Zr的添加对稀燃天然气汽车尾气净化催化剂Pd-Pt/Al2O3性能的影响
胡 伟 王 云 尚鸿燕 徐海迪 钟 琳,*陈建军 龚茂初 陈耀强,,*
(1四川大学化学工程学院, 成都 610064; 2四川大学新能源与低碳技术研究院, 成都 610064;3四川大学化学学院, 绿色化学与技术教育部重点实验室, 成都 610064)
Zr的添加对稀燃天然气汽车尾气净化催化剂Pd-Pt/Al2O3性能的影响
胡 伟1王 云1尚鸿燕1徐海迪2,3钟 琳1,*陈建军2龚茂初2陈耀强1,2,3,*
(1四川大学化学工程学院, 成都 610064;2四川大学新能源与低碳技术研究院, 成都 610064;3四川大学化学学院, 绿色化学与技术教育部重点实验室, 成都 610064)
Pd-Pt双金属基甲烷氧化催化剂的催化活性、抗水热老化性和耐硫性在一个通有模拟稀燃天然气汽车尾气成分的固定床反应器中进行检测. 研究发现Zr掺杂的Pd-Pt/Al2O3(Pd-Pt/ZrxAl(1–x)O(3+x)/2)提高了催化的催化活性、抗水热老化性和耐硫性. 以共沉淀法制备Zr : Al的摩尔比分别为0 : 1、0.25 : 0.75、0.5 : 0.5、0.75 : 0.25和1 : 0的材料为载体材料. 双金属催化剂的活性组分分别为1.5% (w, 质量分数)的Pd和0.3% (w)的Pt, 活性组分Pd、Pt通过共浸渍的方法浸渍到以上载体材料上制备得到一系列整体式催化剂. 分别采用低温N2吸脱附、X射线衍射(XRD)、H2程序升温还原(H2-TPR)、O2程序升温氧化(O2-TPD)及X射线光电子能谱对制备的催化剂进行表征. 结果显示Zr的加入使催化剂的载体材料结晶度提高, 活性组分的分散度也得到了相应的提高. 同时二价Pd物种与周围电子密度分别增加. 相比于Pd-Pt/Al2O3和Pd-Pt/ZrO2催化剂, 在不同条件预处理后, Zr的添加对催化剂的性能有明显的提高, 其中催化剂Pd-Pt/Zr0.5Al0.5O1.75展现了最好的催化活性、抗水热老化性以及耐硫性.
铝; 锆; 不同摩尔比; 载体; 天然气燃料发动机; 催化性能
1 Introduction
Natural gas (NG) resources are abundant, and used widely as energy source in industry and automotive vehicle owing to their low exhaust emission.1,2With the development of the natural gas fields, the number of natural gas vehicles is increasing synchronously.3Besides the economic needs, compressed natural gas vehicles (NGVs) with low CO and low NOxemissions have more eco-friendly advantages than gasoline and diesel vehicles.4,5Especially, the operation of NGVs under lean-burn conditions could reduce the pollutants more effectively and improve thermal efficiency compared with the stoichiometric condition.5–7Although engine technologies have been improved to increase combustion efficiency and decrease emission, the main ingredients of exhaust still contain HC, CO, and NOx; moreover, methane is the main component (90%–95%) of the total hydrocarbon that makes more contributions to global climate warming than carbon dioxide when the emission rates are equality.5,6,8Therefore, it is very urgent to reduce methane emission effectively in the NGV exhaust.
Noble metal (Pt, Pd, Rh) catalysts, especially palladium catalysts, have been recognized as one of the best catalysts to reduce methane emissions.9,10The NVG catalysts are usually working in the harsh environment (the high temperature, high water content, and high sulfur content), which makes the active site of catalysts be easy to be poisoned and sintered.5About 10%–15% (volume fraction) water vapor which exists in the NVG exhaust would induce sintering of active site, leading to the decrease of CH4conversion under lean-burn conditions.11–16The sulfides contained in the exhaust gas also cause deactivation of the catalysts.5,17–19In fact, the synergistic effect existing between water and sulfur in the exhaust of NGVs can accelerate the deactivation of catalysts.20,21Thus, the hydrothermal aging resistance and sulfur tolerance of palladium-supported catalysts for methane conversion need to be improved. Lapisardi,22Persson,23Yang,24Corro,25and Narui26et al. reported that the addition of Pt to Pd could improve the catalytic activity, thermostability, and sulfur resistance. They found that the addition of Pt to the Pd catalysts could weaken the Pd-O bond and affect the adsorption of methane and oxygen. Also the probability of sulfur dioxide interaction with PdO was weakened upon addition of Pt. Schmal et al.27reported that Pt/ZrO2/Al2O3had higher activity and stability than Pt/ZrO2and Pt/Al2O3for the methane oxidation. The enhanced performance may be due to the acidity, alkalinity, redox ability, and the high rate of oxygen transfer of ZrO2.28Guo et al.29also demonstrated that Pd/Al2O3with ZrO2addition showed higher catalytic activity than Pd/Al2O3for the same reaction. Nevertheless, further improvement for the hydrothermal aging resistance and sulfur tolerance by addition of ZrO2to monometallic catalyst supported on Al2O3remains very challenging. Work on Al2O3modified with Zr to support Pd-Pt catalyst for highly efficient aftertreatment of methane emission from natural gas vehicles is highly desired. However, to the best of our knowledge, there is no report about enhancing low-temperature methane oxidation over Zr-modified Pd-Pt/Al2O3under lean-burn condition.
The aim of this study was to explore the catalytic activity, hydrothermal aging resistance, and sulfur tolerance of the catalyst by addition of ZrO2to Al2O3. The Pd-Pt/ZrxAl(1–x)O(3+x)/2was prepared, whose physicochemical property was characterized by N2absorption-desorption, X-ray diffraction (XRD), H2temperature programmed reduction (H2-TPR), O2temperature programmed desorption (O2-TPD), and X-ray photoelectron spectroscopy (XPS). The catalysts were tested for methane oxidation under lean-burn condition.
2 Experimental
2.1 Support preparation The ZrxAl(1–x)O(3+x)/2supports were prepared by co-precipitation method. Briefly, the desired mixture aqueous solution of ZrO(NO3)2(CP, Jiangsu Yi Xing Xin Xing Zirconium Co., Ltd.) and Al(NO3)39H2O (CP) at the appropriate molar ratios (the different molar ratios of Zr to Al were 1 : 0, 3 : 1, 1 : 1, 1 : 3, and 0 : 1, respectively) was precipitated by ammonia at pH ≈10. After the co-precipitation process, the prepared precipitates were filtered, washed, dried, and then calcined at 950 °C for 3 h to obtain the required supports.30The Al2O3and ZrO2supports were prepared by using the same method. The supports were marked as ZrO2, Zr0.75Al0.25O1.875, Zr0.5Al0.5O1.75, Zr0.25Al0.75O1.625, and Al2O3, respectively.
2.2 Catalyst preparation
The Pd-Pt/ZrxAl(1–x)O(3+x)/2catalyst was prepared using impregnation method. A mixture solution of Pd(NO3)2(AR, Chengdu Bright Photoelectric Information Co., Ltd.) and Pt(NO3)2(AR, Chengdu Bright Photoelectric Information Co., Ltd.) were impregnated on the support materials with the Pd: ZrxAl(1–x)O(3+x)/2and Pt:ZrxAl(1–x)O(3+x)/2ratios of 1.5% and 0.3% (w), respectively. The catalysts were dried at 120 °C for 12 h and then calcined at 550 °C for 3 h. Each prepared catalyst powder and a proper amount of deionized water was mixed to obtain homogeneous slurry, and then the slurry was coated onto a honeycomb cordierite (2.5 cm3, Corning, USA). The cordierites were dried at 120 °C for 12 h, and then calcined at 550 °C for 2 h to obtain monolithic catalysts. The prepared catalysts were named as Pd-Pt/ZrO2, Pd-Pt/Zr0.75Al0.25O1.875, Pd-Pt/Zr0.5Al0.5O1.75, Pd-Pt/Zr0.25Al0.75O1.625, and Pd-Pt/Al2O3, respectively.
2.3 Catalyst activity measurements
The activity test of the monolithic catalysts was carried out in a multiple fixed-bed continuous flow micro-reactor from 280 to 520 °C with an interval of 20 °C. Each of the catalysts was pretreated at 550 °C for 1 h with the reaction gas mixture before test. The volume fraction of the gas mixture was controlled by mass-flow controller and the simulated exhaust was consisted of 0.075% (volume fraction, φ) CH4, 0.10% (φ) CO, 5.0% (φ) O2, 12.0% (φ) CO2, 12% (φ) water vapor (when used), and N2as balance gas, the simulated exhaust gas hourly space velocity (GHSV) in the reactor was 50000 h–1. The fresh catalysts were aged at 750 °C for 10 h with 10% water vapor to simulate the water resistance, and also pretreated in the presence of 0.005% (φ) sulfur at 650 °C for 10 h as sulfur tolerance. The volume concentration of CH4was detected by the on-line gas chromatograph equipped with an flame ionization detector (FID).
2.4 Characterization of supports and catalysts
The nitrogen adsorption-desorption isotherms were performed on a QUADRASORB SI automatic surface area and pore size analyzer (Autosorb SI, Quantachrome, Boynton Beach, FL, USA) under liquid nitrogen temperature. The specific surface area and pore size measurements were calculated by Brunauer-Emmett-Teller (BET) method and Barret-Joyner-Halenda (BJH) method, respectively. Before measurements, the supports were degassed in vacuum at 300 °C for 3 h.
The crystal structures of catalysts were detected by X-ray powder diffraction, and the X-ray diffraction data were obtained by power XRD on D/Max-rA using Cu Kαradiation (λ = 0.15406 nm) that operating at 40 kV and 25 mA. The samples were scanned from 10° to 70° with an interval of 0.06°.
H2-TPR and O2-TPD characterizations were performed on a quartz tubular microreactor (a thermal conductivity detector was equipped to conductivity the temperature), 100 mg catalyst was pretreated in the flow of N2(30 mLmin–1) at 500 °C for 60 min, then let it cool to room temperature in the flow of N2(30 mLmin–1). After that the sample was heated from 25 to 850 °C at a heating rate of 8 °Cmin–1in a H2-N2gas mixture (5% (φ) H2, 10 mLmin–1). Before the O2-TPD characterization was performed, 100 mg samples was pretreated in the flow of N2(30 mLmin–1) at 450 °C for 60 min, and cooling down to 80 °C. The pretreated catalyst was pretreated in the flow of O2(30 mLmin–1) at 80 °C for 60 min again. Finally, the catalyst was heated from 80 to 880 °C at the rate of 10 °Cmin–1for O2desorption.
The X-ray photoelectron spectroscopy data were determined by an electron spectrometer (XSAM-800, KRATOS Co.) equipped with an Al Kαradiation as a primary excitation. Binding energies were calculated on the basis of C 1s at 284.8 eV.
3 Results and discussion
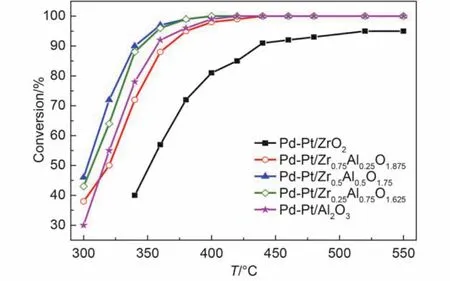
Fig.1 Conversion of CH4over the Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts for feed gas 0.075% (φ) CH4, 0.10% (φ) CO, 5.0% (φ) O2, 12.0% (φ) CO2, and N2as the balance at gas hourly space velocity (GHSV) of 50000 h–1
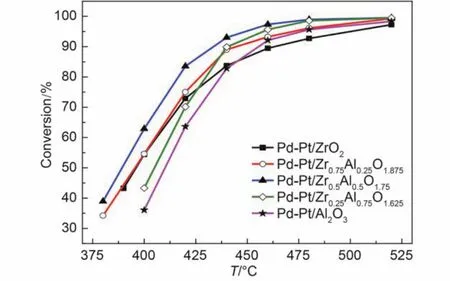
Fig.2 Conversion of CH4over the Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts for feed gas 0.075% (φ) CH4, 0.10% (φ) CO, 5.0% (φ) O2, 12.0% (φ) CO2, 12% (φ) water vapor, and N2as the balance at GHSV of 50000 h–1
3.1 Catalytic performance
3.1.1 Catalytic activity of the fresh samples
The catalytic activity for methane conversion was tested in the simulated exhaust gas of lean-burn natural gas vehicles. The catalytic activity is depicted in Fig.1 and Fig.2. It can be found that the catalytic activity increased firstly and then started to drop in the absence of water vapor in Fig.1. Pd-Pt/Zr0.5Al0.5O1.75had the best catalytic activity among these catalysts, while Pd-Pt/ZrO2showed the worst catalytic activity. The differenceactivity trend can be also observed in the presence of water vapor. Table 1 displayed the temperatures corresponding to 50% and 90% CH4conversions (T50and T90) and the temperature difference (ΔT) between T50and T90. T50and T90of Pd-Pt/Zr0.5Al0.5O1.75were 303 and 340 °C, respectively, which was decreased by 12 and 17 °C compared to those of Pd-Pt/Al2O3catalyst. The Pd-Pt/Zr0.5Al0.5O1.75catalyst also presented the best catalytic activity with water vapor, whose T50and T90were 388 and 433 °C, respectively. Conversion of CH4decreased for all of the samples, which may be due to the competition adsorption of water vapor with methane, active sites sintering by water vapor or formation of stable Pd(OH)2. Water vapor had a greater negative influence on Pd-Pt/Al2O3than other catalysts with ZrO2, which may be attributed to the hydrophobicity of ZrO2.31,32The results indicated clearly that the addition of zirconium to aluminum improved the low-temperature activity and water resistance of the fresh catalysts.

Table1 Light-off temperature (T50), complete conversion temperature (T90), and ΔT for methane oxidation over the Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts
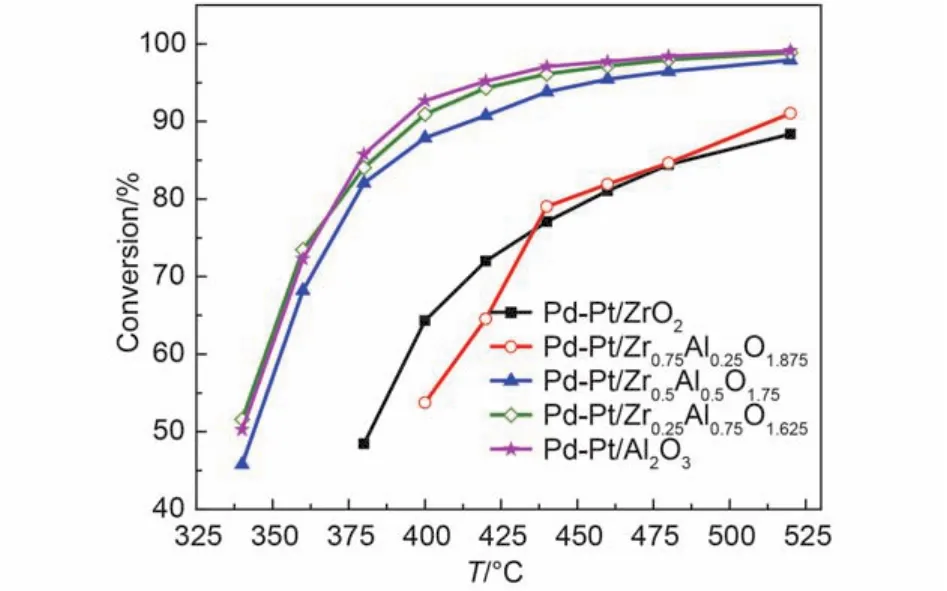
Fig.3 Conversion of CH4over the Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts after hydrothermal aging for feed gas 0.075% (φ) CH4, 0.10% (φ) CO, 5.0% (φ) O2, 12.0% (φ) CO2, and N2as the balance at GHSV of 50000 h–1
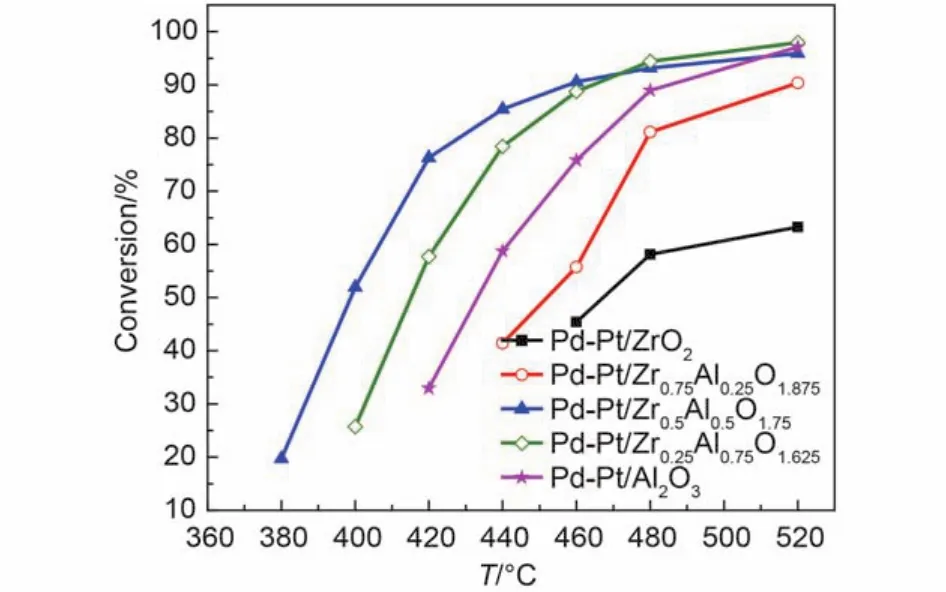
Fig.4 Conversion of CH4over the Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts after hydrothermal-aging for feed gas 0.075% (φ) CH4, 0.10% (φ) CO, 5.0% (φ) O2, 12.0% (φ) CO2,12% (φ) water vapor, and N2as the GHSV of 50000 h–1
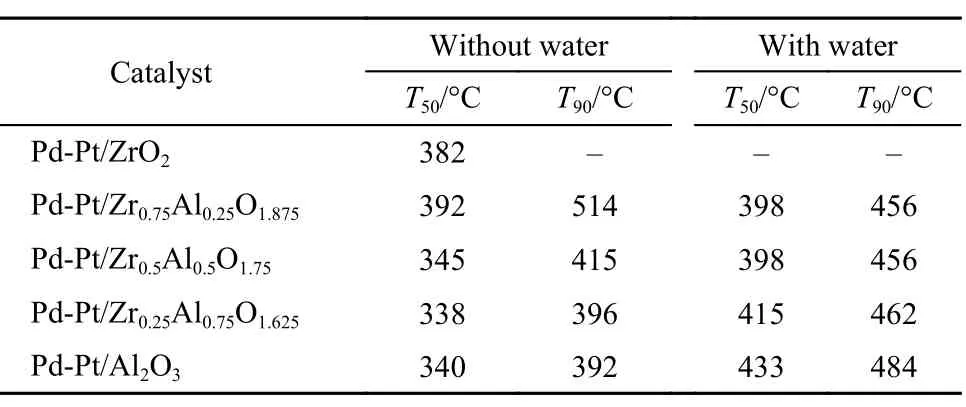
Table2 Light-off temperature, complete conversion temperature for methane oxidation over the Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts after hydrothermal aging
3.1.2 Catalytic activity of hydrothermal aging samples
Fig.3 and Fig.4 show the methane oxidation after hydrothermal aging of the catalysts, the values of T50, T90are shown in Table 2. From Fig.3, we can see that the activity of the hydrothermal aging catalysts follows the order: Pd-Pt/Al2O3> Pd-Pt/ Zr0.25Al0.75O1.625> Pd-Pt/Zr0.5Al0.5O1.75> Pd-Pt/Zr0.75Al0.25O1.875> Pd-Pt/ZrO2. It indicates that the Pd-Pt/Al2O3, Pd-Pt/Zr0.25Al0.75O1.625, and Pd-Pt/Zr0.5Al0.5O1.75catalysts show excellent and similar catalytic activity. Obviously, the catalytic activity of Pd-Pt/ZrO2and Pd-Pt/Zr0.75Al0.25O1.875declined remarkably. The catalytic activity of the hydrothermal aging catalysts increases firstly until the molar ratio of Zr and Al is 1 : 1, and then starts to drop by further enhancing the content of Zr from Fig.4. It seems that the suitable addition of Zr is beneficial to increase the hydrothermal aging resistance of Pd-Pt/Al2O3.
3.1.3 Sulfur tolerance
The catalytic activities after sulfur pretreatment are shown in Fig.5 and Fig.6, and the T50, T90are listed in Table 3. The sulfides react with palladium generate palladium sulfate that makes catalysts deactivate. The influence of sulfur on Pd-Pt/Zr0.5Al0.5O1.75catalyst was the weakest among the investigated catalysts. The light-off temperature and complete conversion temperature for Pd-Pt/Zr0.5Al0.5O1.75only increased from 303 and 340 °C to 328 and 374 °C, respectively. This may be attributed to weaken the interaction between PdO and sulfur dioxide upon addition of Pt. With 12% water vapor feeding, the catalytic activity was affected obviously. Although Pd-Pt/Zr0.5Al0.5O1.75displayed thebest catalytic performance, the T50and T90were 430 and 495 °C, respectively. This may involve a part of PdO reacted with sulfur dioxide to generate palladium sulfate resulting in decreases in the number of active sites.21,33In addition, water vapor adsorbed on the surface of the rest of active sites, which leaded to further decrease for the conversion of methane. The results demonstrated that the different content of Zr had different effects on the catalytic activity after sulfur pretreatment and that the appropriate content of zirconium can enhance the sulfur resistance of Pd-Pt/Al2O3.
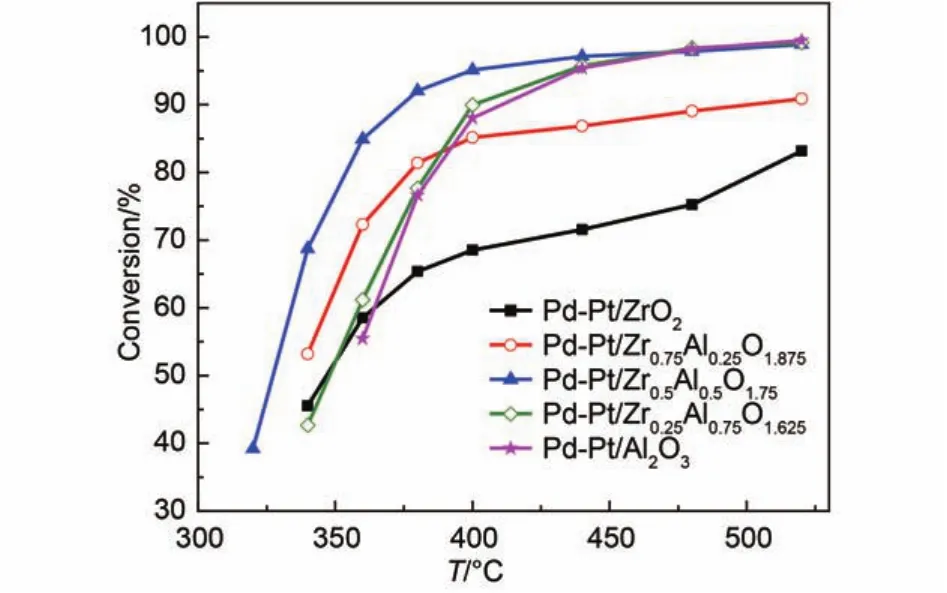
Fig.5 Conversion of CH4over the Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts after sulfur pretreatment for feed gas 0.075% (φ) CH4, 0.10% (φ) CO, 5.0% (φ) O2, 12.0% (φ) CO2, and N2as the balance at the GHSV of 50000 h–1
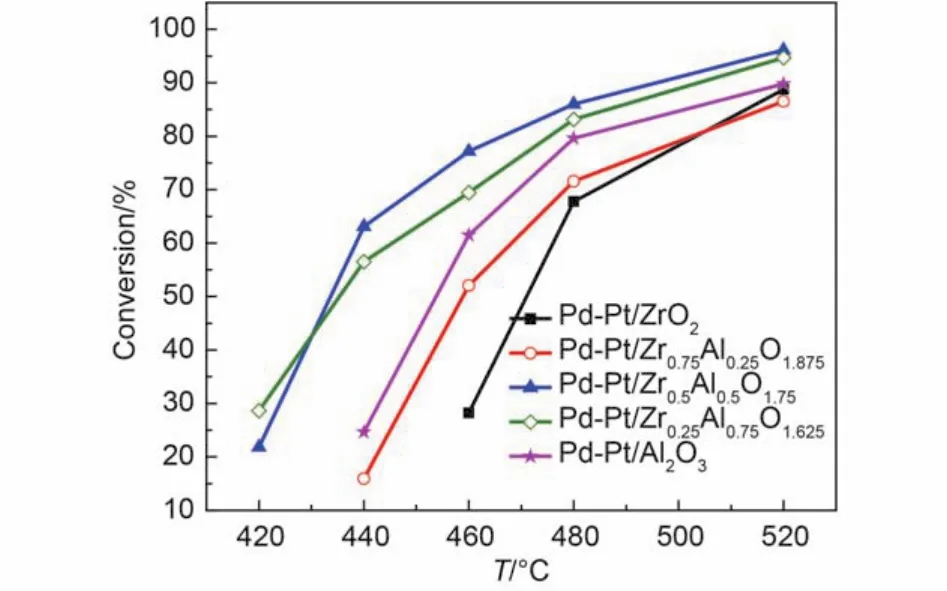
Fig.6 Conversion of CH4over the Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts after sulfur pretreatment for feed gas 0.075% (φ) CH4, 0.10% (φ) CO, 5.0% (φ) O2, 12.0% (φ) CO2,12% (φ) water vapor, and N2as the balance at the GHSV of 50000 h–1
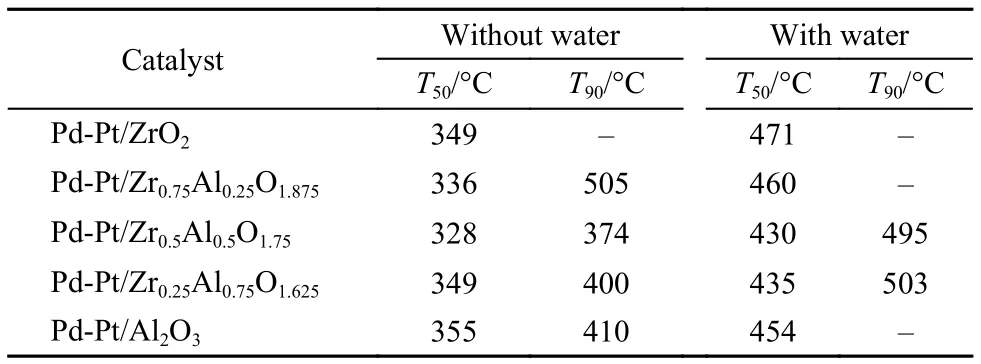
Table3 Light-off temperature and complete conversion temperature for methane conversion over the Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts after sulfur pretreatment
3.2 Textural properties of supports
The textural properties of supports play an important role in the dispersion of the noble metals and the mass transfer in reaction. Thus, the textural properties had significant influence on the catalytic activity. The textural properties of the ZrxAl(1–x)O(3+x)/2were measured by BET method. Their surface area, pore volume, and pore diameter are shown in Table 4. After calcined at 950 °C fo r 3 h, the surfacearea and pore volume of Al2O3are 141 m2g–1and 0.54 mLg–1, respectively. But the decrease of the specific surface area and pore volume and the increase of pore diameter of the ZrxAl(1–x)O(3+x)/2were observed obviously after enhancing the loading of Zr. As reported by Schmal et al.,27the addition of Zr to Al resulted in the blockage of alumina pores by some zirconium crystallites, which can explain the observed in our case. The surface area and pore volume of ZrO2were only 10 m2g–1and 0.11 mLg–1, respectively, which is similar to the literature.34Although a large specific surface area was important for catalytic activity, it was not case in the present system and a suitable specific surface area can improve the catalytic activity, Zr0.5Al0.5O1.75only had a middle texture properties, but its catalyst showed the best catalytic activity.32,35,36
3.3 XRD results
XRD was used to further characterize the structural properties of the catalysts. Fig.7 shows the XRD patterns of the Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts with different molar ratios of Zr and Al. The diffraction peaks of Pd and Pt were not observed, which may be attributed to the well dispersion of active component on the supports or under the limit of detection, the high dispersion of the active component may be helpful to improve the catalytic activity. The peaks at 19.8°, 32.6°, and 46.0° were attributed to the characteristic diffraction peaks of γ-Al2O3. But the diffraction peaks of γ-Al2O3were broad even calcined at 950 °C, implying that even after high temperature calcination, the crystalline grain of Al2O3did not grow up obviously. After the addition of zirconium to aluminum, a new solid solution Zr0.48Al0.52O1.74that may be able to stabilize the textural properties and enhance the dispersion of active component was formed, which can improve the catalytic activity, its diffraction peaks were located at 2θ = 30.4°, 50.5°, 60.2°.34,37As shown in Fig.7(b), the 2θ values of Zr0.48Al0.52O1.74shift to lower values with increasing the content of Zr, indicating that more and more zirconium entered into the Zr0.48Al0.52O1.74, which formed more excellent solid solution, but the solid solution Zr0.48Al0.52O1.74of Pd-Pt/Zr0.75Al0.25O1.625appeared obviously split phase at 2θ = 30.4°.
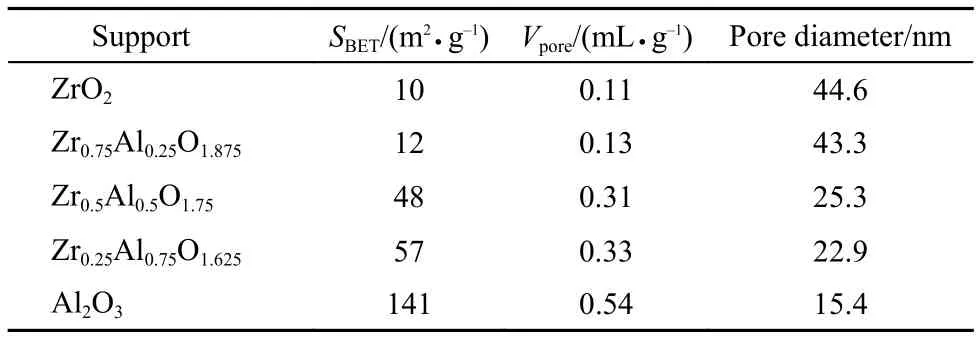
Table4 Textural properties of the ZrxAl(1–x)O(3+x)/2supports
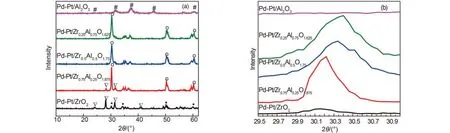
Fig.7 XRD patterns of the prepared Pd-Pt/ZrxAl(1–x)O(3+x)/2catalysts
For Pd-Pt/ZrO2, monoclinic ZrO2and tetragonal ZrO2were presented, and the content of monoclinic ZrO2phase was primary.38The zirconia crystallites may block the pore of some alumina, which can explain the surface area drop from BET.39
3.4 H2-TPR results
H2-TPR experiments were used to evaluate the relationship between the reducibility and the activity of catalysts. It was reported that lower reduction temperature and larger peak area implied higher catalytic activity. The H2-TPR profiles of the Pd-Pt/ZrxAl(1–x)O(3+x)/2are shown in Fig.8. It indicated that the low temperature of reduction peaks concentrated about 100 °C was attributed to the reduction of Pd species.37,40While the peaks at about 150 °C were assigned to the reduction of PtO2.41It can be seen that, with the addition of zirconium to aluminum the reduction temperature of PdO dropped firstly and then started to increase. The lowest reduction temperature was 70 °C, which was attributed to Pd-Pt/Zr0.5Al0.5O1.75sample, and the highest was 103 °C for Pd-Pt/ZrO2sample. For PtO2, the reduction temperature profile showed the similar trend to the law of PdO, and 130 and 150 °C were the lowest and highest reduction temperatures, respectively. It was reported that lower reduction temperature implied higher catalytic activity for the catalyst.42A widely accepted redox mechanism suggested that PdO was the main active species for the oxidation of CH4.43,44The results of H2-TPR were consistent with the results of the catalytic activity. The differences in reduction temperature for the catalysts may be due to different strength interaction between noble metal and the supports. As shown in Fig.8, Pd-Pt/Zr0.5Al0.5O1.75catalyst had the highest peak intensity and the biggest peak area of H2-TPR. The results indicated that the interactions between the noble metal oxide and the supports were the weakest among the five catalysts and that the noble metal oxide can be reduced easily.42,45,46Interestingly, Pd-Pt/ZrO2shown the lowest activity for the methane oxidation had a smallest reduction peak area among the catalysts. Both the reduction temperature and the peak area showed that Pd-Pt/Zr0.5Al0.5O1.75could exhibit better catalytic activity than the others, which was consistent with the above catalytic test results.
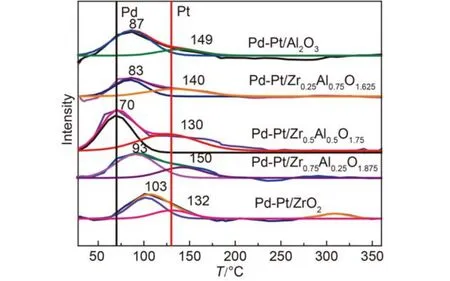
Fig.8 H2-TPR profiles of the five catalysts
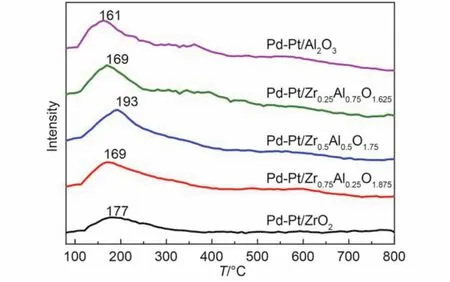
Fig.9 O2-TPD profiles of the five catalysts
3.5 Temperature-programmed desorption
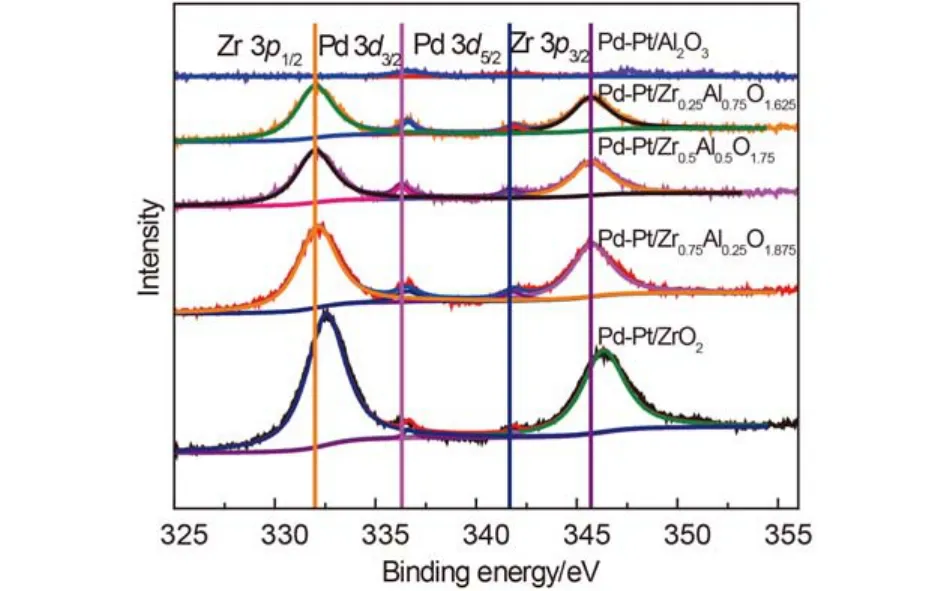
Fig.10 Zr 3p and Pd 3d XPS spectra of the five catalysts
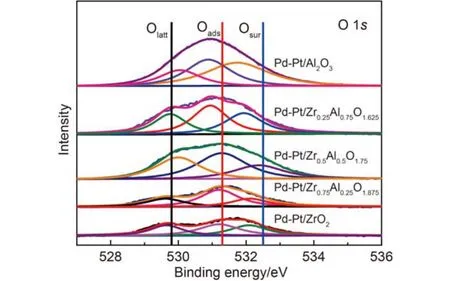
Fig.11 O 1s XPS spectra of the five catalysts
The O2-TPD profiles of the prepared catalysts are illustrated in Fig.9. The previous studies reported that oxygen species were associated with oxidation reaction, which was involved in reactive oxygen species.41Therefore, the catalytic activity was directly related to the behavior of O2adsorption. The desorption peaks of catalysts were obviously different in Fig.9. The peak center of Pd-Pt/Zr0.5Al0.5O1.75(193 °C) was the highest, and the Pd-Pt/Al2O3was at 161 °C. According to the literature,47,48the peak temperature of ordinarily chemically adsorbed oxygen was below 300 °C. The desorption temperature of lattice oxygen was beyond 700 °C. Therefore, there was only ordinarily chemically adsorbed oxygen existing in the catalysts. The interaction between the catalysts and oxygen was stronger and the desorption temperature of oxygen was higher. With increasing the adsorption between the noble metal and oxygen, the O-O bond was in turn weakened. After addition of Zr, the chemically adsorbed oxygen for the catalysts was dissociated easily with enhanced catalytic performance.
3.6 XPS analysis
In order to investigate the effect of the various ratios of zirconium and aluminum on the prepared catalysts, the valence states of Pd and O on the surface of catalysts were studied by XPS. Fig.10 and Fig.11 show the XPS spectra (lines) of Pd 3d and O 1s, respectively. The binding energy of Pd 3d and O 1s are shown in Table 5. The Pt was overlapped partly by Al 3p and the content of Pt was too low to detect.
As shown in Fig.10 and Table 5, the binding energy (BE) values of Pd 3d over different catalysts were different obviously. But the BE values of all Pd 3d in Table 5 indicated that Pd was present at Pd2+species that was usually considered as PdO and the active components.43,49The lowest value of Pd 3d5/2was 336.2 eV that was attributed to Pd-Pt/Zr0.5Al0.5O1.75. Pd-Pt/Al2O3had the highest value of Pd 3d5/2(336.7 eV). And the addition of Zr had influence on the surface atomic concentration of Pd obviously. The Pd-Pt/Zr0.5Al0.5O1.75had the highest surface atomic concentration among the five catalysts, which was 1.4%. The lowest surface atomic concentration of Pd was 0.6% that was attributed to Pd-Pt/Al2O3. The surface atomic concentration of Pd indicated that the addition of zirconium enhanced the dispersion of Pd, which can improve the catalytic performance. The results were consistent with the results of XRD and H2-TPR. From the different BE values of Pd 3d, it can be found that the electron cloud density around the PdO was different due to the interaction between the active components and the supports. According to the shift of the BE, it was considered that a small amount of PdOx(0 < x < 1) located on the surface of PdO or Pd was generated around PdO.34,50PdOxincluded the strong bond of Pd-O, which can enhance the oxygen concentration in the process of reaction.30,51,52So O 1s of XPS is also studied as follows.
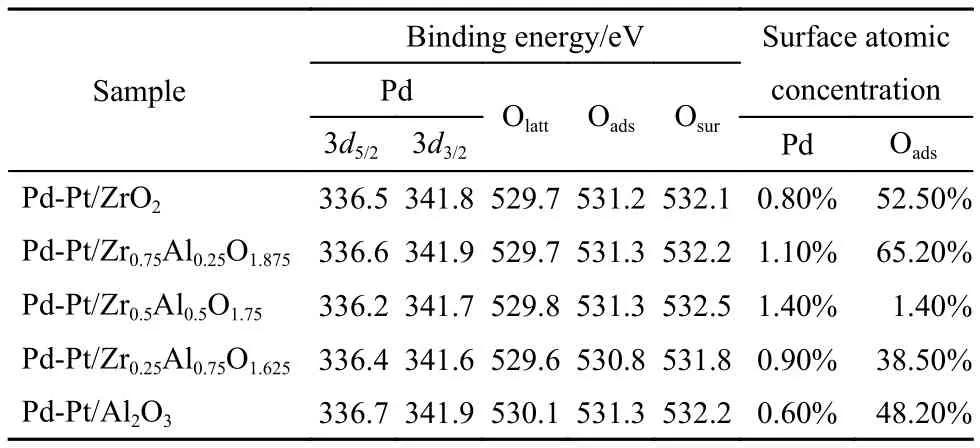
Table5 Binding energies of the characteristic peaks of different atoms in samples
The XPS lines of O 1s indicated that three kinds of surface oxygen species could be recognized in Fig.11. At first, the range of peaks between 530.0 and 531.0 eV were attributed to the lattice oxygen,53the peaks located at 531.0–532.5 eV were assigned to the surface adsorption oxygen,54and the peaks located at 533.0 eV were from hydroxyl groups and adsorbed water molecules.34,55The mechanism of methane oxidation contained the transference of oxygen. Firstly, the O2was absorbed on the surface of metal oxide, and then the Oadscan react with noble metallic particles to form surface metal oxide. Finally, the methane that adsorbed on the oxide surface was oxidized to CO2and H2O.56The activation of methane occurred on surface of PdO, so coming to the conclusion that the Oadswas beneficial to the catalytic activity, and comparing with the Olatt, the Oadsspecies have higher mobility, which was involved the mechanism for methane oxidation.54,55For Pd-Pt/Zr0.5Al0.5O1.75, the concentration of Oadswas 66.0%, which was the highest among the investigated samples. In a word, the results of XPS indicated that the addition of Zr expressed the higher catalytic activity.
4 Conclusions
Compared to Pd-Pt/Al2O3and Pd-Pt/ZrO2, the catalytic performance of zirconium-modified Pd-Pt/ZrxAl(1–x)O(3+x)/2was increased. The catalyst screening proved that Zr0.5Al0.5O1.75was the most effective carrier material for Pd and Pt in the five catalysts. Pd-Pt/Zr0.5Al0.5O1.75had the best low-temperature activity. The T50and T90were only 303 and 340 °C, respectively. Moreover, this catalyst showed the strong water resistance andsulfur tolerance. The excellent catalytic performance for Pd-Pt/Zr0.5Al0.5O1.75may be due to the high crystallinity and dispersion of active components, and ore surface Pd2+species and absorbed oxygen species.
(1)Fan, X.; Wang, F.; Zhu, T.; He, H. Journal of Environmental Sciences 2012, 24, 507. doi: 10.1016/S1001-0742(11)60798-5
(2)Li, J. H.; Liang, X.; Xu, S. C.; Hao, J. M. Applied Catalysis B: Environmental 2009, 90, 307. doi: 10.1016/j.apcatb.2009.03.027
(3)Chen, J. H.; Shi, W. B.; Li, J. H. Catalysis Today 2011, 175, 216. doi: 10.1016/j.cattod.2011.03.061
(4)Choudhary, T. V.; Banerjee, S.; Choudhary, V. R. Applied Catalysis A: General 2002, 234, 1. doi: 10.1016/S0926-860X(02)00231-4
(5)Gélin, P,; Primet, M. Applied Catalysis B: Environmental 2002, 39, 1. doi: 10.1016/S0926-3373(02)00076-0
(6)Liotta, L. F.; Carlo, G. D.; Pantaleo, G.;. Venezia, A. M.; Deganello, G. Topics in Catalysis 2009, 52, 1989. doi: 10.1007/s11244-009-9375-1
(7)Honkanen, M.; Kärkkäinen, M.; Viitanen, V.; Jiang, H.; Kallinen, K.; Huuhtanen, M.; Vippola, M.; Lahtinen, J.; Keiski, R.; Lepistö, T. Topics in Catalysis 2013, 56, 576. doi: 10.1007/s11244-013-0017-2
(8)Salaün, M.; Capela, S.; Costa, S. D.; Gagnepain, L.; Costa, P. D. Topics in Catalysis 2009, 52, 1972. doi: 10.1007/s11244-009-9372-4
(9)Ohtsuka, H. Catalysis Letters 2010, 141, 413.
(10)Fang, H.; Cai, L.; Liu, P.; Zhao, P.; Zhang, L. J.; Gong, M. C.; Chen, Y. Q. Acta Phys. -Chim. Sin. 2006, 22, 1004. [房 华,蔡 黎, 刘 萍, 赵 明, 张丽娟, 龚茂初, 陈耀强. 物理化学学报, 2006, 22, 1004.] doi: 10.3866/PKU.WHXB20060819
(11)Wang, Y.; Shang, H. Y.; Xu, H. D.; Gong, M. C.; Chen, Y. Q. Chinese Journal of Catalysis 2014, 35, 1157. doi: 10.1016/ S1872-2067(14)60062-0
(12)Shang, H. Y.; Hu, W.; Wang, Y. Ren, C. J.; Gong, M. C.; Chen, Y. Q. Acta Phys. -Chim. Sin. 2015, 31, 750. [尚鸿燕, 胡 伟,王 云, 任成军, 龚茂初, 陈耀强. 物理化学学报, 2015, 31, 750.] doi: 10.3866/PKU.WHXB201502051
(13)Persson, K.; Pfefferle, L. D.; Schwartz, W.; Ersson, A.; Järås, S. G. Applied Catalysis B: Environmental 2007, 74, 242. doi: 10.1016/j.apcatb.2007.02.015
(14)Kikuchia, R.; Maedab, S.; Sasakib, K.; Wennerströmc, S.; Eguchia, K. Applied Catalysis A: General 2002, 232, 23. doi: 10.1016/S0926-860X(02)00096-0
(15)Ciuparu, D.; Katsikis, N.; Pfefferle, L. Applied Catalysis A: General 2001, 216, 209. doi: 10.1016/S0926-860X(01)00558-0
(16)Ciuparu, D.; Pfefferle, L. Applied Catalysis A: General 2001, 209, 415. doi: 10.1016/S0926-860X(00)00783-3
(17)Gélin, P.; Urfels, L.; Primet, M.; Tena, E. Catalysis Today 2003, 83, 45. doi: 10.1016/S0920-5861(03)00215-3
(18)Lampert, J. K.; Kazi, M. S.; Farrauto, R. J. Applied Catalysis B: Environmental 1997, 14, 211. doi: 10.1016/S0926-3373(97) 00024-6
(19)Yu, T. C.; Shaw, H. Applied Catalysis B: Environmental 1998, 18, 105. doi: 10.1016/S0926-3373(98)00031-9
(20)Venezia, A. M.; Carlo, G. D.; Pantaleo, G.; Liotta, L.F.; Melaet, G.; Kruse, N. Applied Catalysis B: Environmental 2009, 88, 430. doi: 10.1016/j.apcatb.2008.10.023
(21)Mowery, D. L.; McCormick, R. L. Applied CatalysisB: Environmental 2001, 34, 287. doi: 10.1016/S0926-3373(01) 00222-3
(22)Lapisardi, G.; Urfels, L.; Gélin, P.; Primet, M.; Kaddouri, A.; Garbowski, E.; Toppi, S.; Tena, E. Catalysis Today 2006, 117, 564. doi: 10.1016/j.cattod.2006.06.004
(23)Persson, K.; Ersson, A.; Jansson, K.; Fierro, J.; Jaras,S. Journal of Catalysis 2006, 243, 14. doi: 10.1016/j.jcat. 2006.06.019
(24)Yang, Z. Z.; Zhao, M.; Gong, M. C.; Chen, Y. Q. Acta Phys. -Chim. Sin. 2014, 30, 1187. [杨铮铮, 赵 明, 龚茂初, 陈耀强. 物理化学学报, 2014, 30, 1187.] doi: 10.3866/PKU. WHXB201404281
(25)Corro, G.; Cano, C.; Fierro, J. L. G. Journal of Molecular Catalysis A: Chemical 2010, 315, 35. doi: 10.1016/j.molcata. 2009.08.023
(26)Narui, K.; Yata, H.; Furuta, K.; Nishida, A.; Kohtoku, Y.; Matsuzaki, T. Applied Catalysis A: General 1999, 179, 165. doi: 10.1016/S0926-860X(98)00306-8
(27)Schmal, M.; Souza, M. M. V. M.; Aranda, D. A. G.; Perez, C. A. C. Studies in Surface Science Catalysis 2001, 132, 695. doi: 10.1016/S0167-2991(01)82183-2
(28)Han, X. L.; Liang, Z. Z.; Feng, L.; Wang, W.; Chen, J. F.; Xue, C. Y.; Zhao, H. Ceramics International 2015, 41, 505. doi: 10.1016/j.ceramint.2014.08.098
(29)Guo, Y.; Lu, G. Z.; Zhang, Z. G.; Jiang, L. Z.; Wang,X. H.; Li, S. B.; Zhang, B.; Niu, J. Z. Catalysis Today 2007, 126, 441. doi: 10.1016/j.cattod.2007.06.015
(30)Wang, Y.; Tang, S. Y.; Long, E. Y.; Lin, Z. E.; Gong, M. C.; Chen, Y. Q. Chinese Journal of Catalysis 2011, 32, 303. [王云, 唐石云, 龙恩燕, 林之恩, 龚茂初, 陈耀强. 催化学报, 2011, 32, 303.]
(31)Araya, P.; Guerrero, S.; Robertson, J.; Gracia, F. J. Applied Catalysis A: General 2005, 283, 225. doi: 10.1016/j.apcata. 2005.01.009
(32)Park, J. H.; Cho, J. H.; Kim, Y. J.; Kim, E. S.; Han, H. S.; Shin, C. H. Applied Catalysis B: Environmental 2014, 160–161, 135.
(33)Mowery, D. L.; Graboski, M. S.; Ohnob, T. R.; McCormick, R. L. Applied Catalysis B: Environmental 1999, 21, 157. doi: 10.1016/S0926-3373(99)00017-X
(34)Wang, Y.; Xu, H. D.; Shang, H. Y.; Gong, M. C.; Chen, Y. Q. Journal of Energy Chemistry 2014, 23, 461.
(35)Lin, W.; Zhu, Y. X.; Wu, N. Z.; Xie, Y. C.; Murwani, I.; Kemnitz, E. Applied Catalysis B: Environmental 2004, 50, 59. doi: 10.1016/j.apcatb.2004.03.009
(36)Widjaja, H.; Sekizawa, K.; Eguchi, K.; Arai, H. Catalysis Today 1997, 35, 197.
(37)Shang, H. Y.; Wang, Y.; Gong, M. C.; Chen, Y. Q. Journal of Natural Gas Chemistry 2012, 21, 393. doi: 10.1016/S1003-9953(11)60381-2
(38)Kumar, S.; Bhunia, S.; Ojha, A. K. Physica E: Low-dimensional Systems and Nanostructures 2015, 66, 74. doi: 10.1016/j.physe.2014.09.007
(39)Amairia, C.; Fessi, S.; Ghorbel, A.; Rîves, A. Journal of Molecular Catalysis A: Chemical 2010, 332, 25. doi: 10.1016/j.molcata.2010.08.013
(40)Ren, C. J.; Zhou, X. N.; Shang, H. Y.; Chen, Y. Q. Acta Phys. -Chim. Sin. 2014, 30, 957. [任成君, 周晓娜, 尚鸿燕, 陈耀强. 物理化学学报, 2014, 30, 957.] doi: 10.3866/PKU.WHXB 201403101
(41)Guo, J. X.; Gong, M. C.; Yuan, S. H.; Chen, Y. Q. Journal of Rare Earths 2006, 24, 554. doi: 10.1016/S1002-0721(06)60162-2
(42)Kašpar, J.; Fornasiero, P.; Hickey, N. Catalysis Today 2003, 77, 419. doi: 10.1016/S0920-5861(02)00384-X
(43)Gao, D. N.; Zhang, C. X.; Wang, S.; Yuan, Z. S.; Wang, S. D. Catalysis Communications 2008, 9, 2583. doi: 10.1016/ j.catcom.2008.07.014
(44)Su, C. S.; Carstens, J. N.; Bell, A. T. Journal of Catalysis 1998, 176, 125. doi: 10.1006/jcat.1998.2028
(45)Shao, Y.; Xu, Z. Y.; Wan, H. Q.; Chen, H.; Liu, F. L.; Li, L. Y.; Zheng, S. R. Journal of Hazardous Materials 2010, 179, 135. doi: 10.1016/j.jhazmat.2010.02.070
(46)Gopinath, R.; Babu, N. S.; Kumar, J. V.; Lingaiah, N.; Prasad, P. S. S. Catalysis Letters 2007, 120, 312.
(47)Xue, L.; Zhang, C. B.; He, H.; Teraoka, Y. Applied Catalysis B: Environmental 2007, 75, 167. doi: 10.1016/ j.apcatb.2007.04.013
(48)Zhao, Z.; Yang, X. G.; Wu, Y. Applied Catalysis B: Environmental 1996, 8, 281. doi: 10.1016/0926-3373(95)00067-4
(49)Li, Y. L.; Zhang, X. Y.; Long, E. Y.; Li, H. M.; Wu, D. D.; Cai, L.; Gong, M. C.; Chen, Y. Q. Journal of Natural Gas Chemistry 2009, 18, 415. doi: 10.1016/S1003-9953(08)60136-X
(50)Schmal, M.; Souza, M.; Alegre, V.; Dasilva, M.; Cesar, D.; Perez, C. Catalysis Today 2006, 118, 392. doi: 10.1016/j.cattod. 2006.07.026
(51)Fujimoto, K.; Ribeiro, F. H.; Avalos-Borja, M.; Iglesia, E. Journal of Catalysis 1998, 179, 431. doi: 10.1006/jcat. 1998.2178
(52)Yoshida, H.; Nakajima, T.; Yazawa, Y.; Hattori, T. Applied Catalysis B: Environmental 2007, 71, 70. doi: 10.1016/j.apcatb. 2006.08.010
(53)Chen, H. Y.; Sayari, A.; Adnot, A.; Larachi, F. Applied Catalysis B: Environmental 2001, 32, 195. doi: 10.1016/S0926-3373(01)00136-9
(54)Peluso, M. A.; Gambaro, L. A.; Pronsato, E.; Gazzoli, D.; Thomas, H. J.; Sambeth, J. E. Catalysis Today 2008, 133–135, 487.
(55)Santos, V. P.; Pereira, M. F. R.; Órfão, J. J. M.; Figueiredo, J. L. Applied Catalysis B: Environmental 2010, 99, 353. doi: 10.1016/j.apcatb.2010.07.007
(56)Long, E. Y.; Zhang, X. Y.; Li, Y. L.; Liu, Z. M.; Wang, Y.; Gong, M. C.; Chen, Y. Q. Journal of Natural Gas Chemistry 2010, 19, 134. doi: 10.1016/S1003-9953(09)60044-X
Effects of Zr Addition on the Performance of the Pd-Pt/Al2O3Catalyst for Lean-Burn Natural Gas Vehicle Exhaust Purification
HU Wei1WANG Yun1SHANG Hong-Yan1XU Hai-Di2,3ZHONG Lin1,*CHEN Jian-Jun2GONG Mao-Chu2CHEN Yao-Qiang1,2,3,*
(1College of Chemical Engineering, Sichuan University, Chengdu 610064, P. R. China;2Institute of New Energy and Low-Carbon Technology, Sichuan University, Chengdu 610064, P. R. China;3Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, P. R. China)
The catalytic activity, hydrothermal aging resistance, and sulfur tolerance of a Pd-Pt-based methane oxidation catalyst were evaluated in a fixed fluidized bed reactor containing simulated lean-burn natural gas vehicle exhaust gases. Zirconium-doped Pd-Pt/Al2O3(Pd-Pt/ZrxAl(1–x)O(3+x)/2) was found to significantly improve the catalytic activity, hydrothermal aging resistance, and sulfur tolerance. Zr-modified alumina supports were prepared by co-precipitation with molar ratios of Zr to Al of 0 : 1, 0.25 : 0.75, 0.5 : 0.5, 0.75 : 0.25, and 1 : 0. The Pd-Pt bimetallic catalysts containing 1.5% (w, mass fraction) Pd and 0.3% (w) Pt supported on the above-modified composite supports were prepared by the co-impregnating method.The catalysts were characterized by N2adsorption/desorption, X-ray diffraction(XRD), H2temperatureprogrammed reduction (H2-TPR), O2temperature-programmed desorption, and X-ray photoelectron spectroscopy (XPS). The results show that the crystallinity of the samples, dispersion of the active component, number of Pd2+species, and electron density around Pd2+species increase after addition of ZrO2to Al2O3supports. Compared with the activity results of Pd-Pt/Al2O3and Pd-Pt/ZrO2catalysts after different pretreatment conditions, the performance of the catalyst is greatly enhanced by adding ZrO2in the Al2O3supports, and Pd-Pt/Zr0.5Al0.5O1.75shows the best catalytic activity, strongest hydrothermal aging resistance, and highest sulfur tolerance among the investigated catalysts.
Aluminum; Zirconium; Different molar ratios; Support; Natural gas fuelled vehicle; Catalytic performance
O643
10.3866/PKU.WHXB201507141
Received: May 4, 2015; Revised: July 14, 2015; Published on Web: July 14, 2015.
*Corresponding authors. CHEN Yao-Qiang, Email: nic7501@scu.edu.cn; Tel/Fax: +86-28-85418451. ZHONG Lin, Email: zhonglin@scu.edu.cn.
The project was supported by the National Natural Science Foundation of China (21173153), Project from Sichuan Provincial Environment Office,
China (2011HB002), and Science and Technology Support Project of Science and Technology Department of Sichuan Province, China (2012FZ0008).
国家自然科学基金(21173153), 四川省环境保护厅项目(2011HB002)及四川省科技厅科技支撑项目(2012FZ0008)资助
© Editorial office of Acta Physico-Chimica Sinica

