Fatty acid methyl ester profiles and nutritive values of 20 marine microalgae in Korea
Sung-Suk Suh, So Jung Kim, Jinik Hwang, Mirye Park, Taek-Kyun Lee*, Eui-Joon Kil, Sukchan Lee
1South Sea Environment Research Department, Korea Institute of Ocean Science and Technology, Geoje 656-830, Korea
2Kyeongbuk Institute for Marine Bio-Industry, Wooljin, 767-813, Republic of Korea
3Department of Genetic Engineering, Sungkyunkwan University, Suwon 440-746, Republic of Korea
Fatty acid methyl ester profiles and nutritive values of 20 marine microalgae in Korea
Sung-Suk Suh1, So Jung Kim2, Jinik Hwang1, Mirye Park1, Taek-Kyun Lee1*, Eui-Joon Kil3, Sukchan Lee3
1South Sea Environment Research Department, Korea Institute of Ocean Science and Technology, Geoje 656-830, Korea
2Kyeongbuk Institute for Marine Bio-Industry, Wooljin, 767-813, Republic of Korea
3Department of Genetic Engineering, Sungkyunkwan University, Suwon 440-746, Republic of Korea
ARTICLE INFO
Article history:
Received 15 December 2014
Received in revised form 20 January 2015
Accepted 15 February 2015
Available online 20 March 2015
Microalgae
PUFA
Nutritive values
DHA
EPA
Objective: To screen the fatty acid (FA) composition of 20 marine microalgae species, including seven Diophyceae, six Bacillariophyceae, four Chlorophyceae, two Haptophyceae and one Raphidophyceae species. Methods: Microalgal cells cultured at the Korea Institute of Ocean Science & Technology were harvested during the late exponential growth phase and the FA composition analyzed. Results: The FA composition of microalgae was speciesspecific. For example, seven different species of Dinophyceae were composed primarily of C14:0, C16:0, C18:0, C20:4n-6, C20:5n-3 and C22:6n-3, while C14:0, C16:0, C16:1, C18:0, C20:5n-3 and C22:6n-3 were abundant FAs in six species of Bacillariophyceae. In addition, four Chlorophyceae, two Haptophyceae and one Raphidophyceae species all contained a high degree of C16:1n-7 [(9.28-34.91)% and (34.48-35.04)%], C14:0 [(13.34-25.96)%] and [(26.69-28.24)%], and C16:0 [(5.89-29.15)%] and [(5.70-16.81)%]. Several factors contribute to the nutritional value of microalgae, including the polyunsaturated FA content and n-3 to n-6 FA ratio, which could be used to assess the nutritional quality of microalgae. Conclusions: This study is the first comprehensive assessment of the FA composition and nutritional value of microalgae species in South Korea, and identifies the potential utility of FAs as species-specific biomarkers.
1. Introduction
Microalgae are highly diverse photoautotrophic organisms that play a primary role in the food chain of the open sea[1] as prey for crustaceans and late larval and juvenile fish such as rotifer, copepod, daphnia, and brine shrimp[2,3]. The great diversity in microalgae has led to numerous studies on the effects of fatty acid (FA) composition and lipid class on nutritional quality and algae growth[4,5]. In addition, microalgae produce polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (DHA, 22:6n-3) and eicosapentaenoic acid (EPA, 20:5n-3)[6,7], which have a wide variety of nutraceutical and pharmaceutical applications[8,9].
The biochemical composition of microalgae is one determining factor affecting its nutritional quality and utility as a food for bivalves[10]. Many of the studies performed using juvenile or adult bivalves[11,12] have focused on microalgae PUFAs as indicators of nutritional quality[13]. Thus, the correlation between microalgae PUFA composition and nutritional value has now been established[3,14].
DHA and EPA are important n-3 PUFAs, while arachidonic acid (AA, C20:4n-6) is a vital n-6 PUFA. Modern nutritional theory has focused on the numerous health benefits of maintaining sufficient levels of n-3 PUFAs[15]. The eicosanoids, such as prostaglandin, prostacyclin and leukotriene, derived from n-3 PUFAs, are important for infant development, modulating vascular resistance and wound healing[16]. DHA plays an important role in coronary heart disease,hypertension, type II diabetes, and ocular diseases and is used in the prevention and treatment of chronic diseases such as arthritis and cystic fibrosis[15]. DHA and EPA also help treat atherosclerosis, cancer, rheumatoid arthritis, psoriasis and diseases of old age, such as Alzheimer's and age-related macular degeneration[15], while AA and DHA are particularly important for brain and blood vessels and essential for pre-and postnatal, as well as retinal, development[17].
Physiological studies of FA content and general composition using marine microalgae have been reported elsewhere, although few studies of Korean microalgae exist. The study of Korean microalgae FA composition has great significance for the use of marine bioresources as a basic material. The purpose of this study was to screen the FA composition of 20 species in the marine microalgae culture collection at the Korea Institute of Ocean Science & Technology to characterize the nutritional value of microalgae as a food source.
2. Materials and methods
2.1. Culture and isolation of microalgae
A total of 20 purified cultures from the Korea Institute of Ocean Science & Technology collection were selected, including seven dinoflagellate [Gymnodinium sanguineum, Alexandrium sp., Prorocentrum minimum (P. minimum), Prorocentrum micans (P. micans), Prorocentrum dentatum (P. dentatum), Hetrocapsa triquetra (H triqutra), Scrippsiella trochoidea (S. trochoidea)], six diatom [Chaetoceros sp., Chaetoceros didymus (C. didymus), Chaetoceros affinis (C. affinis), Thalassiosira weissflogii (T. weissflogii), Phaeodactyrum tricornutum (P. tricornutum), Skeletonema costatum (S. costatum)], four Chlorophyceae [Chlamydomonas sp., Sphaerocystis schroeteri (S. schroeteri), Chlorella ellipsoidea (C. ellipsoidea), Nannochloropsis oculata (N. oculata)], two Haptophyta [Isocrysis galbana (I. galbana), Palova gyran (P. gyran)] and one Raphidophyceae species [(Heterosigma akashiwo (H. akashiwo)]. Microalgae were cultured in F/2 media[18] at (20±1) ℃ under a 12-h light/dark cycle at 150 μmol.m-2.s-1. All microalgae were grown under the same conditions and harvested by centrifugation at 3 500 rpm for 5 min at 4 ℃ during the exponential growth phase. The harvested algae were stored at 4 ℃ until all samples were obtained and then concentrated by continuous centrifugation. The concentrated algae were freeze-dried and stored at -80 ℃ until FA analysis. Table 1 lists the strains used in this study along with cell numbers and cell dry weight/mL.
2.2. Lipid and FA extraction
Lipid extraction was performed based on the method of Folch et al[18]. After culturing microalgae, 5 mL CHCl3:MeOH (2:1) was added to 0.5 mL of the pellets collected by centrifugation and sonicated for 20 min in 5 mL 0.58% sodium chloride followed by additional sonication for 10 min. The sample was then centrifuged for 5 min at 2 000-3 000 rpm to remove the upper layer, and the separated lower layer was transferred to another tube using a Pasteur pipet dried using nitrogen gas. A known amount of heneicosanoic acid (C21:0) was used as an internal standard. Next, 0.5 mL toluene and 2 mL 0.5 N NaOH were added to the dried sample, which was incubated for 5 min in a heated bath and cooled. BF3MeOH was then added, and the sample was heated in the bath for 3 min and cooled again. In the final step, 15 mL petroleum ether and 20 mL H2O were added, the sample sonicated, and the supernatant isolated and dried using nitrogen gas.
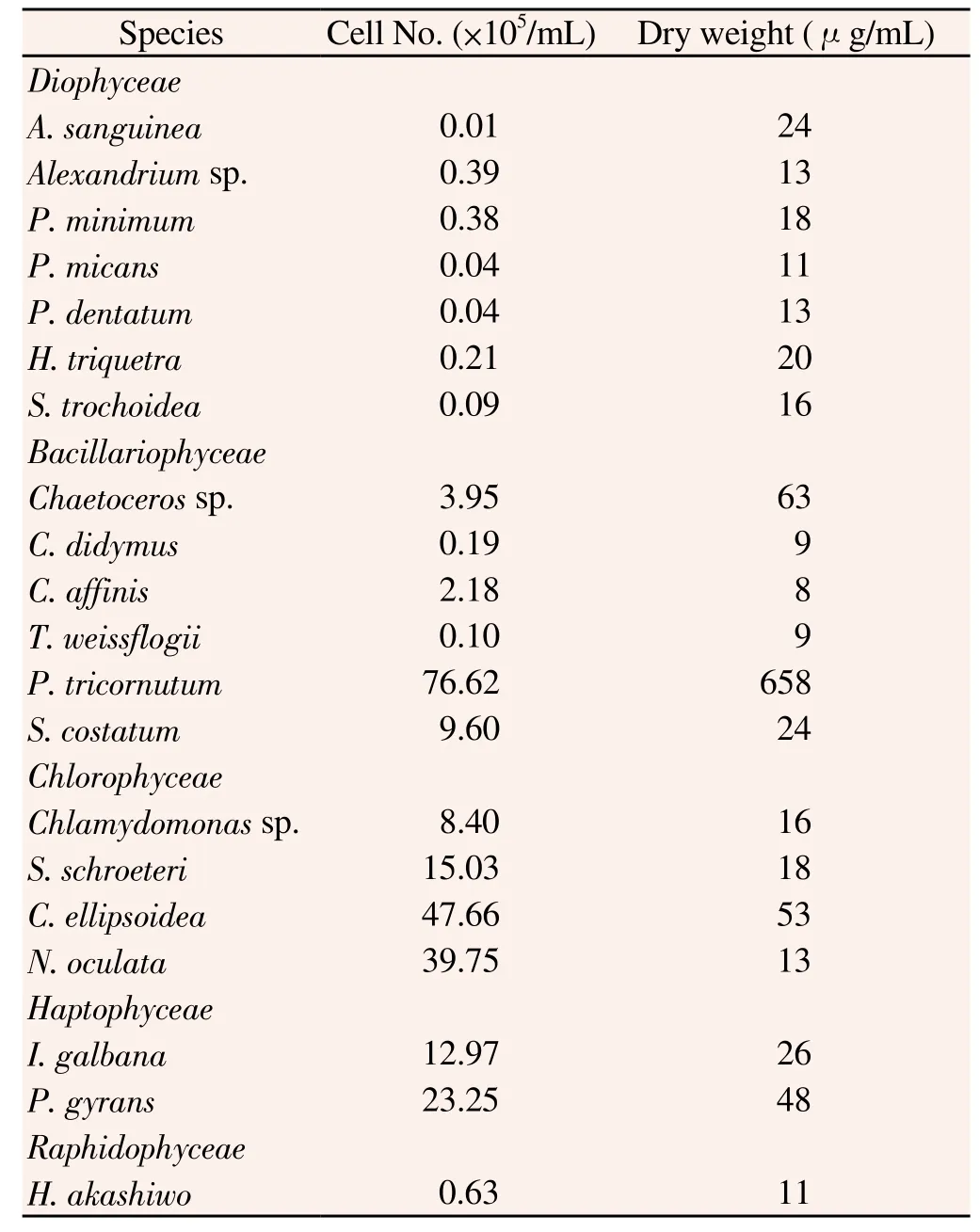
Table 1 Cell density and dry weight of marine microalgae used in the study.
2.3. Fatty acid analysis
Fatty acid methyl esters (FAMEs) were analyzed in a Varian CP-3800 equipped with a HP-Innowax silica capillary column (30 m× 0.25 mm id., 0.25 μm film thickness), using helium as the carrier gas. Samples (1 mL) were injected under the following conditions: the column temperature was held at 50 ℃ for 2 min, then elevated at a rate of 5 ℃/min to 220 ℃, maintained for 30 min and held until all FAME of interest had been eluted. The injector and flame ionization detector temperatures were both 250 ℃. FAMEs were identified by comparing their retention times with those of validated standards (Sigma-Aldrich Co., USA) and quantified using heneicosanoic acid as the internal standard.
2.4. Data analysis
All data have been expressed as weight percentages and representthe means of at least three different samples of each algae species. Statistical analysis was performed using a non-parametric ANOVA followed by a Dunn's pair-wise multiple comparison test with significance indicated at P<0.05. FAs were grouped into saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and PUFAs. Among the PUFAs, 20:5n3 (EPA), 22:6n3 (DHA), total n3 and n6, and n3/n6 ratios were reported.
3. Results
3.1. FAME composition of 20 marine microalgae
FAME compositions of the seven species of Dinophyceae used in this study are shown in Table 2. The major FAs in Dinophyceae were C14:0, C16:0, C16:1, C18:0, C18:1n-9, C20:5n-3 and C22:6n-3 in Alexandrium sanguinea (A. sanguinea) and Alexandrium sp., while the other five species of Dinophyceae (P. minimum, P. micans, P. dentatum, H. triqutra and S. trochoidea) contained C14:0, C16:0, C16:1, C18:0, C18:1n-9, C20:2n-6, C20:4n-6, C20:5n-3 and C22:6n-3. In comparisons of the FAME contents between Alexandrium sp. and the other species, ΣSFA (41.9%) was relatively high and ΣMUFA (36.7%) relatively low. In particular, C16:0 (26.2%) and C18:0 (11.4%) were higher than the other species.

Table 2 Fatty acid composition of Dinophyceae algae species.
The FAME compositions of the six species of Bacillariophyceae used in this study are shown in Table 3. The contents of six major FA tested in Bacillariophyceae (C14:0, C16:0, C18:0, C20:5n-3 and C22:6n-3, and C16:1) were low in C. didymus compared with the other species. Chetocerus sp. had the highest contents of C14:0 (18.2%), C16:0 (23.4%) and C18:3n-6 (2.7%). C. didymus had the highest level of ΣSFA (53.8%) among all Bacillariophyceae, and C22:6n-3 (24.1%) was also high. C. affinis had high contents of C20:2n-6 (6.2%) and C22:6n-3 (18.6%). T. weissflogii was high in C16:0 (23.4%) and C16:1 (34.3%). P. tricornutum was high in C18:2n-6 (2.8%) and C20:5n-3 (52.6%), with the highest ΣPUFA (64.8%) among all Bacillariophyceae species. Furthermore, S. costatum showed the highest level of C16:1 (35.6%) among the Bacillariophyceae.
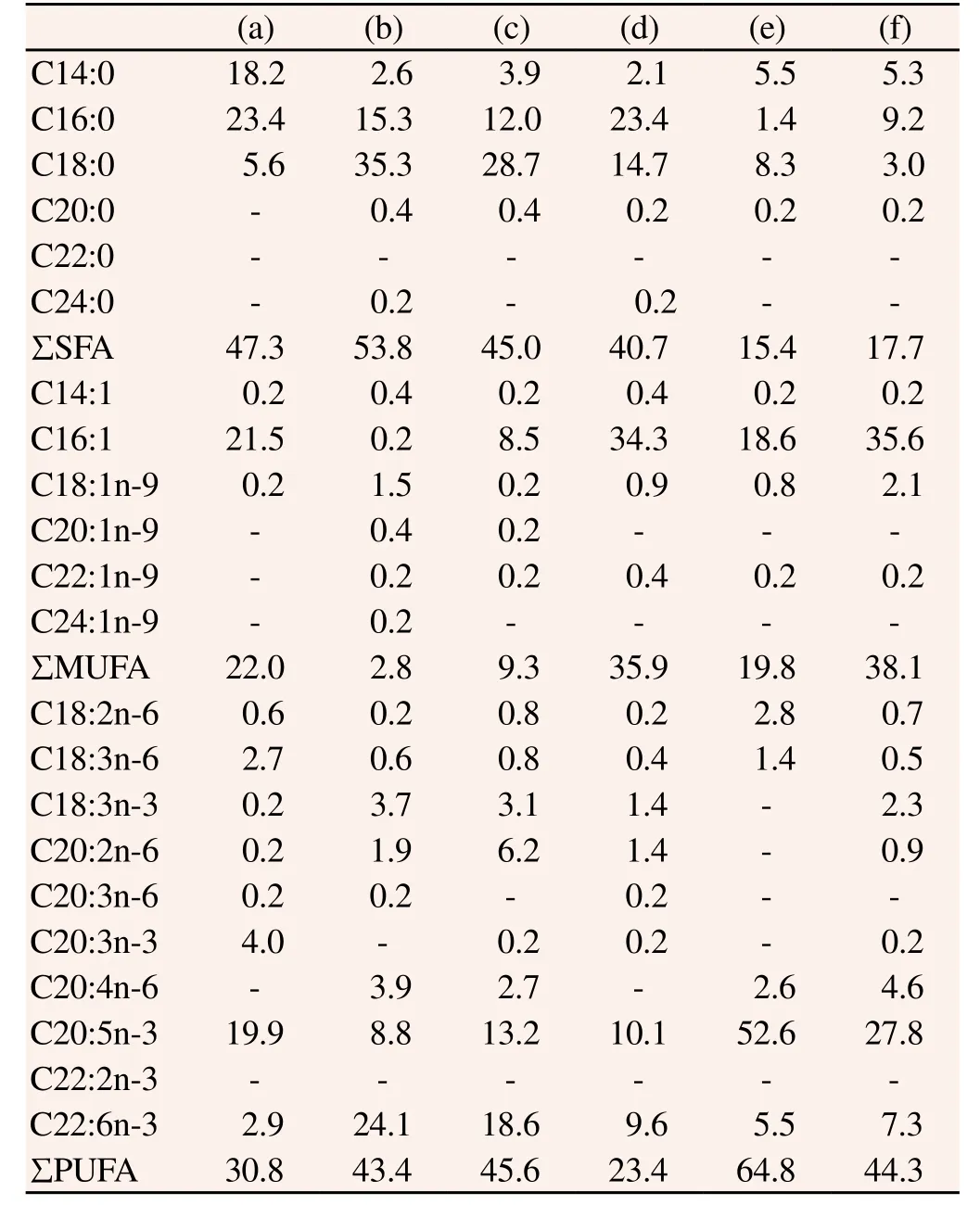
Table 3 Fatty acid composition of Bacillariophyceae algae species.
FAME compositions of the four Chlorophyceae, two Haptophyceae, one Raphidophyceae and one Eugtophyceae species used in this study are shown in Table 4. In this study, the major FAs of Chlorophyceae, including C16:0, C18:0, C16:1, C18:3n-3, C20:4n-6 and C22:6n-3, were low. The major FAs of Chlamydomonas sp. were C16:0 (24.3%), C18:0 (13.9%), C18:3n-3 (26.7%) and C20:5n-3 (19.7%), and S. schroeteri had the highest amount of C18:0 (46.8%) among the Chlorophyceae species evaluated with 26.0% C18:3n-3. C.ellipsoidea showed the highest levels of C16:0 (23.8%), C16:1 (25.3%) and C20:5n-3 (34.6%) among Chlorophyceae, but C18:3n-3 (0.5%) was relatively low. N. oculata had a very high level of C18:0 (35.5%) and C18:3n-3 (29.1%). The major FAs in the two species of Haptophyceae, I. galbana and P. gyrans, were C14:0, C16:1, C20:5n-3 and C22:6n-3. In particular, the C22:6n-3 content of I. ganbana was very high at 33.6%. H. akashiwo had C16:0 (18.3%), C18:0 (9.3%), C16:1 (15.1%), C20:5n-3 (38.8%) and C22:6n-3 (8.7%) as the major FAs.

Table 4 Fatty acid composition of 4 Chlorophyceae, 2 Haptophyceae, and 1 Raphidophyceae algae species.
3.2. Nutritive values of FAME contents from 20 marine microalgae
Next, we investigated the nutritive values of FAME contents from microalgae. The EPA content was high and increased in the order of P. tricornutum, H. akashiwo, C. ellipsoidea, P. gyrans and S. costatum (27.8-52.6%), and the DHA content was highest in I. galbana (33.6%). Dinophyceae, excluding Alexandrium sp., contained significantly high levels DHA from 21.0%-28.1%. The DHA and EPA ratio critical to the survival of marine fish larvae[19] was the highest in I. galbana (3.3) and C. didymus (2.7) (Table 5).
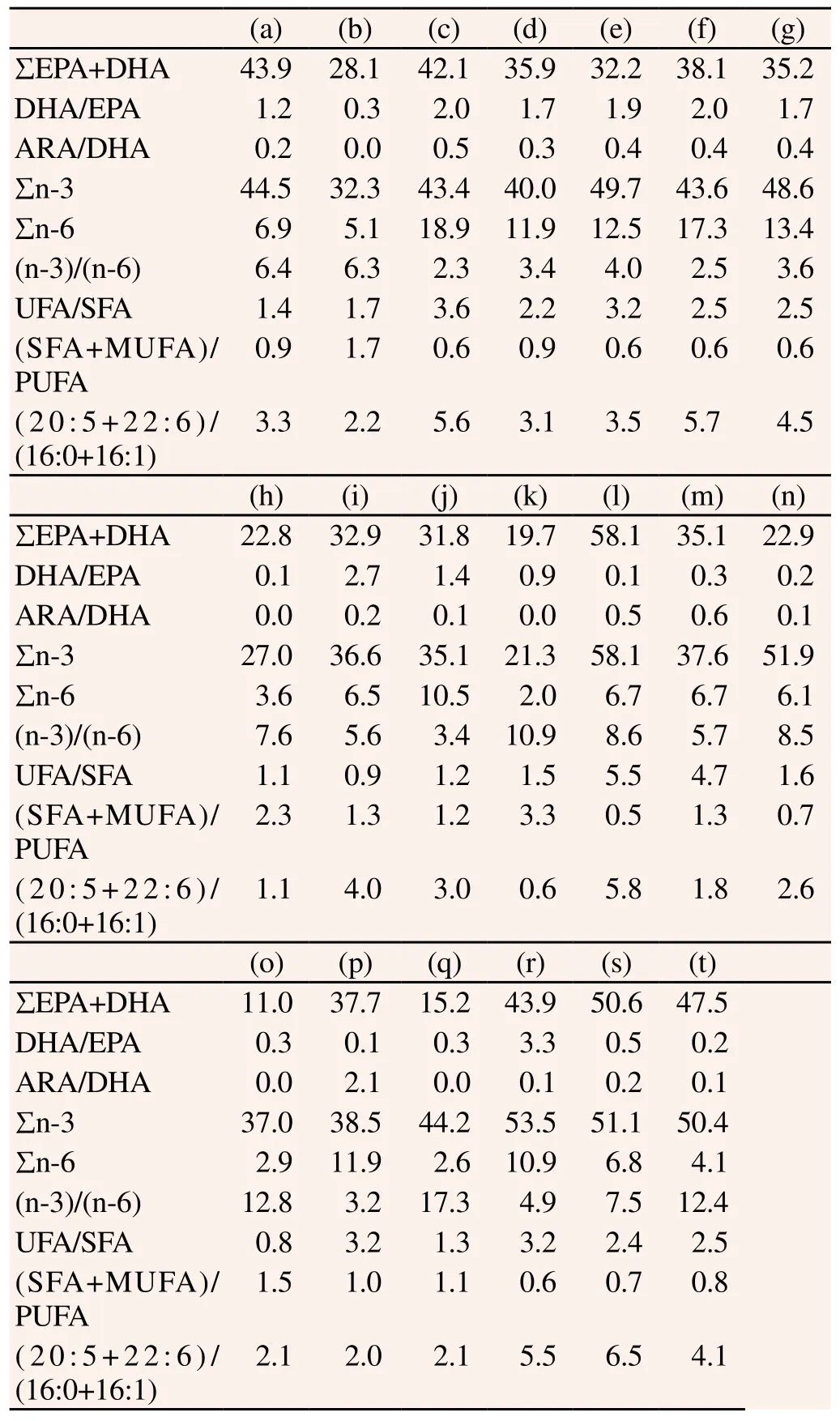
Table 5 Nutritive values of FAME contents from 20 marine microalgae.
The n-3/n-6 ratio was a parameter used to assess the nutritional value of microalgal cells[20]. This study determined that the n-3/n-6 ratios ranging from 2-5 provided sufficient microalgae nutritional value. The authors reported that the microalgae with a high n-3/n-6 ratio included N. oculata (17.3), S. schreoteri (12.8) and H. akashiwo (12.4), although eight species were in the 2-5 range and thus nutritionally suitable, including P. minimum (3.3), P. micans (3.4), P. dentatum (4.0), H. triquitra (2.5). S. trochoidea (3.6), C. affinis (3.4), C. ellipsoidea (3.2) and I. galbana (4.9). In addition, arachidonic acid (AA, C20:4n-6) content was higher in Dinophyceae, similar to DHA, and high in P. minimum (13.8%), H. triqutra (10.8%) and S. trochoidea (8.1%) (Table 2). Interestingly weobserved low levels of (SFA+MUFA)/PUFA and high ratios of UFA/SFA and (C20:5+C22:6)/ (C16:0+C16:1). These values are reported in Table 5 and corresponded to levels in Dinophyceae and P. tricornutum of Bacillariophyceae, indicating high nutritional value among these microalgae (Table 5).
4. Discussion
Our study indicated the distinct patterns of FA composition in twenty different microalgae, showing a species-specific FA composition. For example, C14:0, C16:0, C18:0, C20:4n-6, C20:5n-3 and C22:6n-3 were abundant in Dinophyceae, while C14:0, C16:0, C16:1, C18:0, C20:5n-3 and C22:6n-3 were abundant in Bacillariophyceae. In addition, four Chlorophyceae, two Haptophyceae and one Raphidophyceae species predominantly produce C16:1n-7, C14:0 and C16:0 FAs. In Chlorophyceaen, Raphidophyceae and Haptophyceae members, the prominent fatty acids such as C16:0 and C16:1n-7 were found similar to the earlier report, showing a higher concentration of C16:0 over C16:1n-7. The FA contents in Chlorophyceae were characterized previously by low amounts of 20:5n-3 and C22:6n-3[21]. In addition, previous reports determined that Bacillariophyceae contained high levels of C14:0, C15:0 and C16:1 and low levels of C24:0[20,21]. Moreover, while 20:5 n-3 was high in all species, it was particularly increased in P. tricornutum[22,23]. The Bacillariophyceae species in this study showed similar results.
On the other hand, The optimal nutritional value of microalgae as a food source is affected by the amino acid composition of proteins and the sugar composition of carbohydrates[24,25], but it is influenced to a greater extent by the FA composition of lipids[24]. The lipid profile of microalgae as a food source indicates that PUFA, especially DHA and EPA, are associated with rapid growth of bivalve larvae and juveniles of many other organisms[26]. When EPA and DHA are not present in microalgae, the growth of juvenile oysters is retarded[24,27]. In fact, it has been reported that low levels of (SFA+MUFA)/PUFA and high ratios of UFA/ SFA and (C20:5+C22:6)/ (C16:0+C16:1) have nutritional value in cultured organisms[28]. Young oysters can elongate or desaturate the carbon chain of dietary FAs, but the rate is too slow to maintain optimal growth[29]. Among the n-3 PUFA, DHA (C22:6n-3) and EPA (C20:5n-3) are essential for growth and development of fish and oysters. Our results demonstrate variations in several factors that contribute to the nutritional value of microalgae, including EPA and DHA contents, as well as the ratio n-3/n-6 fatty acids ratio. In particular, the C22:6n-3 content of I. ganbana was very high at 33.6%, which was similar to previous reports[30]. In addition, arachidonic acid (AA, C20:4n-6) is a precursor of eicosanoid synthesis and an essential FA for marine fish. When turbot juveniles, which prefer food with a high DHA content, were fed only AA, they showed higher growth and survival rates than those fed an AA and DHA mixture or DHA only[31]. Taken together, this is the first report on the FA profiles and nutritional value of microalgae species in South Korea, and it provides information regarding the potential utility of FAs as biomarkers and a basis for the development of more effective culture techniques for marine organisms via microalgae identification.
Conflict of interest statement
The authors disclose no conflicts.
Acknowledgements
This research was supported by the Public Welfare & Safety Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Science, ICT & Future Planning (PN65760).
[1] Ariyadej C, Tansakul R, Tansakul P, Angsupanich S. Phytoplankton diversity and its relationships to the physio-chemical environment in the Banglang Reservoir, Yala Province. Songklanakarin J Sci Technol 2004; 26: 595-607.
[2] Ryckebosch E, Bruneel C, Termote-Verhalle R, Goiris K, Muytaert K, Foubert I. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem 2014; 160: 393-400.
[3] Ronquillo JD, Fraser J, McConkey AJ. Effect of mixed microalgal diets on growth and polyunsaturated fatty acid profile of European oyster (Ostrea edulis) juveniles. Aquaculture 2012; 360: 64-68.
[4] Brown M, Robert R. Preparation and assessment of microalgal concentrates as feeds for larval and juvenile Pacific oyster (Crassostrea gigas). Aquaculture 2002; 207: 289-309.
[5] Martinez-Fernandez E, Acosta-Salmon H, Southgate PC. The nutritional value of seven species of tropical microalgae for black-lip pearl oyster (Pinctada margaritifera, L.) larvae. Aquaculture 2006; 257: 491-503.
[6] Ryckebosch E, Bruneel C, Termote-Verhalle R, Goiris K, Muytaert K, Foubert I. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem 2014; 160: 393-400.
[7] Wang JL, Dong XY, Wei F, Zhong J, Liu B, Yao MH, et al. Preparation and characterization of novel lipid carriers containing microalgae oil for food applications. J Food Sci 2014; 79: 167-177.
[8] Sahu A, Pancha I, Jain D, Paliwal C, Ghosh T, Patidar S, et al. Fatty acids as biomarkers of microalgae. Phytochemistry 2013; 89: 53-58.
[9] Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 2008; 155: 751-759.
[10] Martínez-Fernández E, Acosta-Salmón H, Southgate PC. The nutritional value of seven species of tropical microalgae for black-lip pearl oyster (Pinctada margaritifera, L.) larvae. Aquaculture 2006; 257: 491-1503.
[11] Ratha SK, Babu S, Renuka N, Prasanna R, Prasad RB, Saxena Ak. Exploring nutritional modes of cultivation for enhancing lipid accumulation in microalgae. J Basic Microbiol 2013; 53: 440-450.
[12] Garay LA, Boundy-Mills KL, German JB. Accumulation of high-value lipids in single-cell microorganisms: a mechanistic approach and future perspectives. J Agric Food Chem 2014; 62: 2709-2727.
[13] Albentosa M, Perez-Camacho A, Labarta U, Fernandez-Reiriz MJ. Evaluation of live microalgae diets for the seed culture of Ruditapes decussates using physiological and biochemical parameters. Aquaculture 1996; 148: 11-23.
[14] Islam MM, Ahmed ST. Kim YJ, Mun HS, Kim YJ, Yang CJ. Effect of sea tangle (Laminaria japonica) and charcoal supplementation as alternatives to antibiotics on growth performance and meat quality of ducks. Asian-Australas J Anim Sci 2014; 27: 217-224.
[15] Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr 1999; 70: 560-569.
[16] Patil V, Gislerød HR. The importance of omega-3 fatty acids in diet. Curr Sci 2006; 90: 908-909.
[17] Yang DY, Pan HC, Yen YJ, Wang CC, Chuang YH, Chen SY, et al. Detrimental effects of post-treatment with fatty acids on brain injury in ischemic rats. Neurotoxicology 2007; 28: 1220-1229.
[18] Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957; 226: 497-509.
[19] Bell JG, McEvoy LA, Estevez A, Shields RJ, Sargent JR. Optimising lipid nutrition in first-feeding flatfish larvae. Aquaculture 2003; 227: 211-220.
[20] Fernandez-Reiriz MJ, Labarta U, Albentosa M, Perez-Camacho A. Effect of microalgal diets and commercial wheatgerm flours on the lipid profile of Ruditapes decussatus spat. Comp Biochem Physiol A Mol Integr Physiol 1998; 119: 369-377.
[21] Zhukova NV, Aizdaicher NA. Fatty acid composition of 15 species of marine microalgae. Phytochem 1995; 39: 351-356.
[22] Pahl SL, Lewis DM, Chen F, King KD. Heterotrophic growth and nutritional aspects of the diatom Cyclotella cryptica (Bacillariophyceae): Effect of some environmental factors. J Biosci Bioeng 2010; 109: 235-239.
[23] Song M, Pei H, Hu W, Han F, Ji Y, Ma G, et al. Growth and lipid accumulation properties of microalgal Phaeodactylum tricornutum under different gas liquid ratios. Bioresour Technol 2014; 165: 31-37.
[24] Abreu AP, Fernandes B, Vicente AA, Teixeira J, Dragone G. Mixtrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour Technol 2012; 118: 61-66.
[25] Brown MR, Jeffrey SW, Volkman JK, Dunstan GA. Nutritional properties of microalgae for mariculture. Aquaculture 1997; 234: 315-331.
[26] Brown MR, Jeffrey SW, Volkman JK, Dunstan GA. Nutritional properties of microalgae for maricuture. Aquaculture 1997; 151: 315-331.
[27] Thompson PA, Gua M, Harrison PJ. Nutritional value of diets that vary in fatty acid composition for larval Pacific oyster (Crassostrea gigas). Aquaculture 1996; 143: 379-391.
[28] Enright CT, Newkirk GF, Craigie JS, Castell JD. Evaluation of phytoplankton as diets for juvenile Ostrea edulis L. J Exp Mar Biol Ecol 1986; 96: 1-13
[29] Sequineau C, Racotta IS, Palacios E, Delaporte M, Moal J, Soudant P. The influence of dietary supplementation of arachidonic acid on prostaglandin production and oxidative stress in the Pacific oyster Crassostrea gigas. Comp Biochem Physiol A Mol Integr Physiol 2011; 160: 87-93.
[30] Gao Y, Yang M, Wang C. Nutrient deprivation enhances lipid content in marine microalgae. Bioresour Technol 2013; 147: 484-491.
[31] Castell JD, Kennedy EJ, Robinson SMC, Parsons GJ, Blair TJ, Gonzalez-Duran E. Effect of dietary lipids on fatty acid composition and metabolism in juvenile green sea urchins (Strongylocentrotus droebachiensis). Aquaculture 2004; 242: 417-435.
ent heading
10.1016/S1995-7645(14)60313-8
*Corresponding author: Dr. Taek-Kyun Lee, South Sea Environment Research Department, Korea Institute of Ocean Science and Technology, Geoje 656-830, Korea.
Tel: 82-55-639-8530
Fax: 82-55-639-8539
E-mail: tklee@kiost.ac
 Asian Pacific Journal of Tropical Medicine2015年3期
Asian Pacific Journal of Tropical Medicine2015年3期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Statistical estimations for Plasmodium vivax malaria in South Korea
- Evolutionary relationship of 5’-untranslated regions among Thai dengue-3 viruses, Bangkok isolates, during 24 year-evolution
- Depressant effects of Agastache mexicana methanol extract and one of major metabolites tilianin
- Antibiotic susceptibility profiling and virulence potential of Campylobacter jejuni isolates from different sources in Pakistan
- Prevalence of West Nile virus in Mashhad, Iran: A population-based study
- Effects of high glucose on expression of OPG and RANKL in rat aortic vascular smooth muscle cells
