液晶/水界面上的氢键作用诱导液晶取向转变
廖芝建 秦振立 杜思南 李思雨 陈冠侯 左 芳 罗建斌
(西南民族大学化学与环境保护工程学院, 成都 610041)
液晶/水界面上的氢键作用诱导液晶取向转变
廖芝建 秦振立 杜思南 李思雨 陈冠侯 左 芳*罗建斌
(西南民族大学化学与环境保护工程学院, 成都 610041)
本文提出液晶/水界面上氢键作用可以诱导热致型液晶(戊基联苯氰, 简称: 5CB)发生取向转变. 当液晶5CB膜接触酚类(如对硝基苯酚)水溶液的时候, 由于酚类物质的酚羟基与液晶5CB分子中的氰基在液晶水界面上形成了氢键, 在氢键的作用下使得液晶5CB由平行取向转变成了垂直取向. 此外, 还利用了液晶传感器可视化了酚类物质与牛血清蛋白(BSA)之间的相互作用. 本文的研究结果可为研究液晶/水界面上的界面现象提供新的思路, 并且有望开发出基于氢键作用的液晶生物化学传感技术.
液晶; 氢键作用; 取向转变; 界面现象; 光学响应; 酚类; 牛血清蛋白
1 Introduction
Liquid crystals (LCs) are materials typically composed of rod-like molecules, which possess short-range positional but long-range orientational order.1The orientation of LCs is extraordinarily sensitive to the change of the surface which they are in contact with. The surface-induced local order can be amplified over several tens of micrometers in the LC bulk due to the long-range interaction of LCs.2,3According to these features of LCs, the binding events between the interesting molecules and LC molecules at the LC interface can be transduced and amplified into optical signals that are distinguishable through the naked eye under polarized light microscopy.4–25
Recently, the orientational ordering of thermotropic liquid crystals (LCs) at the LC-aqueous interface has attracted substantial attention due to both fundamental and technological interest.4–12Previous studies have demonstrated that the orientational transition of LCs is closely coupled to the presence and organization of amphiphiles which can spontaneously absorb at the interface created between the LC phase and the aqueous phase.26–33For example, Abbott's group reported that the tails of surfactants,26–28lipids,28–31and polymers32,33can orient the transition of nematic 5CB (4-cyano-4'-pentylbiphenyl) as well as induce a dark homeotropic alignment owing to the hydrophobic interaction between 5CB and the tails of amphiphiles. Furthermore, more complex LC-aqueous interfacial phenomena have been imaged, such as the specific binding events involving the hybridization of DNA,10,11,34,35proteins,14–17,30bacteria,18,19viruses,19and enzymatic reactions,8,23–25,36,37which can perturb the orientations ordered by the tails of amphiphiles.
Many past studies were focused on the orientation of LCs at the LC-aqueous interface ordered by the tails of amphiphiles.26–38On the other hand, it was reported that ordering transitions of LC at the LC-solid interface could also be induced by hydrogen-bond (HB) interaction.39–43To the best of our knowledge, an anchoring orientation in LCs induced by the HB interaction at the LC-aqueous interface has not been reported.
Here, we emphasize that the hydrogen-bond (HB) interaction between 5CB (containing -CN as HB acceptor) and a HB donor also plays an important role in the orientational ordering of 5CB at the LC-aqueous interface. In this work, phenols such as p-nitrophenol were selected as HB donors to anchor the orientation of 5CB through the attraction between the -OH group of phenol and the -CN group of 5CB at the LC-aqueous interface. The results presented in this paper will be interesting in that they provide new fundamental insights into the balance of intermolecular interactions that order the orientational transition of LCs at the LC-aqueous interface.
2 Materials and methods
2.1 Materials
4-Cyano-4'-pentylbiphenyl (5CB, 99.5%), p- and m-hydroxybenzaldehyde (> 99%), p-, m-, and o-nitrophenol (> 99%), benzonitrile (> 98.5%), bovine serum albumin (BSA, > 98%) and dichloromethane (> 99%) were obtained from Sinopharm Chemical Reagent Co., Ltd. All chemicals were used without further purification. Glass microscopy slides and transmission electron microscope (TEM) copper grids (100 mesh, 18 μm thickness, 285 μm grid spacing, and 55 μm bar width) were obtained from Beijing Zhongjingkeyi Technology Co., Ltd. All aqueous solutions were prepared with deionized water.
2.2 Preparation of LC optical cells
The LC optical cells were prepared following the procedures reported in the previous studies.12,30,32Briefly, glass slides were cleaned by sonication in ethanol for 15 min and then dried in a 100 °C oven for 30 min. Next, a silicon plate support with holes of 5 mm in diameter and 0.5 mm depth was placed onto the slide and the bubbles between the slide and the silicon plate was removed through pressure. Subsequently, the silicon plate was secured on the slide via adhesion. Meanwhile, TEM copper grids (100 mesh, 18 μm thickness, 285 μm grid spacing, and 55 μm bar width) were first cleaned in methanol, ethanol, and acetone (sonication for 10 min in each solvent), then heated overnight at 45 °C to evaporate residual solvents. The copper grid was then impregnated with about 0.5 μL of 5CB using a capillary tube. Excess of 5CB was removed by contacting the LCs with the other end of the capillary tube. The grids containing LCs were then put on the wells of the plate containing samples of interest. This optical cell was then ready for examination under cross-polarized lighting. The schematic (Fig.1(A)) and real (Fig.1(B)) pictures of the experimental equipment are shown in Fig.1.
2.3 Preparation of the mixture of p-nitrophenol and 5CB
The mixtures of p-nitrophenol and 5CB were prepared by mixing 2 mmol (or 1 mmol) p-nitrophenol with 8 mmol (or 9mmol) 5CB, and then dissolve into CH2Cl2for the uniform mixing of p-nitrophenol and 5CB. Next, the mixture solutions were incubated in a vacuum oven at a temperature of 35 °C for a set time period of 48 h. The mixtures of p-nitrophenol and 5CB were used to characterize the optical response of 5CB, Fourier transform infrared (FTIR) and ultraviolet visible (UV-Vis) absorption spectra.
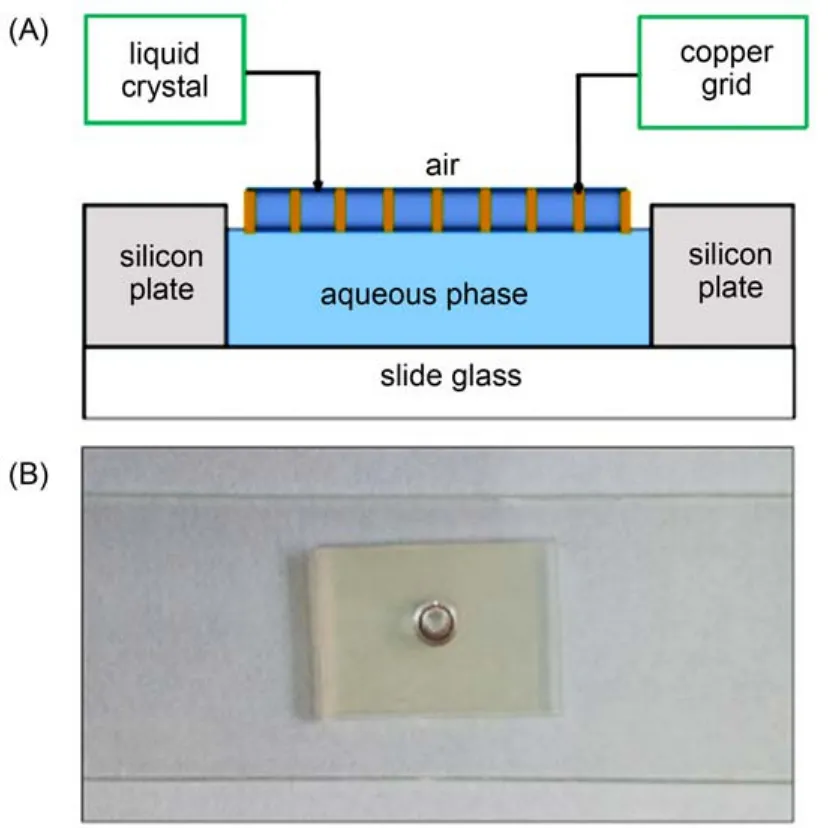
Fig.1 (A) Schematic and (B) real pictures of the experimental equipment
2.4 Characterizations
The optical texture of the 5CB films filled in the pores of the copper grids was examined by using plane-polarized light in transmission mode on an UOP UB200i microscope with crossed polarizer. All optical microscopy images were taken at room temperature with a digital camera (DPIXEL DP330C CCDCamera) mounted on the polarizing optical microscope. Arthroscopic examinations were performed with the source light intensity set to 50% of full illumination and the aperture set to 10% so as to collimate the incident light. Homeotropic alignments were determined by first observing no transmission of light during a 360° rotation of the sample. Insertion of a condenser below the stage and a Bertrand lens above the stage allowed conoscopic examination of the cell. An interference pattern consisting of two crossed isogyres indicated homeotropic alignment (Fig.2A).44,45In-plane birefringence was indicated by the presence of brush textures, typically four-brush textures emanating from a line defect (Fig.2B), when the sample was viewed between crossed polarizers.44,45
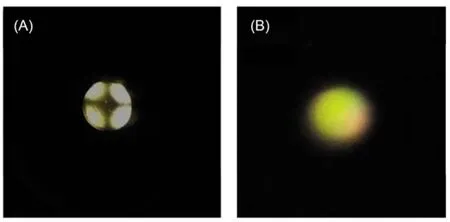
Fig.2 Optical images of 5CB confined with copper grids at different time after exposure to 4 mmolL–1p-nitrophenol (A) and water (B) with insertion of a condenser below the stage and a Bertrand lens above the stage
The FTIR spectra of 5CB, p-nitrophenol, and their mixtures (n(p-nitrophenol) : n(5CB) = 2 : 8 (molar ratio)) were measured at room temperature in the 4000–400 cm–1region with the use of a Thermo Nicolet IR 200 FTIR spectrometer. The spectral slit width was 2–4 cm–1. 5CB, p-nitrophenol, and their mixtures were casted on the KBr slides for testing.
The UV-Vis absorption spectra of p-nitrophenol were measured at room temperature in the 200–800 cm–1region with the use of a Thermo UV-500 spectrophotometer, when pure 5CB was layered onto the surface of a 0.3 mmolL–1p-nitrophenol aqueous solution in a cuvette, or a 3 μL mixture of p-nitrophenol and 5CB (1 : 9, molar ratio) was layered onto the water surface in a cuvette.
3 Results and discussion
3.1 Imaging the HB interaction between 5CB and p-nitrophenol
Driven by the interest in HB interaction on anchoring the orientational transition of 5CB at the LC-aqueous interface, we contacted the nematic phase of nitrile-containing mesogen 5CB with water and aqueous solutions of p-nitrophenol at various concentration. Under illumination with polarized light, nematic 5CB in contact with water exhibits a bright appearance consistent with a LC film that was anchored planar to the interface formed between the nematic 5CB phase and the aqueous phase (Fig.3A). Upon replacing the water with an aqueous solution of p-nitrophenol, the optical appearance 5CB changed from bright to dark over time (Fig.3(B–D)), corresponding to the planar to homeotropic orientational transition of 5CB, and the time required for 5CB to turn fully dark depended on the concentration of p-nitrophenol. The homeotropic alignment of the LC film was also confirmed by conoscopic examination (see Section 2.4).
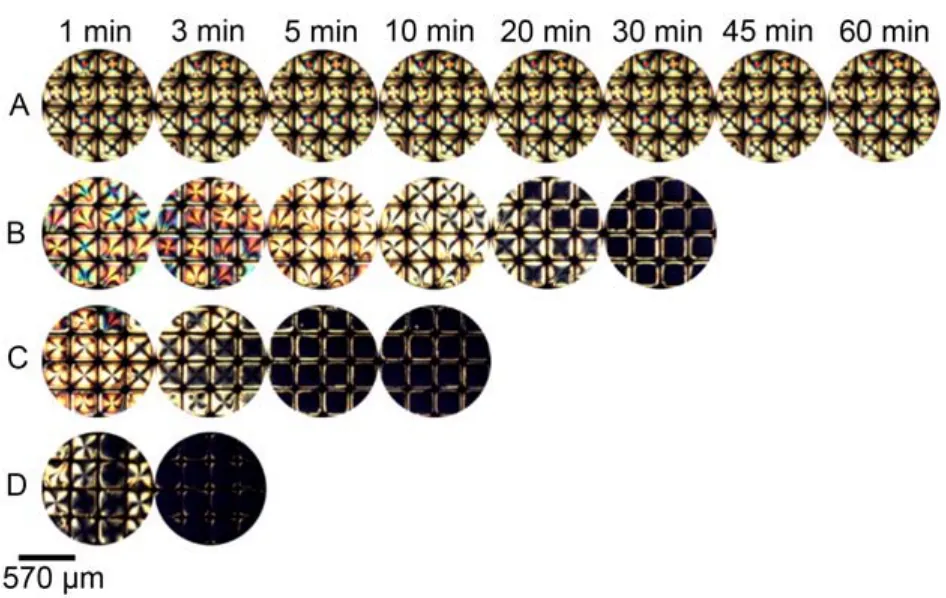
Fig.3 Optical images of 5CB confined with copper grids at different time after exposure to aqueous solutions of p-nitrophenol atvarious concentrations
Previous studies have demonstrated the presence of intermolecular hydrogen bonds between -OH groups and -CN groups.46–48According to the above-reported results, a hypothesis can be proposed that the -OH group of p-nitrophenol molecules promotes an attractive interaction with the -CN of 5CB molecules via intermolecular HB interaction such that pnitrophenol can be absorbed into the 5CB phase (Fig.4, route A) or be attracted and assembled at interface created between the 5CB phase and the aqueous phase (Fig.4, route B), which facilitates the homeotropic orientation of nematic 5CB molecules.
3.2 Determination of HB interaction between 5CB and nitrophenol at the LC-aqueous interface
With a view to confirming the existence of hydrogen bonds between 5CB and p-nitrophenol, a series of FTIR characterizations were performed. Fig.5 shows the FTIR spectra of p-nitrophenol (A), 5CB (B), and a mixture of p-nitrophenol-doped 5CB (2 : 8, molar ratio) (C). The O-H stretch of p-nitrophenol appears at 3327.97 cm–1(Fig.5A), and the spectrum of 5CB has C≡N stretch peak at 2226.30 cm–1(Fig.5B). In the FTIR spectrum of p-nitrophenol-doped 5CB (Fig.5C), the O-H stretch shifted from 3327.97 to 3343.65 cm–1indicating an intermolecular HB interaction with the -CN groups of 5CB.47In addition, the C≡N stretch of 5CB changed from 2226.30 to 2227.35 cm–1in the mixture due to the intermolecular HB interaction with the OH groups of p-nitrophenol.47These results reveal the presence of intermolecular hydrogen bonds between the hydroxyl groups and the cyanogen groups (-CN…HO-),which are the essential driving force for homeotropic alignment of nematic 5CB.
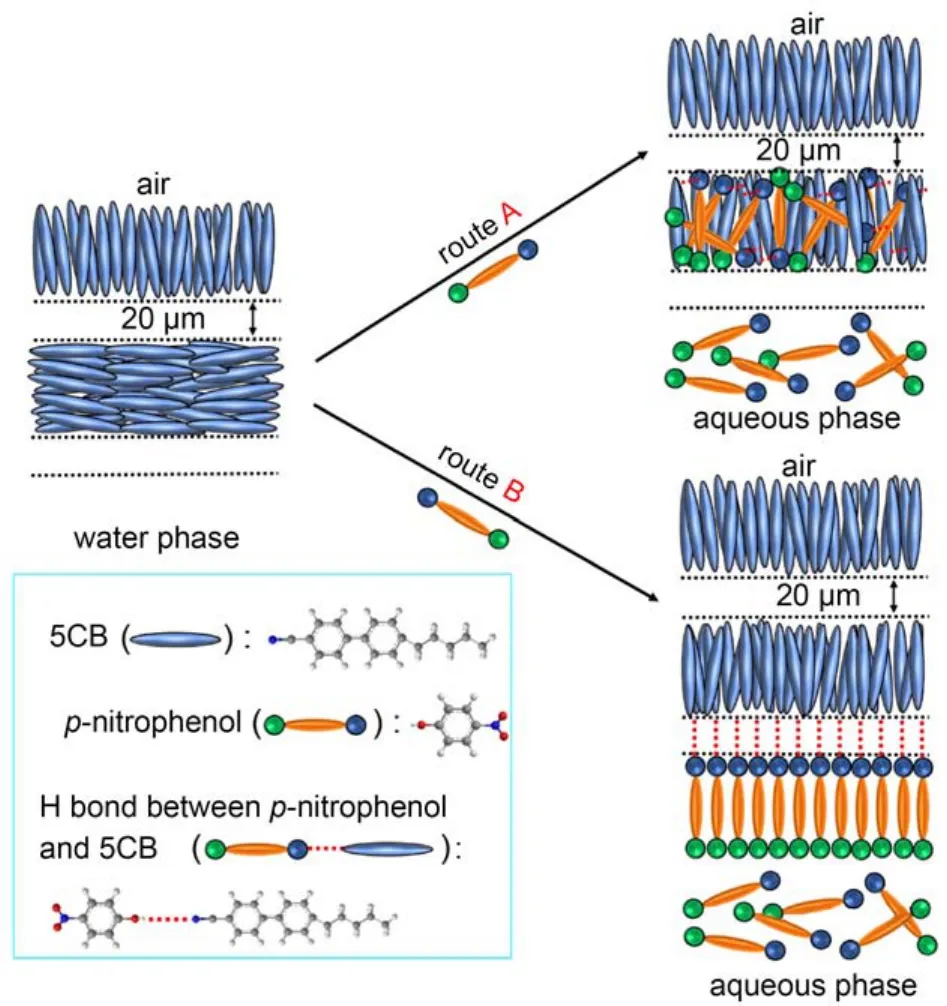
Fig.4 A model for the hypothesis that HB interaction between 5CB and p-nitrophenol orients the original alignment of 5CB homeotropically at the 5CB phase (route A) and LC-aqueous interface (route B)
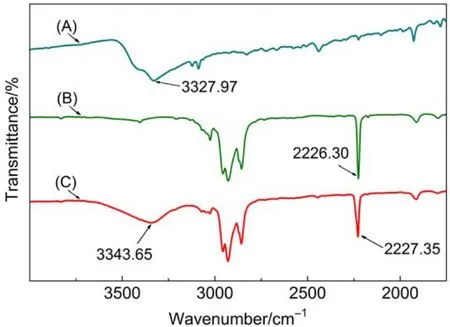
Fig.5 FTIR spectra of p-nitrophenol cast film (A), 5CB cast film (B) and the mixture of p-nitrophenol-doped 5CB (2 : 8, molar ratio) film (C) on a KBr slide
Considering the possibility that the origin of the orientational transition of 5CB is caused by the HB interaction between 5CB and p-nitrophenol which enters into 5CB phase (as shown in Fig.4, route A), we prepared a film from a mixture of pnitrophenol and 5CB (1 : 9, molar ratio) to examine the optical response of 5CB at the aqueous interface. Fig.6 shows that the p-nitrophenol-doped 5CB had a dark appearance up to 3 min after the mixed monolayer was introduced onto the aqueous interface, indicating a homeotropic orientation of 5CB induced by intermolecular HB interaction at the 5CB phase. Subsequent observation revealed that the optical appearance shifted from dark to bright, and became almost completely bright at 5 min, suggesting that the intermolecular HB interaction between 5CB and p-nitrophenol at the 5CB phase was disrupted and then gave rise to an orientational transition of 5CB from a homeotropic to a planar state. Surprisingly, a gradual reorientation of 5CB from a planar to a homeotropic alignment was observed after 30 min, implying that a new intermolecular HB interaction was rebuilt between 5CB and p-nitrophenol.
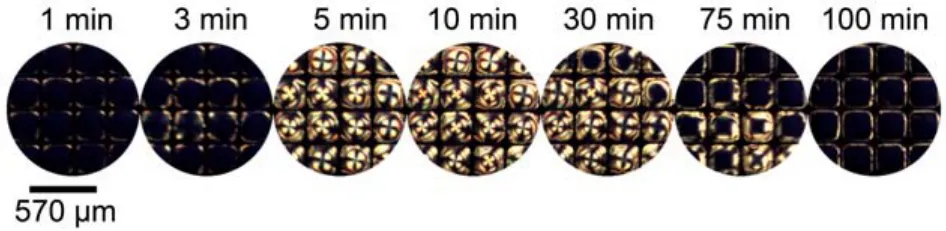
Fig.6 Optical images of the mixture of p-nitrophenol and 5CB (1 : 9 molar ratio) confined with copper grids at different times afterexposure to water
The above phenomena may be explained by the mechanism proposed in Fig.7. At the 5CB phase dark alignment of 5CB is triggered by the HB interaction between 5CB and p-nitrophenol (Fig.7a). When the p-nitrophenol-doped 5CB film contacts with water, p-nitrophenol transfers into the aqueous phase leading to the bright alignment (Fig.7b). Subsequently, p-nitrophenol assembles at the LC-aqueous interface inducing a homeotropic orientation of 5CB (Fig.7c). These results are consistent with Fig.4, route B, which suggests that when contacting with p-nitrophenol aqueous solution, the homeotropic orientation of 5CB is caused by the HB interaction from p-nitrophenol which assembles at the LC-aqueous interface, as opposed to entering into the 5CB phase (as shown in Fig.4, route A).
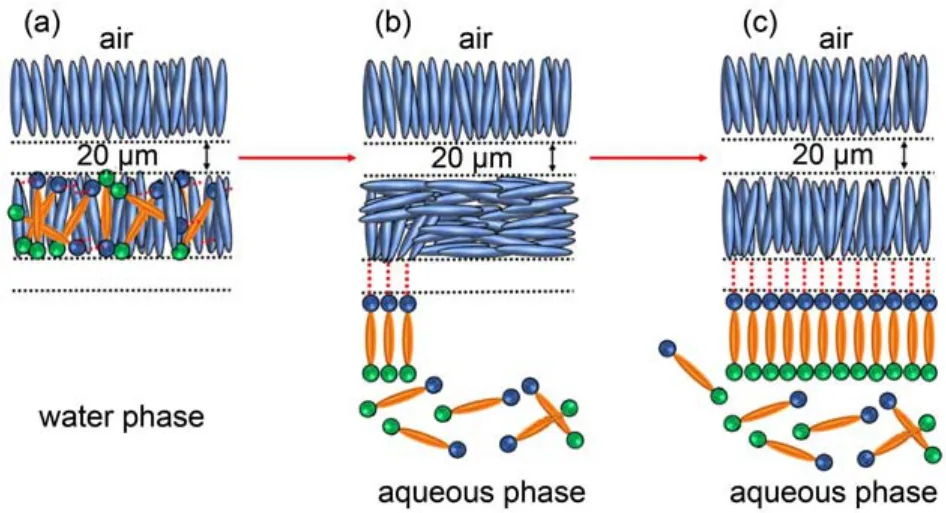
Fig.7 A model for explaining the transfer progress of p-nitrophenol doped in 5CB phase
To provide further insight into the proposed HB interactiondriven orientational transition of 5CB at the LC-aqueous interface, a series of UV-Vis characterizations was performed. A 3-μL mixture of p-nitrophenol and 5CB (1 : 9, molar ratio) was layered onto the water surface in a cuvette to monitor the timedependent process by which p-nitrophenol transfers into the aqueous phase from the 5CB phase. Here the mixture layer was above the beam of UV-Vis light through the cuvette. It was observed that the intensity of the absorption peaks of p-nitrophenol located at 226 and 319 nm increased during the first 30 min,and there was no significant change in intensity after 60 min (Fig.8). This result showed that p-nitrophenol did in fact transfer into the aqueous phase from the 5CB phase, causing the orientational transition of 5CB from dark to bright (Fig.6) by the disruption of the HB interaction between 5CB and p-nitrophenol at the 5CB phase. This was consistent with the explanation that p-nitrophenol transferred from the 5CB phase into the aqueous interface and assembled at the LC-aqueous interface leading to a dark-to-bright-to-dark appearance (as shown in Fig.6 and Fig.7).
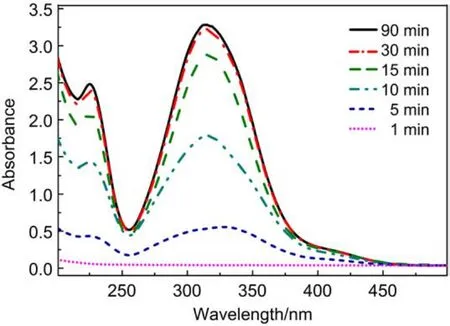
Fig.8 UV-Vis absorption spectra of p-nitrophenol transferring into the aqueous phase from the p-nitophenol-5CB phase at different time intervals
In addition, we layered pure 5CB onto the surface of an aqueous solution containing p-nitrophenol in a cuvette to monitor the assembly of p-nitrophenol at the LC-aqueous interface. Here the 5CB layer was above the beam of UV-Vis light through the cuvette. It was observed that the intensity of the absorption peaks of p-nitrophenol located at 226 and 319 nm decreased during the range of time from 0 to 120 min (Fig.9), consistent with the assembly of p-nitrophenol at the LC-aqueous interface through the attraction of the -CN of 5CB, which reduced the concentration of p-nitrophenol in solution. This supports the explanation that the reorientational transition of 5CB from bright to dark shown in Fig.6 was ordered by the intermolecular HB interaction rebuilt between 5CB and p-nitrophenol at the LC-aqueous interface, and the homeotropic orientation of 5CB (as shown in Fig.3(B–D)) caused by the HB interaction between 5CB and p-nitrophenol did in fact occur at the LC-aqueous interface (as shown in Fig.4, route B) not at the 5CB phase (as shown in Fig.4, route A).
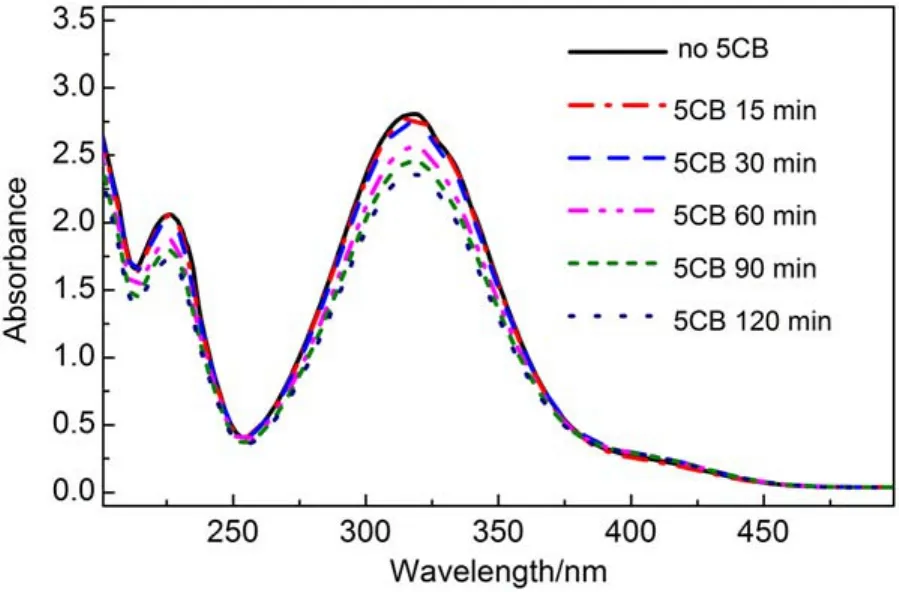
Fig.9 UV-Vis absorption spectra of p-nitrophenol assembling at the LC-aqueous interface from the aqueous phase at different time intervals
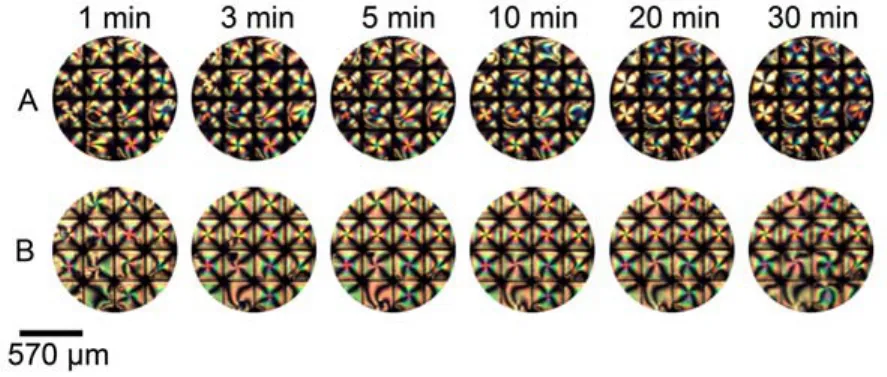
Fig.10 Optical images of 5CB confined with copper grids at different time after exposure to 10 mmolL–1benzonitrile (A), mixture solutions of 4 mmolL–1p-nitrophenol and 10 mmolL–1benzonitrile (B)
Gupta group48have proved that the HB interaction between phenol and benzonitrile was stronger than that between phenol and 5CB. Furthermore, in order to disturb the intermolecular HB interaction between 5CB and p-nitrophenol at the LC-aqueous interface, we contacted 5CB confined within a copper grid with an aqueous solution containing a mixture of 4 mmolL–1p-nitrophenol and 10 mmolL–1benzonitrile. We found that the optical response of 5CB showed a bright appearance (Fig.10), implying that benzonitrile can disrupt the intermolecular HB interaction between 5CB and p-nitrophenol due to the formation of an intermolecular HB interaction between pnitrophenol and benzonitrile, causing the homeotropic dark alignment of 5CB to be shifted to the planar bright alignment (Fig.10).
The results described above lead to the conclusion that intermolecular HB interaction between 5CB and p-nitrophenol triggered the homeotropic orientation of 5CB at the LC-aqueous interface, then gradually propagated this interfacial homeotropic orientation into the bulk 5CB phase, and finally gave rise to an almost uniform homeotropic appearance.
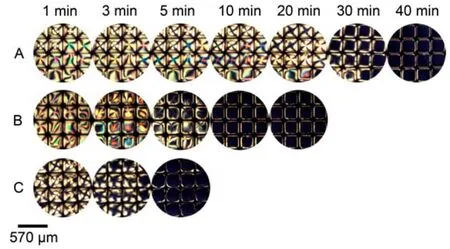
Fig.11 Optical images of 5CB confined with copper grids at different time after exposure to aqueous solutions of m-nitrophenol at various concentrations
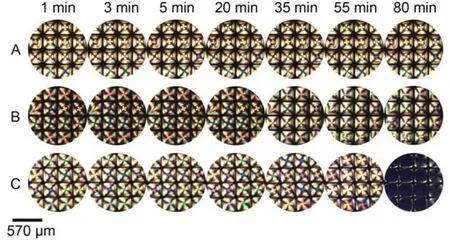
Fig.12 Optical images of 5CB confined with copper grids at different time after exposure to aqueous solutions of o-nitrophenol at various concentrations
3.3 Monitering the optical response of LC couple to other phenols
Here, o- and m-nitrophenol were evaluated as alternative hydrogen-bond donors to anchor the orientational transition of 5CB at LC-aqueous interface. When exposed 5CB confined within copper grids to aqueous solutions containing o- or m-nitrophenol, the optical images of 5CB turned black gradually, indicating the formation of HB interaction between 5CB and o- or m-nitrophenol. It was also found that the response time of 5CB when contracted with m-nitrophenol (Fig.11) solution was shorter than that with o-nitrophenol (Fig.12) at the same concentration, owing to the intramolecular HB interaction in o-nitrophenol which weakened the intermolecular HB interaction between 5CB and o-nitrophenol. Additionally, m- and p-hydroxybenzaldehyde were able to anchor the orientational transition of 5CB at the LC-aqueous interface (Fig.13). These results reinforce the hypothesis that the -OH group of phenols (e.g. p-nitrophenol) can promote an attractive interaction with the -CN of 5CB via intermolecular HB interaction such that phenols can be absorbed and assemble at the interface created between the 5CB phase and the aqueous phase, facilitating the homeotropic orientation of nematic 5CB molecules.
3.4 Imaging interactions between p-nitrophenol and BSA
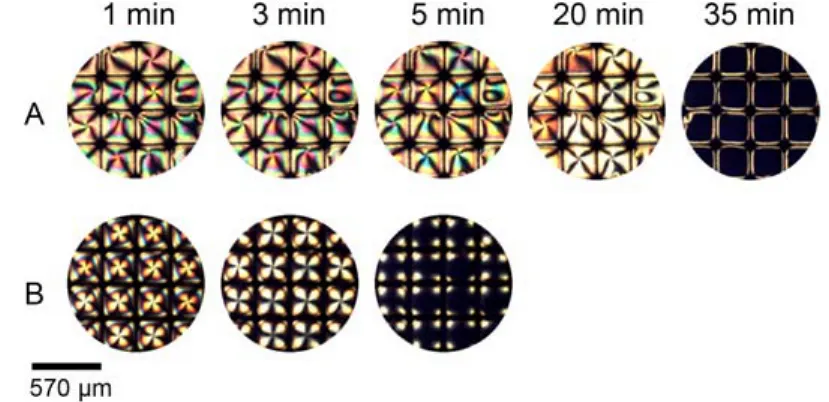
Fig.13 Optical images of 5CB confined with copper grids at different time after exposure to 50 mmolL–1m-hydroxybenzaldehyde (A), 50 mmolL–1p-hydroxybenzaldehyde (B)
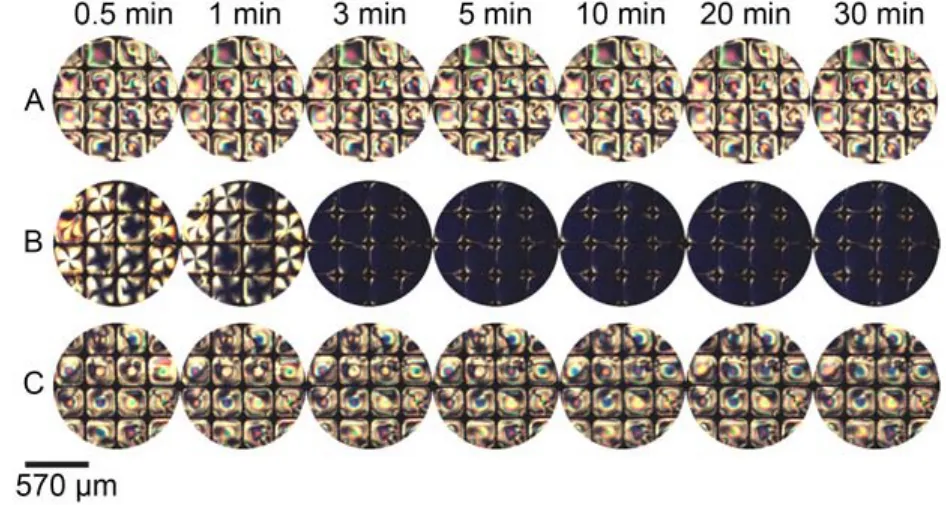
Fig.14 Optical images of 5CB confined with copper grids at different time after exposure to 10 μmolL–1BSA (A), 4 mmolL–1p-nitrophenol(B), mixture solutions of 4 mmolL–1p-nitrophenol and 10 μmolL–1BSA (C)
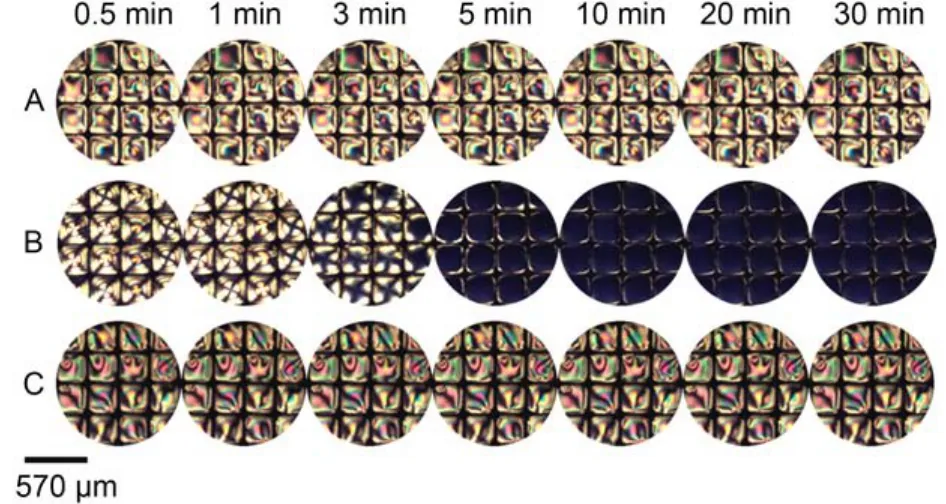
Fig.15 Optical images of 5CB confined with copper grids at different time after exposure to 10 μmolL–1BSA (A), 4 mmolL–1m-nitrophenol(B), mixture solutions of 4 mmolL–1m-nitrophenol and 10 μmolL–1BSA (C)
Furthermore, the interaction between p-nitrophenol and bovine serum albumin was imaged by the LC sensor. When contact with aqueous solutions of 10 μmolL–1BSA, the image of 5CB remained bright in appearance (Fig.14A). The addition of 4 mmolL–1p-nitrophenol into the optical cell resulted in a bright-to-dark change in the optical response of LCs within 5 min (Fig.14B). When exchanged the p-nitrophenol aqueous solution in the optical cell with the mixture aqueous solution of p-nitrophenol and BSA, an obvious optical shift from a dark appearance to a nearly bright appearance (Fig.14C) was observed, which suggests that LCs underwent an orientational transition from the homeotropic to the nearly planar state. The result was consistent with the previous study49that p-nitrophenol interacts with BSA mainly through the hydrogen bonds, which disrupt the interaction between p-nitrophenol and 5CB. The interaction between m-nitrophenol and BSA was also imaged by the LC sensor as shown in Fig.15.
4 Conclusions
In summary, we have demonstrated that the hydrogen-bond interaction could also induce the orientational ordering of 5CBat the LC-aqueous interface. Phenol molecules (such as nitrophenol, p- and m-hydroxybenzaldehyde) could assemble at the interface created between the aqueous phase and the nematic 5CB phase, and anchor the transition of nematic 5CB driven by the HB interactions between 5CB and phenols at the LC-aqueous interface, which facilitated the homeotropic orientation of 5CB molecules. In addition, the interaction of pnitrophenol or m-nitrophenol with BSA was imaged by the LC sensor. The results reported in this paper, therefore, provide new guidance for the design of interfaces that can report chemical and biological interactions.
Acknowledgment: The authors thank Brian McGarvey for revising the manuscript.
(1)De Gennes, P. G. The Physics of Liquid Crystals; Clarendon: Oxford, 1974.
(2)Gupta, V. K.; Skaife, J. J.; Dubrovsky, T. B.; Abbott, N. L. Science 1998, 279, 2077. doi: 10.1126/science.279.5359.2077
(3)Shah, R. R.; Abbott, N. L. Science 2001, 293, 1296. doi: 10.1126/science.1062293
(4)Brake, J. M.; Daschner, M. K.; Luk, Y. Y.; Abbott, N. L. Science 2003, 302, 2094. doi: 10.1126/science.1091749
(5)Mushenheim, P. C.; Trivedi, R. R.; Weibel, D. B.; Abbott, N. L. Biophysical Journal 2014, 107, 255. doi: 10.1016/j.bpj.2014.04.047
(6)Li, X.; Li, G.; Yang, M.; Chen, L. C.; Xiong, X. L. Sensor. Actuat. B 2015, 215, 152. doi: 10.1016/j.snb.2015.03.054
(7)Zhu, Q.; Yang, K. L. Anal. Chim. Acta 2015, 853, 696. doi: 10.1016/j.aca.2014.08.039
(8)Zhang, M.; Jang, C. H. ChemPhysChem 2014, 15, 2569. doi: 10.1002/cphc.201402120
(9)Yang, S.; Liu, Y.; Tan, H.; Wu, C.; Li, X.; Wu, Z.; Shen, G.; Yu, R. Chem. Commun. 2012, 48, 2861. doi: 10.1039/c2cc17861c
(10)Tan, H.; Li, X.; Liao, S.; Yu, R.; Wu, Z. Biosens. Bioelectron. 2014, 62, 84. doi: 10.1016/j.bios.2014.06.029
(11)Noonan, P. S.; Mohan, P.; Goodwin, A. P.; Schwartz, D. K. Adv. Funct. Mater. 2014, 24, 3206. doi: 10.1002/adfm.201303885
(12)Zuo, F.; Liao, Z.; Zhao, C.; Qin, Z.; Li, X.; Zhang, C.; Liu, D. Chem. Commun. 2014, 50, 1857.
(13)Jung, Y. D.; Khan, M.; Park, S. Y. J. Mater. Chem. B 2014, 2, 4922. doi: 10.1039/C4TB00476K
(14)Seo, J. M.; Khan, W.; Park, S. Y. Soft Matter 2012, 8, 198. doi: 10.1039/C1SM06616A
(15)Hartono, D.; Xue, C. Y.; Yang, K. L.; Yung, L. Y. L. Adv. Funct. Mater. 2009, 19, 3574. doi: 10.1002/adfm.v19:22
(16)Tan, L. N.; Orler, V. J.; Abbott, N. L. Langmuir 2012, 28, 6364. doi: 10.1021/la300108f
(17)Das, D.; Sidiq, S.; Pal, S. K. ChemPhysChem 2015, 16, 753. doi: 10.1002/cphc.v16.4
(18)Hu, Q. Z.; Jang, C. H. Analyst 2012, 137, 567. doi: 10.1039/C1AN15743D
(19)Sivvakumar, S.; Wark, K. L.; Gupta, J. K.; Abbott, N. L.; Caruso, F. Adv. Funct. Mater. 2009, 19, 2260. doi: 10.1002/adfm.v19:14
(20)Bai, Y.; Abbott, N. L. Langmuir 2011, 27, 5719. doi: 10.1021/la103301d
(21)Yang, Z.; Gupta, J. K.; Kishimoto, K.; Shoji, Y.; Kato, T.; Abbott, N. L. Adv. Funct. Mater. 2010, 20, 2098.
(22)Xue, C. Y.; Khan, S. A.; Yang, K. L. Adv. Mater. 2009, 21, 198. doi: 10.1002/adma.v21:2
(23)Bi, X.; Hartono, D.; Yang, K. L. Adv. Funct. Mater. 2009, 19, 3760. doi: 10.1002/adfm.v19:23
(24)Hu, Q. Z.; Jang, C. H. Talanta 2012, 99, 36. doi: 10.1016/j.talanta.2012.05.016
(25)Hartono, D.; Bi, X.; Yang, K. L.; Yung, L. Y. L. Adv. Funct. Mater. 2008, 18, 2938. doi: 10.1002/adfm.v18:19
(26)Lockwood, N. A.; Gupta, J. K.; Abbott, N. L. Surf. Sci. Rep.2008, 63, 255. doi: 10.1016/j.surfrep.2008.02.002
(27)Lockwood, N. A.; Pablo, J. J.; Abbott, N. L. Langmuir 2005, 21, 6805. doi: 10.1021/la050231p
(28)Lockwood, N. A.; Abbott, N. L. Current Opinion Colloid Interface 2005, 10, 111. doi: 10.1016/j.cocis.2005.06.002
(29)Brake, J. M.; Daschner, M. K.; Abbott, N. L. Langmuir 2005, 21, 2218. doi: 10.1021/la0482397
(30)Brake, J. M.; Abbott, N. L. Langmuir 2007, 23, 8497. doi: 10.1021/la0634286
(31)Liu, D.; Hu, Q. Z.; Jang, C. H. Colloid Surface B 2013, 108, 142. doi: 10.1016/j.colsurfb.2013.02.047
(32)Kinsinger, M. I.; Sun, B.; Abbott, N. L.; Lynn, D. M. Adv. Mater. 2007, 19, 4208.
(33)Kinsinger, M. I.; Buck, M. E.; Meli, M. V.; Abbott, N. L.; Lynn, D. M. J. Colloid Interface Sci. 2010, 341, 124. doi: 10.1016/j.jcis.2009.09.026
(34)Price, A. D.; Schwartz, D. K. J. Am. Chem. Soc. 2008, 130, 8188. doi: 10.1021/ja0774055
(35)Umber, A. C. M.; Noonan, P. S.; Schwartz, D. K. Soft Matter 2012, 8, 4335. doi: 10.1039/c2sm07483d
(36)Hartono, D., Lai, S. L.; Yang, K. L.; Yung, L. Y. L. Biosens. Bioelectron. 2009, 24, 2289. doi: 10.1016/j.bios.2008.11.021
(37)Park, J. S.; Abbott, N. L. Adv. Mater. 2008, 20, 1185. doi: 10.1002/adma.v20:6
(38)Hu, Q. Z.; Jang, C. H. Appl. Mater. Interfaces 2012, 4, 1791. doi: 10.1021/am300043d
(39)Bai, Y.; Abbott, N. L. J. Am. Chem. Soc. 2012, 134, 548. doi: 10.1021/ja2089475
(40)Luk, Y. Y.; Yang, K. L.; Cadwell, K.; Abbott, N. L. Surf. Sci. 2004, 570, 43. doi: 10.1016/j.susc.2004.06.180
(41)Park, J. S.; Jang, C. H.; Tingey, M. L.; Lowe, A. M.; Abbott, N. L. J. Colloid Interface Sci. 2006, 304, 459. doi: 10.1016/j.jcis.2006.08.063
(42)Hunter, J. T.; Abbott, N. L. Sensor. Actuat. B 2013, 183, 71. doi:10.1016/j.snb.2013.03.094
(43)Ding, X.; Yang, K. L. Sensor. Actuat. B 2012, 173, 607. doi: 10.1016/j.snb.2012.07.067
(44)Brake, J. M.; Mezera, A. D.; Abbott, N. L. Langmuir 2003, 19, 8629. doi: 10.1021/la034469u
(45)Tan, L. N.; Abbott, N. L. J. Colloid Interface Sci. 2015, 449, 452. doi: 10.1016/j.jcis.2015.01.078
(46)Han, S.; Martin, S. M. J. Phys. Chem. B 2009, 113, 12696. doi: 10.1021/jp903726d
(47)Thote, A. J.; Gupta, R. B. Ind. Eng. Chem. Res. 2003, 42, 1129. doi: 10.1021/ie020513+
(48)Thote, A. J.; Gupta, R. B. Fluid Phase Equilibria 2004, 220, 47. doi: 10.1016/j.fluid.2004.01.035
(49)Guo, X.; Li, X.; Jiang, Y.; Yi, L.; Wu, Q.; Chang, H.; Diao, X.; Sun, Y.; Pan, X.; Zhou, N. J. Lumin. 2014, 149, 353. doi: 10.1016/j.jlumin.2014.01.036
Orientational Transitions of Liquid Crystal Driven by Hydrogen-Bond Interaction at the Liquid Crystal-Aqueous Interface
LIAO Zhi-Jian QIN Zhen-Li DU Si-Nan LI Si-Yu CHEN Guan-Hou
Z
UO Fang*LUO Jian-Bin
(College of Chemistry & Environment Protection Engineering, Southwest University for Nationalities, Chengdu 610041, P. R. China)
In this paper, an orientational anchoring transition of the thermotropic nematic liquid crystal (LC) (4-cyano-4'-pentylbiphenyl (5CB)) driven by hydrogen-bond (HB) interaction at the LC-aqueous interface is presented. After a 5CB film is introduced onto an aqueous solution of phenols such as nitrophenol, the alignment of 5CB changes from planar to homeotropic, which is attributed to HB interaction between 5CB and phenols at the LC-aqueous interface. On the other hand, the interaction of pnitrophenol or m-nitrophenol with bovine serum albumin (BSA) is imaged by the LC sensor. Overall, the results provide new insight into interfacial phenomena occurring at the LC-aqueous interface, and hold the potential for biological and chemical sensing techniques based on HB interaction.
Liquid crystal; Hydrogen-bond interaction; Orientational transition; Interfacial phenomenon; Optical response; Phenols; Bovine serum albumin
O647
10.3866/PKU.WHXB201508101
Received: June 5, 2015; Revised: August 7, 2015; Published on Web: August 10, 2015.
*Corresponding author. Email: polymerzf@swun.cn; Tel: +86-28-85522315.
The project was supported by the National Natural Science Foundation of China (51273220) and Fundamental Research Funds for the Central Universities, China (11NZYQN23).
国家自然科学基金(51273220)和中央高校基本科研基金(11NZYQN23)资助项目
© Editorial office of Acta Physico-Chimica Sinica

