degrees C, where methanol vapour may be used as a fuel in DMFCs.
Keywords: NAFION; polymer membrane; direct liquid methanol fuel cell; proton conductivity; electroosmotic drag; permeation; proton diffusion
来源出版物:Journal of Membrane Science, 2001, 185(1): 29-39
被引频次:1656
Polymer Electrolyte Fuel-Cell Model
Springer, TE
Abstract: 参见本期“经典文献推荐”栏目.
被引频次:1332
A high-performance cathode for the next generation of solid-oxide fuel cells
Shao, ZP; Haile, SM
Abstract: Fuel cells directly and efficiently convert chemical energy to electrical energy (1). Of the various fuel cell types, solid-oxide fuel cells (SOFCs)combine the benefits of environmentally benign power generation with fuel flexibility. However, the necessity for high operating temperatures (800-1000 degrees C)has resulted in high costs and materials compatibility challenges (2). As a consequence,significant effort has been devoted to the development of intermediate-temperature (500-700 degrees C)SOFCs. A key obstacle to reduced-temperature operation of SOFCs is the poor activity of traditional cathode materials for electrochemical reduction of oxygen in this temperature regime (2). Here we present Ba0.5Sr0.5Co0.8Fe0.2O3-delta (BSCF)as a new cathode material for reduced-temperature SOFCoperation. BSCF, incorporated into a thin-film doped ceria fuel cell, exhibits high power densities (1010 mW cm-2and 402 mW cm-2at 600 degrees C and 500 degrees C, respectively)when operated with humidified hydrogen as the fuel and air as the cathode gas. We further demonstrate that BSCF is ideally suited to ‘single-chamber’ fuel-cell operation, where anode and cathode reactions take place within the same physical chamber (3). The high power output of BSCF cathodes results from the high rate of oxygen diffusion through the material. By enabling operation at reduced temperatures, BSCF cathodes may result in widespread practical implementation of SOFCs.
Keywords: oxygen permeation; membranes; electrolyte; stability
来源出版物:Nature, 2004, 431(7005): 170-173
被引频次:1034
Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells
Qu, Liangti; Liu, Yong; Baek, Jong-Beom
Abstract: Nitrogen-doped graphene (N-graphene)was synthesized by chemical vapor deposition of methane in the presence of ammonia. The resultant N-graphene was demonstrated to act as a metal-free electrode with a much better electrocatalytic activity, long-term operation stability, and tolerance to crossover effect than platinum for oxygen reduction via a four-electron pathway in alkaline fuel cells. To the best of our knowledge, this is the first report on the use of graphene and its derivatives as metal-free catalysts for oxygen reduction. The important role of N-doping to oxygen reduction reaction (ORR)can be applied to various carbon materials for the development of other metal-free efficient ORR catalysts for fuel cell applications, even new catalytic materials for applications beyond fuel cells.
Keywords: graphene; N-doping; oxygen reduction; fuel cell
来源出版物:ACS Nano, 2010, 4(3): 1321-1326
被引频次:1023
Origin of the over potential for oxygen reduction at a fuel-cell cathode
Norskov, JK; Rossmeisl, J; Logadottir, A; et al.
Abstract: We present a method for calculating the stability of reaction intermediates of electrochemical processes on the basis of electronic structure calculations. We used that method in combination with detailed density functional calculations to develop a detailed description of the free-energy landscape of the electrochemical oxygen reduction reaction over Pt (111)as a function of applied bias. This allowed us to identify the origin of the over potential found for this reaction. Adsorbed oxygen and hydroxyl are found to be very stable intermediates at potentials close to equilibrium, and the calculated rate constant for the activated proton/electron transfer to adsorbed oxygen or hydroxyl can account quantitatively for the observed kinetics. On the basis of a database of calculated oxygen and hydroxyl adsorption energies, the trends in the oxygen reduction rate for a large number of different transition and noble metals can be accounted for. Alternative reaction mechanisms involving proton/electron transfer to adsorbed molecular oxygen were also considered, and this peroxide mechanism was found to dominate for the most noble metals. The model suggests ways to improve the electrocatalytic properties of fuel-cell cathodes.
Keywords: total-energy calculations; alloy surfaces; electrocatalysis; kinetics; CO; oxidation; platinum; temperature; electrodes;adsorption
来源出版物:Journal of Physical Chemistry B, 2004, 108(46): 17886-17892
被引频次:940
Direct oxidation of hydrocarbons in a solid-oxide fuel cell
Park, SD; Vohs, JM; Gorte, RJ
Abstract: The direct electrochemical oxidation of dry hydrocarbon fuels to generate electrical power has the potential to accelerate substantially the use of fuel cells in transportation and distributed-power applications (1). Most fuel-cell research has involved the use of hydrogen as the fuel, although the practical generation and storage of hydrogen remains an important technological hurdle (2). Methane has been successfully oxidized electrochemically (3-6), but the susceptibility to carbon formation from other hydrocarbons that may be present or poor power densities have prevented the application of this simple fuel in practical applications (1). Here we report the direct,electrochemical oxidation of various hydrocarbons (methane, ethane, 1-butene, n-butane and toluene)using a solid-oxide fuel cell at 973 and 1073 K with a composite anode of copper and ceria (or samaria-doped ceria). We demonstrate that the final products of the oxidation are CO2and water, and that reasonable power densities can be achieved. The observation that a solid-oxide fuel cell can be operated on dry hydrocarbons, including liquid fuels, without reforming suggests that this type of fuel cell could provide an alternative to hydrogen-based fuel-cell technologies.
来源出版物:Nature, 2000, 404(6775): 265-267
被引频次:875
A Mathematical-Model of the Solid-Polymer-Electrolyte Fuel-Cell
Bernardi, DM; Verbrugge, MW
Abstract: 参见本期“经典文献推荐”栏目.
被引频次:827
Iron-Based Catalysts with Improved Oxygen Reduction Activity in Polymer Electrolyte Fuel Cells
Lefevre, Michel; Proietti, Eric; Jaouen, Frederic; et al.
Abstract: Iron-based catalysts for the oxygen-reduction reaction in polymer electrolyte membrane fuel cells have been poorly competitive with platinum catalysts, in part because they have a comparatively low number of active sites per unit volume. We produced microporous carbon-supported iron-based catalysts with active sites believed to contain iron cations coordinated by pyridinic nitrogen functionalities in the interstices of graphitic sheets within the micropores. We found that the greatest increase in site density was obtained when a mixture of carbon support, phenanthroline, and ferrous acetate was ball-milled and then pyrolyzed twice, first in argon, then in ammonia. The current density of a cathode made with the best iron-based electrocatalyst reported here can equal that of a platinum-based cathode with a loading of 0.4 milligram of platinum per square centimeter at a cell voltage of >=0.9 volt.
Keywords: heat-treatment affect; cathode catalyst; nonnoble electrocatalysts; sputter-deposition; Fe/N/C catalysts; O-2 reduction;carbon-blacks; site; ORR; electroreduction
来源出版物:Science, 2009, 324(5923): 71-74
被引频次:815
A class of non-precious metal composite catalysts for fuel cells
Bashyam, Rajesh; Zelenay, Piotr
Abstract: Fuel cells, as devices for direct conversion of the chemical energy of a fuel into electricity by electrochemical reactions, are among the key enabling technologies for the transition to a hydrogen-based economy (1-3). Of several different types of fuel cells under development today, polymer electrolyte fuel cells (PEFCs)have been recognized as a potential future power source for zero-emission vehicles (4, 5). However, to become commercially viable, PEFCs have to overcome the barrier of high catalyst cost caused by the exclusive use of platinum and platinum-based catalysts (6-8)in the fuel-cell electrodes. Here we demonstrate a new class of low-cost (non-precious metal)/(heteroatomic polymer)nanocomposite catalysts for the PEFC cathode, capable of combining high oxygen-reduction activity with good performance durability. Without any optimization, the cobalt-polypyrrole composite catalyst enables power densities of about 0.15 W cm-2in H-2-O-2 fuel cells and displays no signs of performance degradation for more than 100 hours. The results of this study show that heteroatomic polymers can be used not only to stabilize the non-precious metal in the acidic environment of the PEFC cathode but also to generate active sites for oxygen reduction reaction.
Keywords: Fe-based catalysts; oxygen reduction; platinum monolayer; O-2 reduction; carbon-black; electrocatalysts; cathode; polypyrrole;proton; energy
来源出版物:Nature, 2006, 443(7107): 63-66
·推荐论文摘要·
低温固体氧化物燃料电池电解质材料
韩达,吴天植,辛显双,等
摘要:低温化是固体氧化物燃料电池(SOFC)发电技术的重要发展趋势.SOFC工作温度的降低不仅可极大地降低材料及制备成本,更重要的是可极大地提高其长期运行的稳定性.电解质是SOFC的核心部件,可以采用电解质薄膜化或新型电解质材料来降低SOFC的工作温度.本文概述了目前被广泛研究的低温SOFC的电解质材料,并从其结构及性能出发,重点阐述了它们各自的优点和局限性.
关键词:低温SOFC;新型电解质;离子电导率
来源出版物:中国工程科学,2013, 15(2): 66-71联系邮箱:占忠亮,zzhan@mail.sic.ac.cn
吡啶掺杂碳载钴酞菁催化氧还原的电化学性能及在燃料电池中的应用
戴先逢,郑明富,徐攀,等
摘要:以碳黑(VulcanXC-72R)为载体,吡啶(Py)和钴酞菁(CoPc)为催化剂前驱体,经溶剂分散法制备了Py掺杂碳负载纳米钴酞菁复合催化剂(Py-CoPc/C).通过扫描电镜-能谱分析(SEM-EDS)、X射线光电子能谱(XPS)分析和X射线衍射(XRD)分析技术对催化剂的组成和微观结构进行了表征,并运用线性扫描循环伏安法(LSV)和旋转圆盘电极(RDE)技术考察了不同Py掺杂含量对碳载钴酞菁(CoPc/C)催化氧还原反应(ORR)活性的影响及稳定性.结果显示:Py掺杂可以明显改善CoPc/C对ORR的电催化性能,其中掺杂20%Py下所制备的20%Py-20%CoPc/C催化剂对ORR表现出最佳的催化活性,以其制备的气体扩散电极在O2气氛饱和的0.1 mol·L-1KOH电解质溶液中,0.2 V(相对于标准氢电极)即可产生明显的氧还原电流,半波电位为-0.03 V.相比于40%Py/C和未掺杂的40%CoPc/C,20%Py-20%CoPc/C催化剂的半波电位分别正移了160和15 mV.进一步运用RDE理论研究表明,在Py-CoPc/C电极上ORR的电子转移总数为2.38,高于CoPc/C电极上的电子转移总数1.96,从而使ORR的选择性明显提高.SEM-EDS和XRD分析表明Py掺杂提高了CoPc/C催化剂的分散性和N含量,更利于O2的吸附.XPS分析表明:吡啶结构的N与石墨结构的N均存在于Py-CoPc/C催化剂中,与催化剂表面的Co离子配位可能是促使ORR活性提高的原因.最后以20%Py-20%CoPc/C制备了膜电极组装(MEA)电极,应用于 H2/O2燃料电池单电池发电,室温下获得最大发电功率密度为21 mW·cm-2,相对于CoPc/C提高至2.4倍.
关键词:碳载钴酞菁;吡啶氮掺杂;氧还原反应;膜电极组装;H2-O2单电池
来源出版物:物理化学学报,2013, 29(8): 1753-1761联系邮箱:乔锦丽,qiaojl@dhu.edu.cn
质子交换膜燃料电池Pt纳米线电催化剂研究现状
严泽宇,李冰,杨代军,等
摘要:质子交换膜燃料电池(PEMFC)能直接将化学能转换为电能,具有能量转换效率高、环境友好、启动快等优点.其中电催化剂是决定PEMFC性能、寿命及成本的关键材料之一.目前所采用的Pt催化剂成本较高,是阻碍其商业化的主要因素.而Pt纳米线电催化剂的Pt利用率和催化剂活性高,抗CO毒性以及耐久性好.本文综述了Pt纳米线电催化剂的制备及其电化学催化性能的研究现状.
关键词:质子交换膜燃料电池;电催化剂;铂;纳米线
来源出版物:催化学报,2013, 34(8): 1471-1481联系邮箱:李冰,libing210@tongji.edu.cn
质子交换膜燃料电池阴极风扇系统实验研究
朱星光,贾秋红,陈唐龙
摘要:对自制的阴极开放式自增湿型质子交换膜燃料电池阴极风扇系统不同工作模式下电池的空气流量分布及温度分布开展了实验研究.采用testo435多功能测量仪测量不同工作模式下电池阴极的空气流速;采用FLUKE Ti25红外温度成像仪测量不同操作模式下电池的表面温度分布.实验结果表明:阴极风扇系统不同的工作模式(“吸”和“吹”)会造成空气流量分布及温度分布不同.风扇工作在“吸”模式下,燃料电池的表面工作温度分布和空气流量分布更均匀,性能更好;电池表面工作温度分布与流过电池阴极的空气流量具有一致性.该研究对于阴极开放式燃料电池性能研究及寻求电池系统效率、性能、温湿度等整体最优具有一定的指导和参考价值.
关键词:质子交换膜燃料电池;阴极风扇系统;不同工作模式;空气流量分布;表面工作温度分布
来源出版物:中国电机工程学报,2013, 33(11): 47-53联系邮箱:贾秋红,jqh.01@163.com
改性石墨烯用作燃料电池阴极催化剂
钟轶良,莫再勇,杨莉君,等
摘要:石墨烯材料以其独特的超薄片层结构、超高比表面积、良好的导电性等重要特性,而被认为在制备高性能燃料电池催化剂方面具有重要的潜在应用价值.最近的一些研究工作表明,通过选择合适的制备方法和前驱体制备的改性石墨烯,对于氧还原反应具有一定的活性,可用作燃料电池阴极催化剂.目前有关石墨烯应用于燃料电池阴极催化剂的研究工作主要集中在两个方面:一是通过表面改性后直接作为燃料电池非贵金属阴极催化剂;二是将改性石墨烯作阴极催化剂载体而制备活性组分高度分散的高性能催化剂.尽管有关改性石墨烯的氧还原活性中心的结构尚不明确,然而由于这类材料在酸性及碱性环境下对氧还原的良好的催化性能,对改性石墨烯的研究已成为探索燃料电池非铂催化剂的新途径.随着这类材料的催化性能的不断提高和对表面-活性关系认识的不断深入,改性石墨烯材料在燃料电池方面将具有广阔的应用前景.
关键词:石墨烯改性;燃料电池;氧还原反应;阴极催化剂
来源出版物:化学进展,2013, 25(5): 717-725联系邮箱:廖世军,chsiliao@scut.edu.cn
新型钴-聚吡咯-碳载Pt燃料电池催化剂的制备与表征
范仁杰,林瑞,黄真,等
摘要:采用脉冲微波辅助化学还原法制备了钴-聚吡咯-碳(Co-PPy-C)载Pt催化剂(Pt/Co-PPy-C),其中Pt的总质量占20%.利用透射电镜(TEM)、光电子射线能谱分析(XPS)和X射线衍射(XRD)研究了催化剂的结构,用循环伏安(CV)、线性扫描伏安(LSV)等方法考察了其电化学活性及氧还原反应(ORR)动力学特性及耐久性.Pt/Co-PPy-C电催化剂的金属颗粒直径约1.8 nm,略小于商用催化剂Pt/C(JM)颗粒尺寸(约2.5 nm);催化剂在载体上分散均匀,粒径分布范围较窄.Pt/Co-PPy-C的电化学活性比表面积(ECSA)(75.1 m2·g-1)高于商用催化剂的ECSA(51.3 m2·g-1).XPS测试表明,自制催化剂表面的Pt主要以零价形式存在.而XRD结果显示,自制催化剂中Pt(111)峰最强,Pt主要为面心立方晶格.Pt/Co-PPy-C具有与Pt/C(JM)相同的半波电位;在0.9 V下.Pt/Co-PPy-C的比活性(1.21 mA·cm-2)高于商用催化剂的比活性(1.04 mA·cm-2),表现出更好的ORR催化活性.动力学性能测试表明催化剂的ORR反应以四电子路线进行.CV测试1000圈后,Pt/Co-PPy-C和Pt/C(JM)的ECSA分别衰减了13.0%和24.0%,可见自制催化剂的耐久性高于商用Pt/C(JM),在质子交换膜燃料电池(PEMFC)领域有一定的应用前景.
关键词:质子交换膜燃料电池;催化剂;钴-聚吡咯-碳;氧还原反应;微波化学还原
来源出版物:物理化学学报,2014, 30(7): 1259-1266联系邮箱:林瑞,ruilin@tongji.edu.cn
通过溅射与退火制备的用于固体氧化物燃料电池的氧化钆掺杂氧化铈电解质隔层
武卫明,刘中波,赵哲,等
摘要:采用溅射或溅射与退火相结合的方法制备了一系列氧化钆掺杂的氧化铈(GDC)隔层,并考察了其对固体氧化燃料电池性能的影响.结果表明,200°C下溅射获得了立方结构氧化钆掺杂的氧化铈均匀薄膜,在900~1100°C范围内的退火处理使得 GDC薄膜致密,从而有效阻止了氧化钇掺杂的氧化锆电解质与阴极材料之间的反应,大幅度提高了电池的电化学性能.
关键词:固体氧化物燃料电池;稀土金属氧化物;氧化钆掺杂的氧化铈;隔层;溅射;退火
来源出版物:催化学报,2014, 35(8): 1376-1384联系邮箱:程谟杰,mjcheng@dicp.ac.cn
固体氧化物燃料电池平板式电池堆的研究进展
宋世栋,韩敏芳,孙再洪
摘要:燃料电池可以直接将燃料的化学能转化为电能,其发电效率高、污染物排放少,是一种高效、洁净的发电装置.固体氧化物燃料电池(SOFC)的燃料适用性强、稳定性好,被认为是现阶段最有应用前景的绿色发电系统.本文介绍了SOFC的平板式单电池及电池堆的最新研究进展,以及国际上代表性研发单位的技术现状,并提出了在平板式SOFC商业化进程中亟待解决的问题.
关键词:固体氧化物燃料电池;电池堆;发电系统;平板式;碳氢燃料
来源出版物:科学通报,2014, 59(15):1405-1416联系邮箱:韩敏芳,hanminfang@sina.com
交流阻抗技术在质子交换膜燃料电池上的研究进展
蔡光旭,郭建伟,王佳
摘要:质子交换膜燃料电池(PEMFC)具有低温、高效、零排放等特点,是有效解决环境污染和能源危机的发电装置,然而其内在电化学、传输机理不明确限制了其发展.交流阻抗技术(EIS)作为研究电极过程动力学和表面现象的重要手段,应用在PEMFC上受到高度重视.本文概括介绍了EIS的应用原理以及对于PEMFC的测量方式,并重点结合电池电极中典型的阻抗谱解析,总结了近来EIS在电池和材料两个方面的研究进展,从原位极化分析、材料性能评估及反应机理剖析等几个方面予以深入,详细分析了各阻抗元件参数对电池和材料改进的指导作用,进而展望了EIS在燃料电池上的应用前景,指出除了采用等效电路加以分析以外,结合数学模型推导将更加完美呈现出阻抗谱数据的特点.
关键词:交流阻抗技术;电极;材料;质子交换膜燃料电池
来源出版物:化工进展,2014, 33(1): 56-63联系邮箱:郭建伟,jwguo@mail.tsinghua.edu.cn
共流延法制备固体氧化物燃料电池阳极的优化
骆婷,史坚,王绍荣,等
摘要:采用共流延成型、共烧结法制备了以Ni-YSZ阳极支撑的氧化钪稳定的氧化锆(SSZ)电解质膜.为提高电化学活性在支撑阳极与电解质膜之间引入了Ni-SSZ活性阳极.通过调整活性阳极的厚度和SSZ:NiO的质量比优化了阳极活性;通过比较支撑阳极中添加不同造孔剂含量时的性能,优化了支撑阳极的孔隙率.研究结果表明,当活性层厚度为35 μm,质量比为 w(SSZ):w(NiO)=1:1,支撑层造孔剂含量为10 wt%时,阳极活性最佳;采用丝网印刷并烧结LSM-SSZ复合阴极后,所得单电池在750℃的最高功率密度达到0.96 W/cm2,比优化前本课题组前期报道的性能提高了2.3倍.
关键词:固体氧化物燃料电池;活性层;优化;流延法
来源出版物:无机材料学报,2014, 29(2): 203-208联系邮箱:王绍荣,srwang@mail.sic.ac.cn
碳材料的掺杂改性及其用于燃料电池催化剂的研究
郑锐萍,廖世军
摘要:开发掺杂改性的碳材料用作燃料电池的非贵金属氧还原催化剂已成为燃料电池领域的重要研究课题,相关研究对于降低燃料电池成本、促进燃料电池的商业化具有十分重要的意义.大量研究工作表明,对碳材料进行掺杂改性可以实现其形貌、微观结构、组成及其他表面物理化学性质的优化,从而得到具有较高催化活性、选择性和稳定性的氧还原催化剂.人们在这类催化剂的制备方法、性能优化和催化机理等方面进行了大量研究工作.综述了对碳纳米管、石墨烯、介孔碳、大孔碳、碳微球等碳材料进行掺杂改性的最新进展.并基于目前的研究结果,展望了掺杂碳材料作为燃料电池非贵金属氧还原催化剂的应用前景和未来的发展趋势.
关键词:掺杂;改性;碳材料;催化剂;燃料电池
来源出版物:表面技术,2015, 44(1): 34-40
以木片气为燃料的中温型固体氧化物燃料电池/燃气轮机混合动力系统性能研究
吕小静,耿孝儒,朱新坚,等
摘要:以木片气化气为燃料,建立中温型固体氧化物燃料电池(intermediate temperature solid oxide fuel cell, IT-SOFC)/燃气轮机(gas turbine, GT)混合动力系统的详细模型,分析混合动力系统的运行性能,研究生物质气的组分和水碳比的变化对混合动力系统性能的影响.结果表明,在设计工况下,以木片气化气为燃料的IT-SOFC/GT混合动力系统的发电效率高达59.24%,具有较好的系统性能.生物质气组分的变化对混合动力系统性能影响很大,H2百分比的变化使系统输出功率变化幅度最大,CO和 CH4相近,系统的发电效率随H2百分比增加略有上升,随CO和CH4百分比的增加下降明显.研究还表明,当水碳摩尔比([S]/[C])改变时,系统输出功率和发电效率随着[S]/[C]的减小而逐渐增加,但从系统运行安全性和寿命方面考虑,应选择适当的[S]/[C]值.
关键词:中温型固体氧化物燃料电池;燃气轮机;混合动力系统;生物质气;组分变化;水碳比变化
来源出版物:中国电机工程学报,2015, 35(1): 133-141
直接碳燃料电池燃料的研究进展
刘国阳,张亚婷,蔡江涛,等
摘要:直接碳燃料电池(DCFC)具有能量转化效率高、污染低、燃料来源广等优点,是缓解能源危机和环境污染的一种有效途径,其性能与所使用的燃料密切相关.本文介绍DCFC的发展历史、研究现状及发展动态,评述了煤、焦炭、活性炭、石墨等含碳物质作为DCFC燃料的优缺点,分析讨论了碳燃料的晶体结构缺陷、表面含氧官能团对阳极电化学反应的促进作用,以及碳燃料的电解质润湿能力、孔隙结构、电导率、粒径大小对阳极电化学反应的质量传递与电荷传递的相互关系;探讨了阳极催化剂促进阳极反应并提高电池性能的机制;简要讨论了DCFC碳燃料的未来发展趋势.
关键词:直接碳燃料电池;燃料;煤;发展趋势
来源出版物:新型炭材料,2015, 30(1): 12-18联系邮箱:邱介山,jqiu@ dlut.edu.cn
质子交换膜燃料电池电源系统停机特性及控制策略
彭跃进,彭赟,李伦,等
摘要:质子交换膜燃料电池(PEMFC)电源系统在停机后,燃料电池开路高电压被认为是造成电池性能下降和寿命缩短的重要因素.这主要是因为PEMFC电源系统停机后,燃料电池处于开路状态,阳极侧残留的氢气和阴极侧的空气发生电化学反应,电池电压为开路高电压且维持在开路电压的时间比较长,这容易引起催化剂碳载体发生氧化,使分布在载体上的铂(Pt)颗粒脱落,造成燃料电池性能衰减以及寿命缩短.以最大程度缩短停机后开路高电压的时间和加快阳极侧残留氢气的消耗速度为目标,提出了一种PEMFC电源系统的停机策略,通过实验分别研究了直接停机和停机策略停机对PEMFC输出特性的影响.以该停机控制策略为基础,通过实验验证了该停机策略的有效性,为提出保护性的PEMFC电源系统停机控制策略提供了参考性指导.
关键词:质子交换膜燃料电池;氧化;碳载体;腐蚀;停机策略
来源出版物:化工学报,2015, 66(3): 1178-1184联系邮箱:刘志祥,liuzhixiang@swjtu.edu.cn
车用质子交换膜燃料电池材料部件
王诚,王树博,张剑波,等
摘要:车用燃料电池主要包括质子交换膜燃料电池、金属-空气燃料电池等,其中质子交换膜燃料电池是目前车用燃料电池的主要开发对象(以下简称车用燃料电池).经过全球范围内近十年的持续研发,车用燃料电池在能量效率、功率密度与比功率、低温启动等功能特性方面已经取得了突破性进展,新一轮的燃料电池汽车产业化浪潮正在迫近.然而,车用燃料电池的耐久性和成本还没达到预期商业化目标,是其产业化的最后障碍.探索和研发燃料电池用新型关键材料部件是解决这两大问题、推进其商业化进程的关键所在,也是车用燃料电池长期的研究重点和热点.本文系统地梳理了近几年来车用燃料电池质子交换膜、催化层、气体扩散层、双板板关键材料部件的研究进展和成果,并分类进行了简要评述,分析了其性能与商业化目标的差距.最后展望了车用燃料电池关键材料部件今后的发展方向.
关键词:氢能;燃料电池汽车;催化剂;质子交换膜;扩散层;双极板
来源出版物:化学进展,2015, 37(2/3): 310-320联系邮箱:王诚,wangcheng@tsinghua.edu.cn
Electrocatalyst approaches and challenges for automotive fuel cells
Mark K. Debe
Abstract: Fuel cells powered by hydrogen from secure and renewable sources are the ideal solution for non-polluting vehicles, and extensive research and development on all aspects of this technology over the past fifteen years has delivered prototype cars with impressive performances. But taking the step towards successful commercialization requires oxygen reduction electrocatalysts-crucial components at the heart of fuel cells-that meet exacting performance targets. In addition, these catalyst systems will need to be highly durable,fault-tolerant and amenable to high-volume production with high yields and exceptional quality. Not all the catalyst approaches currently being pursued will meet those demands.
来源出版物:Nature, 2012, 486: 43-51联系邮箱:M. K. Debe; mkdebe1@mmm.com
High temperature (HT)polymer electrolyte membrane fuel cells (PEMFC)-A review
Chandan, Amrit; Hattenberger, Mariska; El-Kharouf, Ahmad; et al.
Abstract: One possible solution of combating issues posed by climate change is the use of the High Temperature (HT)Polymer ElectrolyteMembrane (PEM)Fuel Cell (FC)in some applications. The typical HT-PEMFC operating temperatures are in the range of 100-200 degrees C which allows for co-generation of heat and power, high tolerance to fuel impurities and simpler system design. This paper reviews the current literature concerning the HT-PEMFC, ranging from cell materials to stack and stack testing. Only acid doped PBI membranes meet the US DOE (Department of Energy)targets for high temperature membranes operating under no humidification on both anode and cathode sides (barring the durability). This eliminates the stringent requirement for humidity however, they have many potential drawbacks including increased degradation, leaching of acid and incompatibility with current state-of-the-art fuel cell materials. In this type of fuel cell, the choice of membrane material determines the other fuel cell component material composition, for example when using an acid doped system,the flow field plate material must be carefully selected to take into account the advanced degradation. Novel research is required in all aspects of the fuel cell components in order to ensure that they meet stringent durability requirements for mobile applications.
Keywords: Fuel cell; Intermediate/high temperature PEM; Stack; Bipolar plate; Catalyst layer; Gas diffusion layer
来源出版物:Journal of Power Sources, 2013, 231: 264-278联系邮箱:Bujalski, W; w.bujalski@bham.ac.uk
Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction
Yang, Zhi; Nie, Huagui; Chen, Xi’an; et al.
Abstract: The fuel cell (FC), as a clean and high-efficiency device, has drawn a great deal of attention in terms of both fundamentals and applications. However, the high cost and scarcity of the requisite platinum catalyst as well as a sluggish oxygen reduction reaction (ORR)at the cathode in FC have become the greatest barrier to large-scale industrial application of FC. The development of novel non-precious metal catalysts (NPMC)with excellent electrocatalytic performance has been viewed as an important strategy to promote the development of FC. Recent studies have proven that metal free carbon materials doped with heteroatom (e.g. N, B, P, S or Se)have also shown striking electrocatalytic performance for ORR and become an important category of potential candidates for replacing Pt-based catalysts. This review summarizes recent achievements in heteroatom doped carbon materials as ORR catalysts, and will be beneficial to future development of other novel low-cost NPMCs with high activities and long lifetimes for practical FC applications.
Keywords: Doping; Oxygen reduction; Fuel cell; Graphene; Carbon nanotubes
来源出版物:Journal of Power Sources, 2013, 236: 238-249联系邮箱:Yang, Zhi; yang201079@126.com
PdAg Nanorings Supported on Graphene Nanosheets: Highly Methanol-Tolerant Cathode Electrocatalyst for Alkaline Fuel Cells
Liu, Minmin; Lu, Yizhong; Chen, Wei
Abstract: Due to the high costs, slow reaction kinetics, and methanol poisoning of platinum-based cathode catalysts, designing and exploring non-Pt or low-Pt cathode electrocatalysts with a low cost, high catalytic performance, and high methanol-tolerance are crucial for the commercialization of fuel cells. Here, a facile method to fabricate a system of PdAg nanorings supported by graphene nanosheets is demonstrated; the fabrication is based on the galvanic displacement reaction between pre-synthesized Ag nanoparticles and palladium ions. X-ray diffraction and high-resolution transmission electron microscopy show that the synthesized PdAg nanocrystals exhibit a ring-shaped hollow structure with an average size of 27.49 nm and a wall thickness of 5.5 nm. Compared to the commercial PdC catalyst, the PdAg nanorings exhibit superior properties as a cathode electrocatalyst for oxygen reduction. Based on structural and electrochemical studies,these advantageous properties include efficient usage of noble metals and a high surface area because of the effective utilization of both the exterior and interior surfaces, high electrocatalytic performance for oxygen reduction from the synergistic effect of the alloyed PdAg crystalline phase, and most importantly, excellent tolerance of methanol crossover at high concentrations. It is anticipated that this synthesis of graphene-based PdAg nanorings will open up a new avenue for designing advanced electrocatalysts that are low in cost and that exhibit high catalytic performance for alkaline fuel cells.
Keywords: nanorings; palladium; oxygen reduction reaction; electrocatalysts; fuel cells
来源出版物:Advanced Functional Materials, 2013, 23(10): 1289-1296联系邮箱:Liu, Minmin; weichen@ciac.jl.cn
Alkaline polymer electrolyte membranes for fuel cell applications
Wang, Yan-Jie; Qiao, Jinli; Baker, Rya; et al.
Abstract: In this review, we examine the most recent progress and research trends in the area of alkaline polymer electrolyte membrane(PEM)development in terms of material selection, synthesis, characterization, and theoretical approach, as well as their fabrication into alkaline PEM- based membrane electrode assemblies (MEAs)and the corresponding performance/durability in alkaline polymer electrolyte membrane fuel cells (PEMFCs). Respective advantages and challenges are also reviewed. To overcome challenges hindering alkaline PEM technology advancement and commercialization, several research directions are then proposed.
Keywords: anion-exchange membranes; quaternized poly(vinyl alcohol); cross-linking; conducting membranes; transport-properties; oxy-gen reduction; performance; stability; hydroxide; radiation
来源出版物:Chemical Society Reviews, 2013, 42(13): 5768-5787联系邮箱:Qiao, JL; qiaojl@dhu.edu.cn
Engineering Interface and Surface of Noble Metal Nanoparticle Nanotubes toward Enhanced Catalytic Activity for Fuel Cell Applications
Cui, Chun-Hua; Yu, Shu-Hong
Abstract: In order for fuel cells to have commercial viability as alternative fuel sources, researchers need to develop highly active and robust fuel cell electrocatalysts. In recent years, the focus has been on the design and synthesis of novel catalytic materials with controlled interface and surface structures. Another goal is to uncover potential catalytic activity and selectivity, as well as understand their fundamental catalytic mechanisms. Scientists have achieved great progress in the experimental and theoretical investigation due to the urgent demand for broad commercialization of fuel cells in automotive applications. However, there are still three main problems: cost, performance, and stability. To meet these targets, the catalyst needs to have multisynergic functions. In addition, the composition and structure changes of the catalysts during the reactions still need to be explored.
Activity in catalytic nanomaterials is generally controlled by the size, shape, composition, and interface and surface engineering. As such,one-dimensional nanostructures such as nanowires and nanotubes are of special interest However, these structures tend to lose the nanoparticle morphology and inhibit the use of catalysts in both fuel cell anodes and cathodes. In 2003, Rubinstein and co-workers proposed the idea of nanoparticle nanotubes (NNs), which combine the geometry of nanotubes and the morphology of nanoparticles. This concept gives both the high surface-to-volume ratio and the size effect, which are both appealing in electrocatalyst design.
In this Account, we describe our developments in the construction of highly active NNs with unique surface and heterogeneous interface structures. We try to clarify enhanced activity and stability in catalytic systems by taking into account the activity Impact factors. We briefly introduce material structural effects on the electrocatalytic reactivity including metal oxide/metal and metal/metal interfaces, dealloyed pure Pt, and mixed Pt/Pd surfaces. In addition, we discuss the geometric structure and surface composition changes and evolutions on the activity, selectivity, and stability under fuel cell operation conditions. We expect that these nanostructured materials with particular nanostructured characteristics, physical and chemical properties, and remarkable structure changes will offer new opportunities for wide scientific communities.
Keywords: ternary Pt/Pd/Cu electrocatalyst; oxygen reduction reaction; high-aspect-ratio; atomic redistribution; tellurium nanowires; methanol oxidation; platinum; tubes; Au; design
来源出版物:Accounts of Chemical Research, 2013, 46(7): 1427-1437联系邮箱:Yu, SH; shyu@ustc.edu.cn
A Review of Graphene-Based Nanostructural Materials for Both Catalyst Supports and Metal-Free Catalysts in PEM Fuel Cell Oxygen Reduction Reactions
Zhou, Xuejun; Qiao, Jinli; Yang, Lin; et al.
Abstract: A comprehensive overview and description of graphene-based nanomaterials explored in recent years for catalyst supports and metal-free catalysts for polymer electrolyte membrane (PEM)fuel cell oxygen reduction reactions (ORR)is presented. The catalyst material structures/morphologies, material selection, and design for synthesis, catalytic performance, catalytic mechanisms, and theoretical approaches for catalyst down-selection and catalyzed ORR mechanisms are emphasized with respect to the performance of ORR catalysts in terms of both activity and stability. When graphene-based materials, including graphene and doped graphene, are used as the supporting materials for both Pt/Pt alloy catalysts and non-precious metal catalyst, the resulting ORR catalysts can give superior catalyst activity and stability compared to those of conventional carbon-supported catalysts; when they are used as metal-free ORR catalysts, significant catalytic activity and stability are observed. The nitrogen-doped graphene materials even show superior performance compared to supported metal catalysts. Challenges including the lack of material mass production, unoptimized catalyst structure/morphology, insufficient fundamental understanding, and testing tools/protocols for performance optimization and validation are identified, and approaches to address these challenges are suggested.
Keywords: nitrogen-doped graphene; methanol electrooxidation activity; lithium-ion batteries; one-pot synthesis; electrocatalytic activity;platinum nanoparticles; alloy nanoparticles; efficient electrocatalyst; energy-conversion; carbon nanotubes
来源出版物:Advanced Energy Materials, 2014, 4(8): 1289-1295联系邮箱:Qiao, Jinli; qiaojl@dhu.edu.cn
Numerical thermomechanical modelling of solid oxide fuel cells
Peksen, Murat
Abstract: Over the last decade, many computational models have been presented to describe the complex thermomechanical behaviour of solid oxide fuel cells. The present study elucidates a detailed literature review of the proposed numerical models, ranging from a single channel or unit layer, up to coupled 3D high-end system models. Thermomechanical modelling foundations, including material propertiesand thermomechanical stress sources in SOFCs are emphasized. Employed material models for SOFC components are highlighted. Thermomechanical modelling issues such as geometrical idealisation, initial and boundary conditions for the highly coupled fluid and solid mechanics problem, as well as numerical solutions have been discussed. Thermomechanical stress-strain formulation of the common fuel cell components is highlighted. Finally, an overview of the numerically solved thermomechanical modelling studies in solid oxide fuel cells is given. Case studies are used throughout this review to exemplify and shed light on several modelling aspects.
Keywords: CFD; FEM; Multiphysics; SOFC; Thermal stress; Thermomechanics
来源出版物:Progress in Energy and Combustion Science, 2015, 48: 1-20联系邮箱:Peksen, Murat; m.peksen@fz-juelich.de
Accelerated Membrane Durability Testing of Heavy Duty Fuel Cells
Macauley, Natalia; Alavijeh, Alireza Sadeghi; Watson, Mark; et al.
Abstract: Regular durability testing of heavy duty fuel cell systems for transit bus application requires several thousand hours of operation,which is costly and time consuming. Alternatively, accelerated durability tests are able to generate failure modes observed in field operation in a compressed time period, by applying enhanced levels of stress. The objective of the present work is to design and validate an accelerated membrane durability test (AMDT)for heavy duty fuel cells under bus related conditions. The proposed AMDT generates bus relevant membrane failure modes in a few hundred hours, which is more than an order of magnitude faster than for regular duty cycle testing. Elevated voltage, temperature, and oxidant levels are used to accelerate membrane chemical stress, while relative humidity (RH)cycling is used to induce mechanical stress. RH cycling is found to significantly reduce membrane life-time compared to constant RH conditions. The role of a platinum band in the membrane is investigated and membranes with Pt bands demonstrate a considerable life-time extension under AMDT conditions, with minimal membrane degradation. Overall, this research serves to establish a benchmark AMDT that can rapidly and reliably evaluate membrane stability under simulated heavy duty fuel cell conditions.
Keywords: perfluorosulfonated acid ionomer; polymer electrolyte membranes; active layer degradation; steady-state operation; mechanical-properties; exchange membrane; hydroxyl radicals; pemfc; platinum; hydrogen
来源出版物:Journal of the Electrochemical Society, 2015, 162(1): 98-107联系邮箱:Macauley, Natalia; ekjeang@sfu.ca
Enhancing Hybrid Direct Carbon Fuel Cell anode performance using Ag2O
Deleebeeck, L; Ippolito, D; Hansen, K. Kammer
Abstract: A hybrid-direct carbon fuel cell (HDCFC), consisting of a molten slurry of solid carbon black and LiK2CO3added to the anode chamber of a solid oxide fuel cell, was characterized using current-potentialpower density curves, electrochemical impedance spectroscopy,and cyclic voltammetry. Two types of experimental setups were employed in this study, an anode-supported full cell configuration (two electrodes, two atmospheres setup)and a 3-electrode electrolyte-supported half-cell setup (single atmosphere). Anode processes with and without catalystswere investigated as a function of temperature (700-800 degrees C)and anode sweep gas (N-2, 4-100% CO2in N-2-CO2). It was shown that the addition of silver based catalysts (Ag, Ag2O, Ag2CO3)into the carbon-carbonate slurry enhanced the performance of the HDCFC.
Keywords: Direct carbon fuel cell (DCFC); carbon black; silver oxide; electrochemical performance; cyclic voltammetry (CV)
来源出版物:Electrochimica Acta, 2015, 152: 222-239联系邮箱:Deleebeeck, L; ldel@dtu.dk
编辑:卫夏雯
We present here an isothermal, one-dimensional, steady-state model for a complete polymer electrolyte fuel cell (PEFC)with a 117 Nafion(R)membrane. In this model we employ water diffusion coefficients electro-osmotic drag coefficients, water sorption isotherms,and membrane conductivities, all measured in our laboratory as functions of membrane water content. The model predicts a net-water-per-proton flux ratio of 0.2 H2O/H+under typical operating conditions, which is much less than the measured electro-osmotic drag coefficient for a fully hydrated membrane. It also predicts an increase in membrane resistance with increased current density and demonstrates the great advantage of a thinner membrane in alleviating this resistance problem. Both of these predictions were verified experimentally under certain conditions.
loading electrodes; membranes; platinum; nafion; SPE; technology; reduction; transport
高影响力文章
典
文章题目第一作者来源出版物1Polymer Electrolyte Fuel-Cell ModelSpringer, TEJournal of the Electrochemical Society, 1991,138(8): 2334-2342 2 139(9): 2477-2491 3Two-phase flow and transport in the air cathode of proton exchange membrane fuel cellsWang, ZHJournal of Power Sources, 2001, 94(1): 40-50 A Mathematical-Model of the Bernardi, DM Journal of the Electrochemical Society, 1992,Solid-Polymer-Electrolyte Fuel-Cell 4 A Water and Heat Management Model for Nguyen, TV Journal of the Electrochemical Society, 1993,Proton-Exchange-Membrane Fuel-Cells
Polymer Electrolyte Fuel-Cell Model
Springer, TE; Zawodzinski, TA; Gottesfeld, S
*摘编自《电化学》2012年18卷1期:1~14页

