铜负载量对LNT催化剂CuO-K2CO3/TiO2结构与性能的影响
范丰奇 孟 明,* 田 野,* 郑黎荣 张 静 胡天斗
(1天津大学化工学院, 天津化学化工协同创新中心, 天津市应用催化科学与工程重点实验室, 天津 300072;2中国科学院高能物理研究所, 北京 100049)
铜负载量对LNT催化剂CuO-K2CO3/TiO2结构与性能的影响
范丰奇1孟 明1,*田 野1,*郑黎荣2张 静2胡天斗2
(1天津大学化工学院, 天津化学化工协同创新中心, 天津市应用催化科学与工程重点实验室, 天津 300072;2中国科学院高能物理研究所, 北京 100049)
采用连续浸渍法制备了一系列非贵金属稀燃NOx阱(LNT)催化剂CuO-K2CO3/TiO2, 考察了Cu负载量对催化剂结构和NOx储存还原性能的影响. 发现8% (w) CuO-K2CO3/TiO2催化剂的催化性能最佳, 其对NOx的储存量达到1.559 mmolg–1, 对NOx的还原效率高达99%, 且在NOx还原过程中无副产物N2O产生. 应用粉末X射线衍射(XRD), 高分辩透射电子显微镜(HR-TEM), CO2程序升温脱附(CO2-TPD), 扩展X射线吸收精细结构(EXAFS), 氢气程序升温还原(H2-TPR)和原位漫反射红外光谱(in-situ DRIFTS)等技术详细表征了催化剂的结构. 结果表明, 不同Cu负载量的催化剂中, 铜物种均主要以CuO相存在. 铜的负载量直接影响铜物种、钾物种的存在状态, 高分散的CuO相与表面K2CO3之间存在较强相互作用, 这种相互作用不仅有利于NOx的储存, 而且有利于增强催化剂的稳定性. in-situ DRIFTS结果表明, NOx储存过程中产生的两个负峰(1436和1563 cm–1)缘于碳酸盐的分解, 这间接证明了碳酸盐作为储存介质参与到NOx储存反应中. EXAFS结果表明, 经过15个稀燃/富燃循环测试, 催化剂中的CuO相仍保持稳定. 基于以上表征结果, 提出了CuO和K2CO3在催化剂表面的分布模型, 并探讨了NOx储存还原的可能机理.
NOx; 储存; 还原; 氧化铜; 碳酸钾
1 Introduction
Vehicular engines operating under lean-burn conditions are becoming more and more popular than conventional Otto gasoline engines due to their higher fuel efficiency and lower CO2emission. However, the toxic NOxin lean-burn exhausts cannot be efficiently removed by the classical three-way catalysts.1,2Therefore, an effective technique for lean NOxremoval is necessary. So far, lean NOxtrap (LNT) technique proposed by Toyota company in the mid of 1990s3is still regarded as one of the most promising solutions to lean NOxemission. Although conventional LNT catalysts such as Pt-Ba/Al2O3using noble metals as active phases are highly efficient for lean NOxremoval,4they are rather expensive due to the high price of noble metals. Therefore, low-cost and high-efficiency catalytic materials are expected. By now, several studies have shown that copper oxides are very active for the oxidation of NO to NO2, especially the Cu-zeolite catalysts which have been proved highly active for NO selective reduction or NOxadsorption.5–7It is reported that the copper supported on Ce-doped TiO28and the Na/Cu/TiO2system9are also highly efficient for NO adsorption; the presence of copper led to in-situ oxidation of NO to NO2, thus enhancing NOxadsorption and storage as nitrates or nitrites. Similarly, as copper oxide was deposited on the SrTi0.7Co0.3O3surface, the activity for NO reduction by CO could be greatly increased.10Considering the good performance and low price of copper oxide, in present work, we aim to design a series of Cu-based LNT catalysts using Cu to replace Pt completely. As to the storage components, barium11–14and potassium oxides15–17have been extensively employed in LNT catalysts; in comparison, potassium oxides are more appropriate as NOxstorage components since potassium sulfates could be regenerated by H2reduction at much lower temperature than barium sulfates. In addition, our previous works have shown that the surface dispersed potassium carbonates in K2CO3-based LNT catalysts are also highly efficient for NOxstorage.18,19Therefore, K2CO3was chosen as the precursor of storage components in this study. For LNT catalysts the supports are also important since they can interact with the supported active phases and the storage components, thus influencing their dispersion and catalytic performance. According to the reported works,9,20–22TiO2can well disperse the supported copper oxides, increasing their catalytic performance for CO oxidation and NO oxidation. So, TiO2was selected as the support of the LNT catalysts studied in this work.
Based upon the above analysis, a series of CuO-K2CO3/TiO2catalysts with different Cu loadings were prepared and employed for NOxstorage and reduction. It is found that this series of catalysts exhibit high performance for lean NOxstorage and reduction, especially the one containing 8% (w, mass fraction) CuO which displays the largest NOxstorage capacity as high as 1.559 mmolg–1at lean condition and the highest NOxreduction percentage of 99% in cyclic lean/rich atmospheres. Meanwhile, zero selectivity to the by-product N2O during NOxreduction is achieved. By using X-ray diffraction (XRD), high resolution transmission electron microscopy (HR-TEM), temperature-programmed reduction of H2(H2-TPR), temperature-programmed desorption of CO2(CO2-TPD), extended X-ray absorption fine structure (EXAFS), and in-situ diffuse reflectance Fourier-transform infrared spectra (in-situ DRIFTS), the structures of catalysts, the states of Cu- and K-related species, and the NOxstorage/reduction mechanisms were systematically investigated.
2 Experimental
2.1 Catalysts preparation
The CuO-K2CO3/TiO2catalysts were prepared by sequential impregnation. First, the TiO2support (P25, 57 m2g–1) was impregnated with an aqueous solution of Cu(NO3)2(CuO loading: 2%, 4%, 8%, or 10% (w)), then dried at 120 °C overnight and calcined in air at 500 °C for 3 h to obtain the catalyst precursor. Thereafter, the precursor was impregnated with an aqueous solution of K2CO3(K2CO3loading: 15% (w)). After drying and calcination at the same conditions as above, the final catalysts CuO-K2CO3/TiO2were obtained, which are denoted as xCuOK2CO3/TiO2, where x represents the weight loading of CuO (2%, 4%, 8%, or 10%). The real loadings of Cu and K in the final catalysts measured by the inductively coupled plasma (ICP) method using a Vista MPX instrument could be found in Table 1, which are very close to the theoretical values.
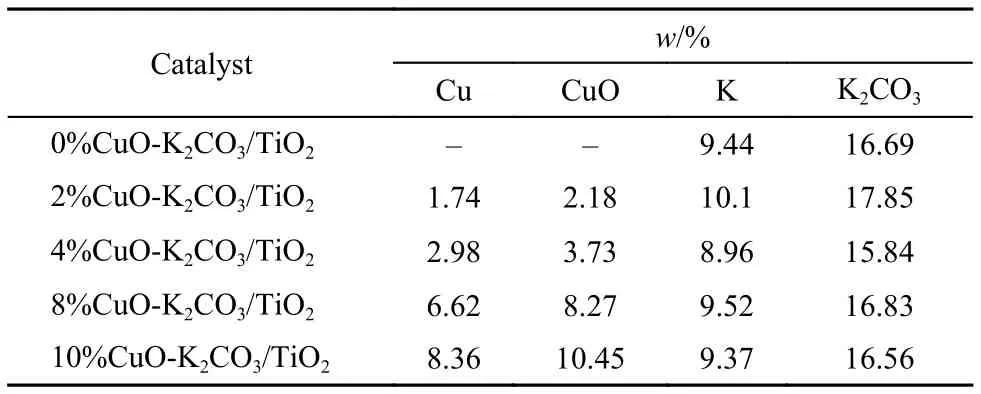
Tabl1 Chemical compositions of xCuO-K2CO3/TiO2catalysts measured by ICP
2.2 Catalyst characterization
The specific surface areas (SBET) of the catalysts were measured by N2adsorption at 77 K using a Quantachrome Quadra-Sorb SI instrument. Before experiments, the samples were degassed in vacuum at 300 °C for 4 h to remove the adsorbed species.
The X-ray diffraction (XRD) patterns of the catalysts were obtained using a Rigaku D/Max-2500 X-ray diffractometer operating at 200 mA and 40 kV using Cu Kαradiation (λ = 0.15418 nm). The diffraction data in the 2θ range from 20° to 90° were collected with a step size of 0.02°.
The HR-TEM images and energy dispersive X-ray (EDX) element mapping images for the catalysts were obtained on a JEM-2100F system operating at 200 kV.
The tests of temperature-programmed desorption of CO2(CO2-TPD) derived from the decomposition of carbonates were carried out on a Thermo-Finnigan TPDRO 1100 instrument. The sample was heated in pure helium fro m room temperature (RT) to 900 °C at a heating rate of 10 °Cmin–1. Before detection by a thermal conductivity detector (TCD), the effluent gas was purified by a trap containing Mg(ClO4)2to remove H2O.
The tests of H2temperature-programmed reduction (H2-TPR) of the catalysts were performed on the TP-5079 TPDRO setup (Tianjin Xianquan Instrument Company) equipped with TCD. In each test, the catalyst was heated from RT to 600 °C at a heating rate of 10 °Cmin–1in the flow atmosphere containing 8% (φ, volume fraction) H2and 92% (φ) N2(30 mLmin–1). Before detection, the gas was purified by a trap containing CaO+NaOH in order to remove the H2O and CO2.
The extended X-ray absorption fine structure (EXAFS) measurements were performed at the XAFS station in the 1W1B beamline of Beijing Synchrotron Radiation Facility (BSRF) operating at about 150 mA and 2.2 GeV. The absorption spectra of the Cu K-edge of the catalysts and reference compounds were recorded at room temperature in transmission mode. A Si(111) double crystal monochromator was used to reduce the harmonic content in monochrome beam. The backsubtracted EXAFS function was converted into k space and weighted by k3in order to compensate for the diminishing amplitude. The Fourier transforming of k3-weighted EXAFS data was performed in the range of k = 25 – 140 nm–1using a Hanning window function.
The in-situ DRIFT spectra were recorded on a Nicolet Nexus spectrometer. Each time, 15 mg powder sample was first loaded into an in-situ chamber and pretreated in highly pure helium (99.999%, 30 mLmin–1) at 350 °C for 30 min to collect the background spectrum, then the sorption gas (400 × 10–6(φ) NO, 5% (φ) O2, and balance N2) was introduced to the sample cell. The in-situ DRIFT spectra were collected at different exposure time up to 60 min at a spectral resolution of 4 cm–1in the range of 650–4000 cm–1.
2.3 NOxstorage capacity (NSC) measurement and cyclic NOxstorage/reduction (NSR) performance evaluation
Isothermal NSC was measured in a quartz-tubular continuous flow reactor (i.d. = 8 mm) loaded with 500 mg fresh catalysts (40–60 mesh). After the temperature reached 350 °C a mixture gas containing 400 × 10–6(φ) NO, 5% (φ) O2, and balance N2with a flow rate of 400 mLmin–1were introduced to the catalyst bed for adsorption. The concentrations of NO, NO2and total NOxin the effluent gas were monitored online by a Chemiluminescence NO-NO2-NOxAnalyzer (Model 42i-HL, Thermo Scientific).
To determine the NOxreduction performance of the catalysts, alternative lean/rich cyclic tests at 350 °C with a lean period of 3 min and a rich period of 1 min were conducted in the same reactor as NSC measurements. In lean period, the mixture gas consisting 400 × 10–6(φ) NO, 5% (φ) O2, and balance N2was introduced to the sample with a flow rate of 150 mLmin–1, while in rich period the atmosphere was changed into the mixture gas of 1000 × 10–6(φ) C3H6and balance N2with the same flow rate. To obtain the stable performance of the catalysts, 15 lean/rich cyclic tests were continuously performed, and the NOxreduction efficiency was calculated on the basis of the last two cycles. Meanwhile, the concentration of N2O was monitored online by a N2O modular gas analyzer (S710, SICK MAIHAK).
3 Results and discussion
3.1 NOxstorage and reduction performance of the catalysts
Fig.1 presents the isothermal NOxstorage curves at 350 °C over the fresh catalysts with different Cu loadings, and the NOxstorage capacities (NSCs) for these catalysts were calculated as listed in Table 2. From Fig.1 it is seen that at the beginning of lean period most NOxcan be quickly adsorbed by the catalysts with its concentration decreasing to the bottom within several minutes. After the dead time for 100% NOxtrapping, the NOxconcentration slowly increases and gradually reaches a steady state. Since the oxidation of NO to NO2is a crucial step for NOxstorage, it is believed that the “fast” NOxstorage mainly takes place on the surface storage sites adjacent to the oxidation components such as CuO, while the “slow” NOxstorage should correspond to the NOxstorage on the remote storage sites, due to the lack of active oxygen species and the diffusion resistancefrom surface to subsurface or bulk structure.23Generally, the highly dispersed surface K species near the oxidation sites are favorable to the fast and complete NOxstorage with little NOxleak.19The data in Table 2 show that the sample K2CO3/TiO2without Cu possesses the lowest NSC of ~0.309 mmolg–1, while those containing Cu display much larger NSC. The sample 8% CuO-K2CO3/TiO2exhibits not only the maximal NOxstorage capacity (1.559 mmolg–1) but also the highest conversion of NO to NO2(31.88%) after reaching the steadystate, showing good correlation between the NSC and NO oxidation capability of the catalyst. Fig.2 displays the NOxconcentration curves during 15 lean/rich cyclic tests over different catalysts. As seen from Fig.2, this series of catalysts display excellent and stable performance for NOxstorage and reduction after 15 lean/rich cyclic tests, giving very low NOxconcentration at the reactor outlet. Upon the basis of the last two lean/rich cycles, the average NOxreduction percentages over all the catalysts are calculated and listed in Table 2. Obviously, the sample 8% CuO-K2CO3/TiO2shows the best NOxreduction performance, displaying a NOxreduction percentage as high as 99%. Compared with the Pt-based LNT catalysts reported in literature,24,25as-prepared Cu-based LNT catalyst 8% CuO-K2CO3/ TiO2exhibits larger NSC and comparable NOxreduction percentage (Table 2). It is worth mentioning that during the whole lean period (3 min) and rich period (1 min) the concentration of the by-product N2O is nearly undetectable. As an example, the curve of N2O concentration for 8% CuO-K2CO3/TiO2is selected and displayed in Fig.2(c). It is found that on this sample the N2O concentration is 0, which indirectly demonstrates that this series of catalysts possess weak capability for the dissociation of NO molecules, since N2O mainly comes from the combination of NO-dissociation produced N (ads) atom and the NO molecule in gaseous or adsorbed states.
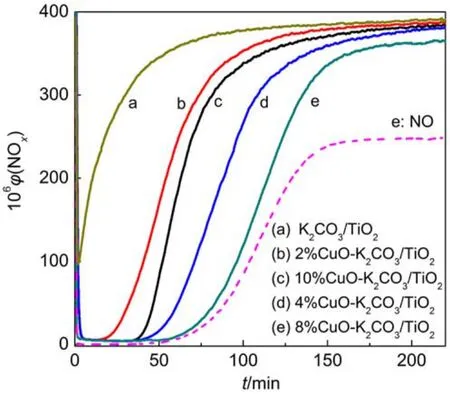
Fig.1 NOxstorage curves of xCuO-K2CO3/TiO2at 350 °C in the flow atmosphere of 400 × 10–6NO, 5% O2, and balance N2with a flow rate of 400 mLmin–1
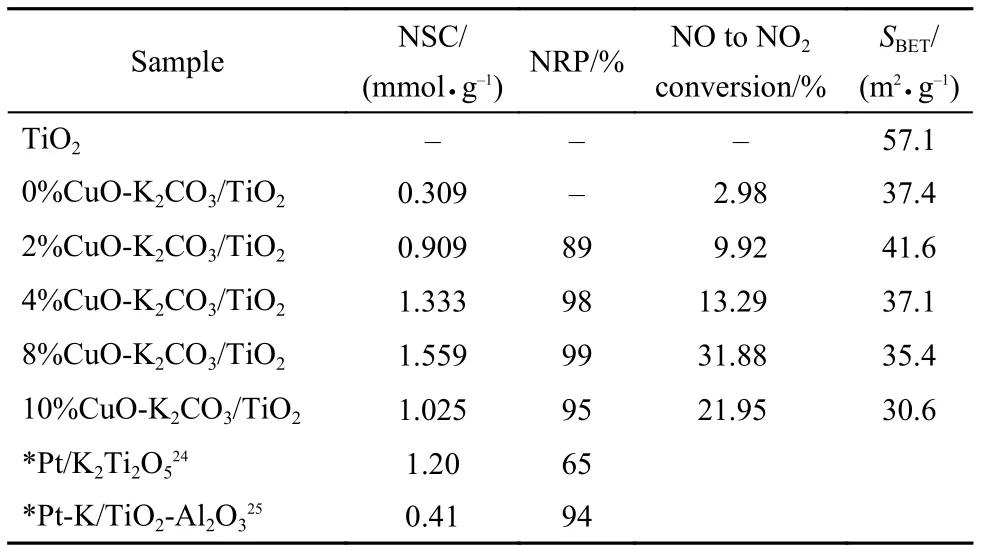
Tabl2 NOxstorage capacity (NSC) of the fresh xCuO-K2CO3/TiO2, NO to NO2conversion (%), specific surface area (SBET), and the NOxreduction percentage (NRP) of the catalysts after 15 cyclic lean/rich NOxstorage-reduction tests
3.2 Catalyst characterization
3.2.1 Specific surface area of the samples
The specific surface areas (SBET) of the catalysts xCuOK2CO3/TiO2are listed in Table 2. It is found that with the addition of K2CO3to TiO2the SBETremarkably decreases from 57.1to 37.4 m2g–1. After a small amount of Cu is supported, the sample 2%CuO-K2CO3/TiO2shows an increased BET surface area of 41.6 m2g–1, in a reported work on MnOx-Ce/TiO2catalyst, a similar slight increase of specific surface area was also observed after doped some transition metals.26However, further elevation of Cu loading to 4%, 8%, and 10% decreases the SBETto 37.1, 35.4, and 30.6 m2g–1, respectively. Combining with the performance of these catalysts, it is deduced that that the SBETis not the main determining factor on the catalytic performance of the catalysts.
3.2.2 XRD characterization
In order to determine the bulk structure of the catalysts and reveal the effects of K2CO3on the crystalline of TiO2and the dispersion of Cu species, X-ray diffraction experiments were performed on the catalysts, the results of which are shown in Fig.3. It is seen that besides the diffraction peaks contributed by the support P25 consisting of anatase and rutile phases there are still other two detectable peaks at 2θ values of 25.309° and 29.306° which could be attributed to K2O phase [JCPDS No. 77-2176] (Fig.3(b–e)). However, no diffraction peaks corresponding to K2CO3are identified, suggesting that the K2CO3may have totally decomposed into K2O or exist in dispersed or amorphous states, as reported for the cases of ZrO2or K2Ti8O17supported K2CO3-based LNT catalysts.19,27For the catalysts containing 2% or 4% CuO, no diffraction peaks of CuO or Cu2O are observed, but with the increase of CuO content to 8% and 10%, the diffraction peaks associated with the (002), (111), and (200) planes of CuO [JCPDS No. 80-1917] are identified at 2θ of 35.504°, 38.735°, and 38.932° due to the growth of CuO crystallites. It was ever pointed out that beyond a critical quantity, additional amounts of copper would readily accumulate to form bulk copper oxide species,28similar to the case of this work.
3.2.3 HR-TEM images and energy dispersive X-ray element mapping
The morphological and structural features of the catalysts xCuO-K2CO3/TiO2are investigated by HR-TEM, as shown in Fig.4. The HR-TEM images clearly reveal the existence of anamorphous material covering the surface of the support with the (101) plane of anatase exposed, which may correspond to the dispersed K2CO3species.19,27When the CuO loading is no more than 4%, CuO crystallites can hardly be observed (Fig.4a); however, with the increase of Cu loading to 8% (Fig.4(b, c)), the CuO crystallites with an average particle size of 2.2 nm are clearly found, showing the lattice spacing of 0.22 nm for (111) plane. These CuO crystallites are closely contacted with the amorphous K2CO3, suggesting the potential interaction between them. As the Cu loading is further increased to 10%, bigger agglomerated CuO crystallites appear as shown in Fig.4d. The results of HR-TEM are in good agreement with the XRD results above. Besides, the EDX element mapping results on the catalyst 8%CuO-K2CO3/TiO2shown in Fig.4e clearly indicate that the O, Cu, K, and Ti elements uniformly exist in the catalyst, suggesting the homogeneous distribution of these components.
3.2.4 CO2-TPD
To characterize the kinds and stability of K2CO3species in different catalysts, the temperature-programmed desorption of CO2(CO2-TPD) derived from the decomposition of K2CO3was performed on the catalysts, the results of which are displayed in Fig.5. The profiles could be divided into three temperature regions: 70–180 °C, 180–700 °C, and 700–880 °C. It is known that pure K2CO3often decomposes at high temperature around 900 °C; however, the interaction between the dispersed K2CO3and surface hydroxyl groups on TiO2can remarkably lower the decomposition temperature of K2CO3.11In Fig.5, the low-temperature peaks from 70 to 180 °C may correspond to the desorption of CO2adsorbed on -OK groups which were generated through the direct interaction between highly dispersed K2CO3and the surface hydroxyl groups on TiO2during catalyst calcination at 500 °C, as described in the following reaction (1):
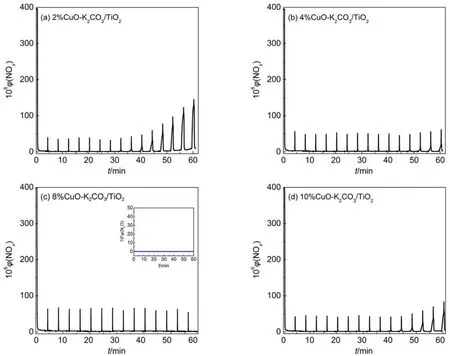
Fig.2 NOxconcentration curves of xCuO-K2CO3/TiO2during storage and reduction tests in the cyclic lean/rich atmospheres at 350 °C with aspace velocity of 36000 h–1
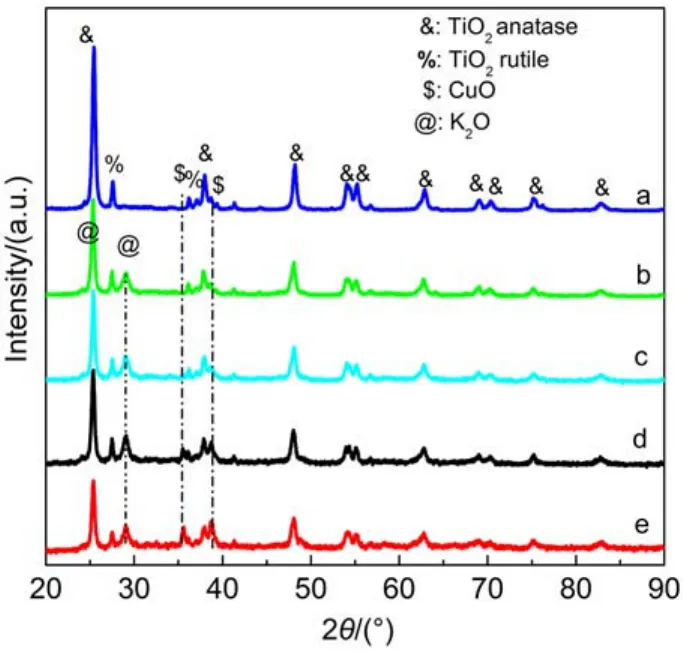
Fig.3 XRD patterns of different samples
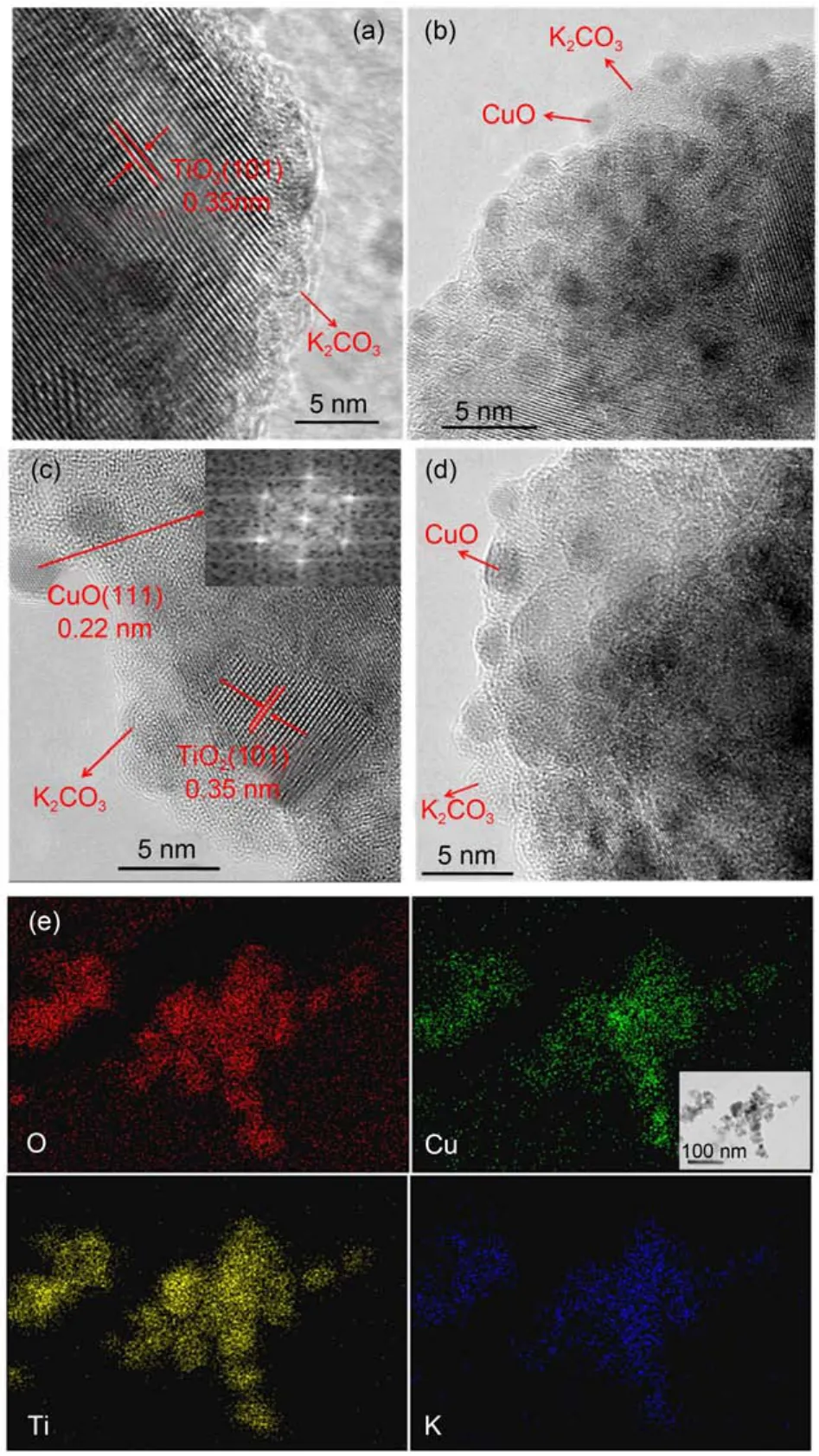
Fig.4 HRTEM images of (a) 4%CuO-K2CO3/TiO2, (b, c) 8%CuOK2CO3/TiO2, and (d) 10%CuO-K2CO3/TiO2; (e) element mapping images of 8%CuO-K2CO3/TiO2
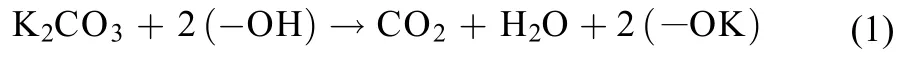
While the middle-temperature peaks between 180 and 700 °C could be attributed to the decomposition of surface K2CO3phase dispersed on the support, as expressed by the following reaction (2):

Although during catalyst calcination at 500 °C some surface K2CO3phase may have decomposed to form K2O, the re-formation of surface carbonates are very potential after exposure to air due to the presence of CO2in air. The high-temperature peaks above 700 °C should be resulted from the decomposition of bulk or bulk-like K2CO3species since at the calcination temperature (500 °C) such bulk carbonates can hardly decompose, and thus still retain on the catalysts. With the increase of Cu loading, the low-temperature peaks dramatically decrease, suggesting the decreased amounts of -OK groups; the covering effect of the increased Cu species on the support can account for this phenomenon. Similarly, the middle-temperature peaks are also weakened and broadened, implying the enhanced dispersion of surface K2CO3species. However, further increase of Cu loading to 10% results in the formation of some bulk or bulk-like K2CO3species which decompose at the high temperature above 700 °C. Apparently, too much supported Cu species are unfavorable to the dispersion of K2CO3. The increased CuO species would occupy more surface of the support, naturally decreasing the dispersion of K2CO3species. Meanwhile, excess amount of Cu can facilitate the agglomeration of CuO species, thus more amounts of bulk copper oxides species are formed, decreasing its enhancement effect on K2CO3dispersion. It has ever been reported that the activity of copper-based catalysts was primarily contributed by the finely dispersed copper species interacting with the supports; bulk copper oxides often showed negligible contribution to the activity.29In present work, as CuO loading is elevated to 10%, both the finely dispersed copper species and the surface K2CO3species would decrease, thus making the corresponding catalyst less active for NOxstorage and reduction than the one containing 8% CuO (see Table 2).
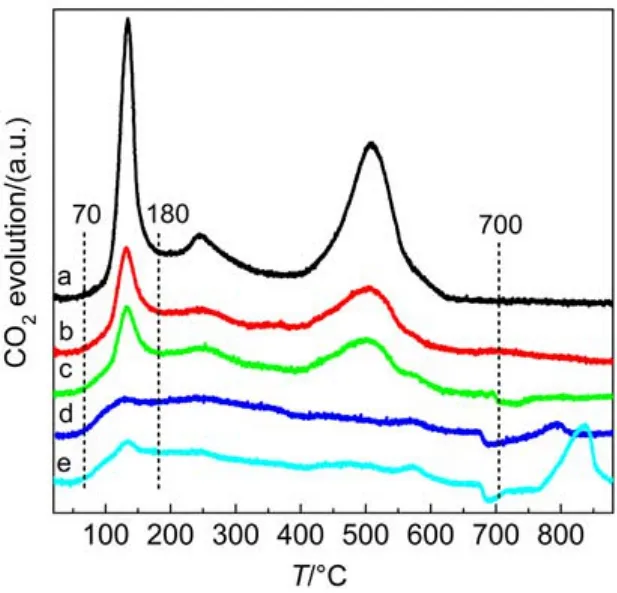
Fig.5 CO2-TPD profiles of the precursors of xCuO-K2CO3/TiO2
3.2.5 XAFS
To get the information of the local microstructures and chemical states of Cu, the catalysts and reference materials (Cu foil, Cu2O and CuO) were characterized by XAFS including X-ray absorption near-edge structure (XANES) and EXAFS. The Cu K-edge XANES spectra of the fresh catalysts are shown in Fig.6. It can be seen that the profiles for xCuO-K2CO3/TiO2are close to that of CuO with the main absorption peaks (1s → 4p) appearing at about 8984 and 8991 eV. In addition, there is also a pre-edge absorption peak appearing at 8977 eV, which is assigned to 1s–3d transition.30These results suggest that the copper species in the fresh catalysts possess the local coordination environment similar to CuO phase, which is consistent with the XRD results. Fig.7 displays the radial structure functions (RSFs) of Cu K-edge derived from the EXAFS spectra of the samples. It is found that the Cu K-edge RSFs of the fresh catalysts are also analogous to that of CuO, further confirming that the copper species mainly exist as CuO phase. The strongestcoordination peak at about 0.15 nm (not corrected by phase scattering shift) is attributed to the first Cu–O coordination in CuO.31After NOxstorage or 15 lean/rich cyclic NOxstorage and reduction tests, the RSFs of the catalyst 8%CuO-K2CO3/TiO2change little (dotted lines in Fig.7) as compared with that of the fresh sample, which demonstrates the high stability of this catalyst.
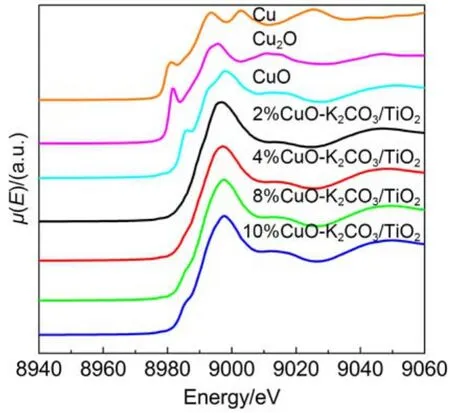
Fig.6 Cu K-edge XANES spectra of xCuO-K2CO3/TiO2catalysts and references
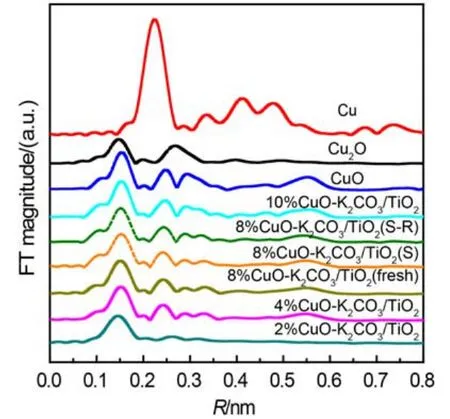
Fig.7 Cu K-edge radial structure functions (RSFs) of references and fresh xCuO-K2CO3/TiO2catalysts as well as the RSFs of 8%CuOK2CO3/TiO2after used in NOxstorage (S) or NOxstorage and reduction (S-R)
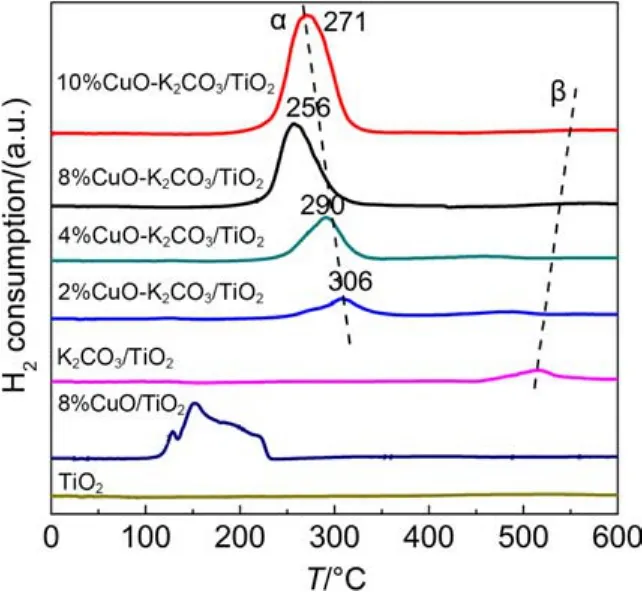
Fig.8 H2-TPR profiles of different samples
3.2.6 H2-TPR
The above characterizations have confirmed that CuO is the main active species in xCuO-K2CO3/TiO2catalysts. To investigate the reducibility of CuO and reveal its correlation with the catalytic performance, H2temperature-programmed reduction tests were performed on them as displayed in Fig.8. As expected, the TiO2support is nearly inert to H2. After loaded with K2CO3, a weak and broad reduction peak around 516 °C is identified, which may be related to the decomposition of K2CO3, since the released CO2can dilute the feed gas stream and decrease the concentration of H2, causing the appearance of pseudo H2consumption peak.32The above CO2-TPD profile (Fig.5) has proved that the sample K2CO3/TiO2does have a K2CO3decomposition peak around 510 °C approaching to the temperature in TPR. After loaded with 2% CuO on the support, the sample displays a reduction peak around 306 °C, which is lower than that for reference CuO (350 °C, not shown), since the highly dispersed CuO species with smaller size could be reduced easier than bulk CuO.33As Cu loading increases from 2% to 8%, the reduction peak gradually increases and shifts to lower temperature, suggesting the improved reducibility of copper species. However, for the sample containing 10% CuO, the reduction temperature is a little higher than that for the one with 8% CuO. The decrease of CuO dispersion or the increase of CuO crystallites is a plausible interpretation for the increased temperature of the sample with 10% CuO. So, the optimal Cu loading in xCuO-K2CO3/TiO2catalysts is 8%, which is in good agreement with the results of catalytic activity. To investigate the effect of K2CO3on the reduction behavior of Cu species and the interaction between them, the H2-TPR test was also carried out on 8%CuO/TiO2containing no K2CO3just for a direct comparison. It is found that the sample 8%CuO-K2CO3/TiO2shows much higher reduction temperature than 8%CuO/TiO2, suggesting the strong interaction between the CuO species and the K species. Such strong interaction would favor the adsorption of NOxsince the basic K species as electron donor can increase the electron density of Cu species, enhancing their ability for the trapping of acidic NO and/or NO2species. Based upon the TPR peak area, we calculated the actual H2consumption and compared with the theoretical values, as listed in Table 3. It is seen that for the same catalyst the actual H2consumption is a little larger than the theoretical value, which suggests that all the Cu oxides have good reducibility; as to the larger H2consumption, it may be also resulted from the decomposition of surface carbonates as analyzed in literature.32
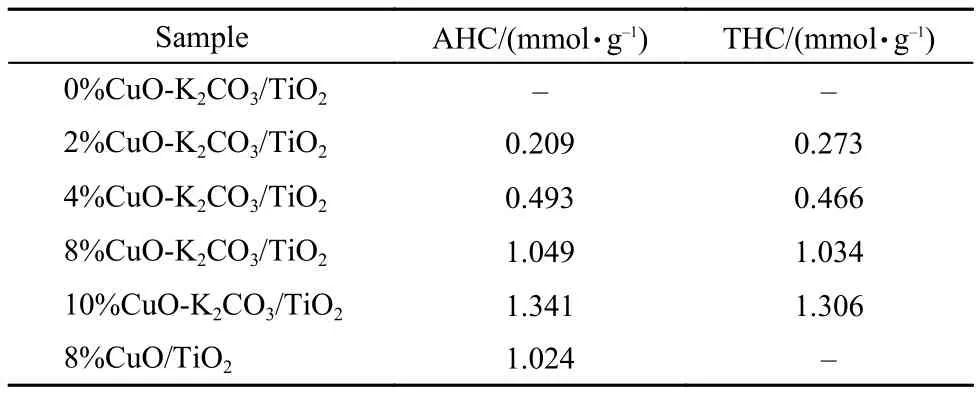
Tabl3 Amounts of actual H2consumption (AHC) in H2-TPR and the theoretical H2consumption (THC) based upon the ICP results for Cu contents
3.2.7 In-situ DRIFTS characterization on NOxstorage species
To investigate the NOxstorage species at lean condition, the time-dependent in-situ DRIFT spectra of the catalysts after exposed to 400 × 10–6NO/5% O2/N2were recorded, as shown in Fig.9. For the sample K2CO3/TiO2, as seen in Fig.9a, only nitrite species are detected, showing the bands around 1248 cm–1(-NO2asymmetric stretch of bidentate nitrite);34the absence of nitrate species suggests the poor oxidation performance of K2CO3/TiO2; the two negative bands around 1456 and 1563 cm–1corresponding to surface bicarbonate species (-CO2asymmetric stretch)35and bidentate carbonate species (-C=O stretch)34are resulted from the decomposition of carbonates induced by NOxstorage. After loading 2% Cu on K2CO3/TiO2, the band of ionic bidentate nitrite species around 1260 cm–1(-NO2asymmetric stretch)34is still retained, as seen in Fig.9b; however, after 20 min this band gradually decreases with a new band around 1350 cm–1attributed to ionic nitrate species (-NO3asymmetric stretch)36simultaneously appearing, which suggests the transformation of nitrites to nitrates. The spectra of the sample with 4% Cu shown in Fig.9c are similar to those for the sample with 2% Cu; at the beginning the bidentate nitrite species (1248 cm–1) are the main storage species, but with the time going the ionic nitrate species (1350 cm–1) become the dominant ones. Similarly, the observed two negative bands around 1436 and 1563 cm–1attributable to -CO2asymmetric stretch in surface bicarbonate species37and -C=O stretch in bidentate carbonates are also resulted from the decomposition of carbonates induced by NOxstorage. As the CuO loading is elevated to 8%, the spectra become more complicated as displayed in Fig.9d. A couple bands at 1340 and 1440 cm–1corresponding to surface monodentate nitrate38develop quickly after 5 min, and the characteristic band of bidentate nitrite around 1242 cm–134gradually disappears after 10 min. Here, it should be noted that during NOxstorage only one negative band around 1563 cm–1related to carbonate decomposition is detected; another negative band around 1436 cm–1is absent, which may be compensated by the quickly developed band of nitrates at the similar position (1440 cm–1). For the sample 10%CuO-K2CO3/ TiO2, the band of bidentate nitrite at 1242 cm–1is the dominant one in the first 10 min; afterwards, the bands around 1350 and 1418 cm–1attributed to ionic nitrate and monodentate nitrate (-NO2asymmetric stretch)34grow successively, dominating the spectra. Upon the basis of these results, it is summarized that the NOxstorage species over CuO-K2CO3/TiO2catalysts are related to Cu loading; in the sample CuO-K2CO3/TiO2containing 2% or 4% Cu, NOxis mainly stored as bidentate nitrite species; as the Cu loading is increased to 8%, NOxis stored as monodentate nitrate species very quickly, suggesting the improved oxidation performance of this catalyst; however, further increase of copper content to 10%, bidentate nitrite species increase due to the decrease of oxidation capability of the catalyst. Therefore, the optimal loading of Cu in the catalysts xCuO-K2CO3/TiO2should be 8% by weight, which is totally consistent with the results of H2-TPR and the catalytic activity.
3.3 Models of the existing states of CuO species and potential NOxstorage pathways
By combining all the results above, models describing the distribution states of CuO species and the NOxstorage species on the samples with different Cu loadings are proposed, as displayed in Fig.10. During the lean period, CuO phase oxidizes the NO to NO2, both of which can therefore undergo adsorption. The highly dispersed surface K species adjacent to the oxidation sites (CuO) are favorable to fast and complete NOxstorage. The slow NOxstorage process possibly corresponds to the NOxstorage on the storage sites far from the CuO phase; during this process the lack of active oxygen species and the diffusion resistance decreases the NOxstorage rate and effciency. As described in Fig.10(a), in the catalyst K2CO3/TiO2, nitrites are the only NOxstorage species as identified by in-situ DRIFTS. It has been widely accepted that the oxidation of NO(ad) to NO2(ad) is a key step for NOxstorage,1the higher NO to NO2conversion, the higher NOxstorage capacity.39In the absence of CuO, although NO can also be trapped, the NOxstorage is much more diffcult, as compared with those samples containing Cu (see Fig.1). After addition of 2% or 4% CuO, as described in Fig.10(b), the NOxstorage is enhanced, but the contents of copper are still low, the NOxstorage is still very slow. During such “slow” storage process, the oxidation capability of the catalyst is not high enough, making the NOxmainly stored as nitrites species as seen from Fig.9(b, c). As CuO loading is elevated to 8%, NOxis mainly stored as nitrates species due to the increased oxidation capability of this catalyst as depicted in Fig.10(d), but further increase of Cu would result in obvious decrease of the dispersion for both CuO and K2CO3species, thus reducing oxidation performance of this catalyst; as a result, the NOxis mainly stored as nitrite species, as described in Fig.10(d).
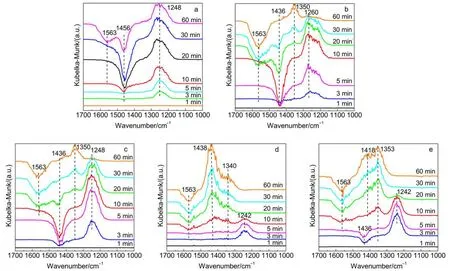
Fig.9 Isothermal time-dependent in-situ DRIFT spectra of NO/O2adsorption at 350 °C over (a) K2CO3/TiO2, (b) 2%CuO-K2CO3/TiO2, (c) 4%CuO-K2CO3/TiO2, (d) 8%CuO-K2CO3/TiO2, and (e) 10%CuO-K2CO3/TiO2
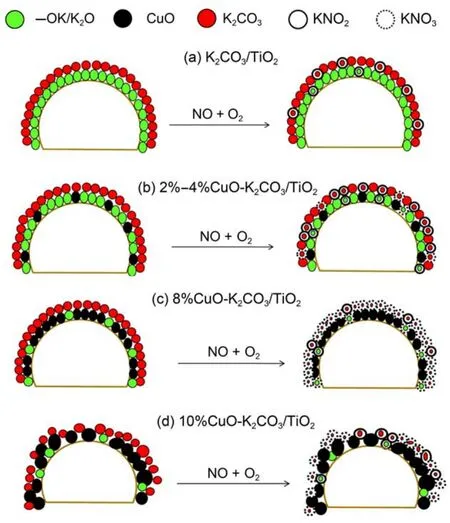
Fig.10 Distribution models for CuO and K2CO3species, and the potential NOxstorage pathways
On the basis of above analysis, the potential reaction pathways for NOxstorage in lean period and reduction in rich period are summarized, as listed below (S refers to support).
Lean period:
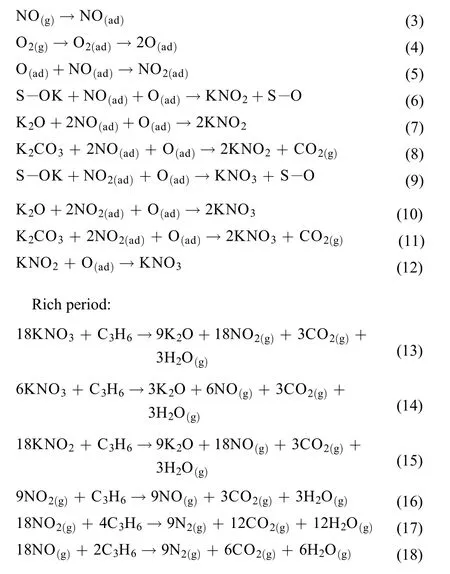
4 Conclusions
The series of TiO2supported Cu-based non-platinic LNT catalysts xCuO-K2CO3/TiO2with different loadings of Cu exhibit very excellent NOxstorage and reduction performance, especially the one containing 8% CuO, which displays the largest NSC of 1.559 mmolg–1, the highest NOxreduction percentage of 99%, and the zero selectivity of NOxto N2O. The Cu loading influences not only the reducibility of the catalysts but also the distributing states of K species in the catalysts. The strong interaction between copper and potassium species enhances the NOxstorage and reduction. In-situ DRIFTS reveals that the catalyst CuO-K2CO3/TiO2with 8% CuO can quickly store the NOxas monodentate nitrate species, showing the best oxidation performance. After 15 lean/rich cyclic NSR tests, the catalyst structure and the high catalytic performance of 8%CuOK2CO3/TiO2can be well retained. The outstanding catalytic performance and stability of this sample make it potential as a candidate of low-cost LNT catalysts containing no noble metals.
(1)Roy, S.; Baiker, A. Chem. Rev. 2009, 109, 4054. doi: 10.1021/cr800496f
(2)Klingstedt, F.; Arve, K.; Eränen, K.; Murzin, D. Y. Accounts Chem. Res. 2006, 39, 273. doi: 10.1021/ar050185k
(3)Takahashi, N.; Shinjoh, H.; Iijima, T.; Suzuki, T.; Yamazaki, K.; Yokota, K.; Suzuki, H.; Miyoshi, N.; Matsumoto, S.; Tanizawa, T.; Tanaka, T.; Tateishi, S.; Kasahara, K. Catal. Today 1996, 27, 63.
(4)Xian, H.; Ma, A. J.; Meng, M.; Li, X. G. Acta Phys. -Chim. Sin. 2013, 29, 2437. [贤 晖, 马爱静, 孟 明, 李新刚. 物理化学学报, 2013, 29, 2437.] doi: 10.3866/PKU.WHXB201309052
(5)Hadjiivanov, K.; Klissurski, D.; Ramis, G.; Busca, G. Appl. Catal. B 1996, 7, 251. doi: 10.1016/0926-3373(95)00034-8
(6)Sultana, A.; Haneda, M.; Hamada, H. Appl. Catal. B 2009, 88, 180. doi: 10.1016/j.apcatb.2008.08.028
(7)Zhang, W. X.; Yahiro, H.; Mizuno, N.; Izumi, J.; Iwamoto, M. Langmuir 1993, 9, 2337. doi: 10.1021/la00033a015
(8)Liu, J.; Li, X. Y.; Zhao, Q. D.; Zhang, D. K.; Ndokoye, P. J. Mol. Catal. A 2013, 378, 115.
(9)Guerrero, S.; Guzmán, I.; Aguila, G.; Chornik, B.; Araya, P. Appl. Catal. B 2012, 123–124, 282.
(10)Glisenti, A.; Natile, M. M.; Carlotto, S.; Vittadini, A. Catal. Lett. 2014, 144, 1466.
(11)Matsumoto, S. I.; Ikeda, Y.; Suzuki, H.; Ogai, M.; Miyoshi, N. Appl. Catal. B 2000, 25, 115. doi: 10.1016/S0926-3373(99) 00124-1
(12)Frola, F.; Manzoli, M.; Prinetto, F.; Ghiotti, G.; Castoldi, L.; Lietti, L. J. Phys. Chem. C 2008, 112, 12869. doi: 10.1021/ jp801480t
(13)Lietti, L.; Forzatti, P.; Nova, I.; Tronconi, E. J. Catal. 2001, 204, 175. doi: 10.1006/jcat.2001.3370
(14)Nova, I.; Castoldi, L.; Lietti, L.; Tronconi, E.; Forzatti, P.; Prinetto, F.; Ghiotti, G. J. Catal. 2004, 222, 377. doi: 10.1016/j.jcat.2003.11.013
(15)Delucasconsuegra, A.; Caravaca, A.; Sanchez, P.; Dorado, F.; Valverde, J. J. Catal. 2008, 259, 54.
(16)Shen, W. H.; Nitta, A.; Chen, Z.; Eda, T.; Yoshida, A.; Naito, S. C. J. Catal. 2011, 280, 161. doi: 10.1016/j.jcat.2011.03.014
(17)Castoldi, L.; Lietti, L.; Forzatti, P.; Morandi, S.; Ghiotti, G.; Vindigni, F. J. Catal. 2010, 276, 335. doi: 10.1016/j.jcat. 2010.09.026
(18)Liu, Y.; Meng, M.; Li, X. G.; Guo, L. H.; Zha, Y. Q. Chem. Eng. Res. Des. 2008, 86, 932. doi: 10.1016/j.cherd.2008.02.010
(19)Hou, N. N.; Zhang, Y. X.; Meng, M. J. Phys. Chem. C 2013, 117, 4089.
(20)Li, W. B.; Yang, R. T.; Krist, K.; Regalbuto, J. R. Energ. Fuel 1997, 11, 428. doi: 10.1021/ef960128v
(21)Huang, J.; Wang, S. R.; Zhao, Y. Q.; Wang, X. Y.; Wang, S. P.; Wu, S. H.; Zhang, S. M.; Huang, W. P. Catal. Commun. 2006, 7, 1029. doi: 10.1016/j.catcom.2006.05.001
(22)Chen, C. S.; Chen, T. C.; Chen, C. C.; Lai, Y. T.; You, J. H.; Chou, T. M.; Chen, C. H.; Lee, J. F. Langmuir 2012, 28, 9996.
(23)You, R.; Zhang, Y. X; Liu, D. S.; Meng, M.; Zheng, L. R.; Zhang, J.; Hu, T. D. J. Phys. Chem. C 2014, 118, 25403. doi: 10.1021/jp505601x
(24)Wang, Q.; Sohn, J. H.; Chung, J. S. Appl. Catal. B 2009, 89, 97. doi: 10.1016/j.apcatb.2008.12.007
(25)Li, Z. B.; Meng, M.; You, R.; Ding, T.; Li, Z. J. Catal. Lett. 2012, 142, 1067. doi: 10.1007/s10562-012-0864-7
(26)Zhang, L. J.; Cui, S. P.; Guo, H. X.; Ma, X. Y.; Luo, X. G. J. Mol. Catal. A 2014, 390, 14. doi: 10.1016/j.molcata. 2014.02.021
(27)Zhang, Y. X.; Meng, M.; Dai, F. F.; Ding, T.; You, R. J. Phys. Chem. C 2013, 117, 23691. doi: 10.1021/jp406950u
(28)Friedman, R. M.; Freeman, J. J.; Lytle, F. W. J. Catal. 1978, 55, 10. doi: 10.1016/0021-9517(78)90181-1
(29)Liu, W.; Flytzani-Stephanopoulos, M. Chem. Eng. J. 1996, 64, 283.
(30)Bhuiyan, M. M. R.; Lin, S. D.; Hsiao, T. C. Catal. Today 2014, 226, 150. doi: 10.1016/j.cattod.2013.10.053
(31)Fox, E. B.; Velu, S.; Engelhard, M. H.; Chin, Y. H.; Miller, J. T.; Kropf, J.; Song, C. J. Catal. 2008, 260, 358. doi: 10.1016/j.jcat. 2008.08.018
(32)Zhang, Y. X.; Liu, D. S.; Meng, M.; Jiang, Z.; Zhang, S. Ind. Eng. Chem. Res. 2014, 53, 8416. doi: 10.1021/ie501034u
(33)Chen, L. F.; Guo, P. J.; Qiao, M. H.; Yan, S. R.; Li, H. X.; Shen, W.; Xu, H. L.; Fan, K. N. J. Catal. 2008, 257, 172. doi: 10.1016/j.jcat.2008.04.021
(34)Prinetto, F.; Manzoli, M.; Morandi, S.; Frola, F.; Ghiotti, G.; Castoldi, L.; Lietti, L.; Forzatti, P. J. Phys. Chem. C 2009, 114, 1127.
(35)Toops, T. J.; Smith, D. B.; Partridge, W. P. Appl. Catal. B 2005, 58, 245. doi: 10.1016/j.apcatb.2004.10.021
(36)Prinetto, F.; Ghiotti, G.; Nova, I.; Lietti, L.; Tronconi, E.; Forzatti, P. J. Phys. Chem. B 2001, 105, 12732. doi: 10.1021/jp012702w
(37)Montanari, T.; Castoldi, L.; Lietti, L.; Busca, G. Appl. Catal. A 2011, 400, 61. doi: 10.1016/j.apcata.2011.04.016
(38)Fanson, P. T.; Horton, M. R.; Delgass, W. N.; Lauterbach, J. Appl. Catal. B 2003, 46, 393. doi: 10.1016/S0926-3373(03) 00275-3
(39)Qi, G. S.; Li, W. Catal. Lett. 2013, 144, 639.
Effect of Cu Loading on the Structure and Catalytic Performance of the LNT Catalyst CuO-K2CO3/TiO2
FAN Feng-Qi1MENG Ming1,*TIAN Ye1,*ZHENG Li-Rong2ZHANG Jing2HU Tian-Dou2
(1Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin Key Laboratory of Applied Catalysis Science and Engineering, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, P. R. China;2Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, P. R. China)
A series of non-platinic lean NOxtrap (LNT) CuO-K2CO3/TiO2catalysts with different Cu loadings were prepared by sequential impregnation, and they showed relatively good performance for lean NOxstorage and reduction. The catalyst containing 8% (w) CuO showed not only the largest NOxstorage capacity of 1.559 mmolg–1under lean conditions, but also the highest NOxreduction percentage of 99% in cyclic lean/rich atmospheres. Additionally, zero selectivity of NOxto N2O was achieved over this catalyst during NOxreduction. Multiple techniques, including X-ray diffraction (XRD), high-resolution transmission electron microscopy (HR-TEM), temperature-programmed desorption of CO2(CO2-TPD), extended X-ray absorption fine structure (EXAFS), temperature-programmed reduction of H2(H2-TPR), and in-situ diffusereflectance Fourier-transform infrared spectroscopy (DRIFTS), were used for catalyst characterization. The results indicate that highly dispersed CuO is the main active phase for oxidation of NO to NO2and reduction of NOxto N2. The strong interaction between K2CO3and CuO was clearly revealed, which favors NOxadsorption and storage. The appearance of negative bands at around 1436 and 1563 cm–1, corresponding to CO2asymmetric stretching in bicarbonates and -C=O stretching in bidentate carbonates, showed the involvement of carbonates in NOxstorage. After using the catalysts for 15 cycles of NOxstorage and reduction in alternative lean/rich atmospheres, the CuO species in the catalysts showed little change, indicating high catalytic stability. Based on the results of in-situ DRIFTS and the other characterizations, a model describing the NOxstorage processes and the distribution of CuO and K2CO3species is proposed.
NOx; Storage; Reduction; Copper oxide; Potassium carbonate
O643
10.3866/PKU.WHXB201507291
Received: April 17, 2015; Revised: July 29, 2015; Published on Web: July 29, 2015.
*Corresponding authors. MENG Ming, Email: mengm@tju.edu.cn; Tel/Fax: +86-22-27892275. TIAN Ye, Email: tianye@tju.edu.cn.
The project was supported by the National Natural Science Foundation of China (21276184, U1332102, 21476160), Specialized Research Fund for the Doctoral Program of Higher Education of China (20120032110014), Natural Science Foundation of Tianjin, China (12JCYBJC14000,
15JCZDJC37400), and Program for Introducing Talents of Discipline to Universities of China (B06006).
国家自然科学基金(21276184, U1332102, 21476160), 高等学校博士学科点专项科研基金(20120032110014), 天津市自然科学基金
(12JCYBJC14000, 15JCZDJC37400)及中国大学学科人才引进计划(B06006)资助
© Editorial office of Acta Physico-Chimica Sinica

