The effects of O-MWNTs,PEG and PVP additiveson the performances of polyvinylidene fluoride(PVDF) membranes
XU Haipeng, CHEN Xingfan, ZHANG Xuan, LANG Wanzhong
(College of Life and Environmental Sciences,Shanghai Normal University,Shanghai 200234,China)
The effects of O-MWNTs,PEG and PVP additiveson the performances of polyvinylidene fluoride(PVDF) membranes
XU Haipeng, CHEN Xingfan, ZHANG Xuan, LANG Wanzhong
(College of Life and Environmental Sciences,Shanghai Normal University,Shanghai 200234,China)
Abstract:Introducing hydrophilic additives is an effective method to improve the structure and permeation performance of polyvinylidene fluoride (PVDF) membranes.In this work,oxidized multi-wall carbon nanotubes (O-MWNTs) accompanied with polyethylene glycol/polyvinyl pyrrolidone (PEG/PVP) additives were employed to modify PVDF membranes.The effects of additives on the structure,permeation performances,hydrophilicity and crystallization behavior of the prepared PVDF membranes were investigated in detail.The results indicate that the addition of O-MWNTs can enhance the permeation flux,hydrophilicity and mechanical property of PVDF membranes.The pure water flux(PWF) attains 222.9±12.5 L·m-2·h-1·bar-1and 256.9±14.8 L·m-2·h-1·bar-1by adding 0.6 wt.% O-MWNTs accompanied with 5% PEG 200 and 3% PVP in the cast solution,respectively.PEG200 additive can enhance the dispersion of O-MWNTs,and accelerate the exchange rate between solvent and non-solvent.PVP working as pore-forming agent promotes the growth of pores of PVDF membranes and pure water permeation flux.
Key words:polyvinylidene fluoride (PVDF) membrane; ultrafiltration; multi-wall carbon nanotubes (MWNTs)
CLC number: TQ 31Document code: AArticle ID: 1000-5137(2016)01-0041-09
1Introduction
In the recent years,polyvinylidene fluoride (PVDF) has become a very popular material to prepare microfiltration/ultrafiltration(MF/UF) membranes for its superior thermal,chemical stability and oxidation resistance etc.[1-4].However,due to its strong hydrophobicity,PVDF membranes are easily fouled by natural organic matters (NOMs),proteins and other substances.To overcome the shortcomings,various ways were explored to modify PVDF membranes.Among these methods,blending modification is widely used because it makes preparation and modification be accomplished in a single step.And numerous nanofillers were employed to modify PVDF membranes[5-8].
In this work,O-MWNTs were used as fillers to improve PVDF membrane performances.Polyethylene glycol(PEG) and/or polyvinyl pyrrolidone (PVP) worked as an auxiliary is used to enhance the dispersion of O-MWNTs.The influences of different content of O-MWNTs,PEG on the membrane performances were studied in detailed.
2Experimental
2.1Materials
PVDF in powder form was supplied by Shanghai 3F New Material Co.Ltd.(China).Raw multi-wall carbon nanotubes (R-MWNTs,diameter 10~20 nm,length 10~15 μm,purity > 97.0wt.%) were purchased from Shenzhen Nanotech Port Co.Ltd.(China).N-methyl-2-pyrrolidone (NMP) and ethanol were provided by Shanghai Chemical Agent Co.Ltd.(China).PVP (K30,MW=58,000) in powder form and PEG200 were purchased from Shanghai Aladdin Chemical Agent Co.Ltd.(China).Deionized water (DI) was self-produced by a reverse osmosis (RO) system.BSA (MW=67 000) was purchased from Shanghai Bio Life Sci.&Tech.Co.,Ltd.(China).All reagents were not further purified before used.
2.2Preparationand characterization of O-MWNTs
Nitric acid vapor-oxidation method was adopted to prepare O-MWNTs,the detailed preparation procedures and characterizations were presented in our recent works[17-18].
2.3Membrane preparation
ThePVDF/O-MWNTs membranes were prepared by phase inversion method.Different amounts of polymer and solvent were mixed and stirred at 70℃ for 24 h to get homogenous dope solutions.The detailed parameters for membrane preparation were summarized in Table 1.
The membranes were cast on a glass plate.The cast intrinsic films were quickly immersed into water which was used as coagulation bath.After peeled off from the plate,the obtained membranes were immersed into deionized water bath for 48 h to remove the residual solvent.
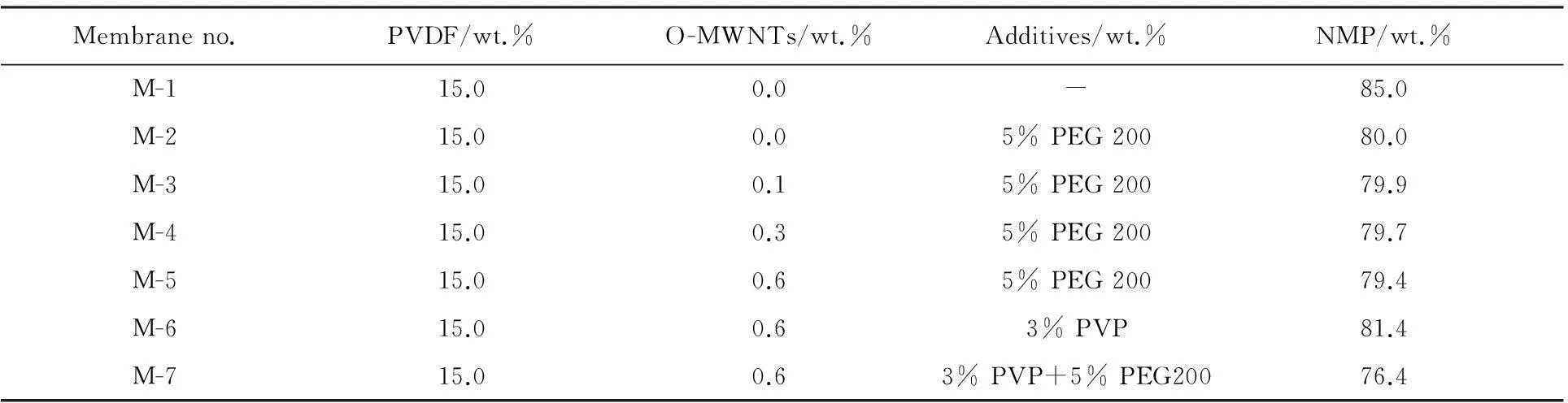
Table 1 The compositions of cast solution for the PVDF/O-MWNTs membranes
2.4Characterizations of PVDF/O-MWNT membranes
2.4.1Morphologies
Themorphologies of PVDF/O-MWNTs membranes were tested by a field emission scanning electron microscopy (FESEM,Hitachi S-4800,Japan).The cross-sections were obtained by fracturing the membranes in liquid nitrogen.The samples were placed onto a copper holder and coated with gold.The morphologies of PVDF/O-MWNTs membranes were observed under different magnifications.
2.4.2Dynamic contact angle
A contact angle analyzer (KRÜSSDSA30,German) was used to detect the dynamic contactangles of PVDF/O-MWNTs membranes.A drop of 3.0 μL DI water was dropped on the top surfaces of PVDF/O-MWNTs membranes at room temperature.A camera enabling image capture at 1 frames/s was coupled with the machine.
2.4.3Permeation performances
The PWFsand BSA rejections of PVDF/O-MWNTs membranes were measured by a self-made device.All membranes were pre-pressured at 2 bar for 30 min before testing.The PWFs of membranes were measured at 25℃ at 1 bar and calculated by
(1)
whereVis the volume of penetrative water (L),A is the effective membrane area (m2),tis the running time (h),and ΔPis the transmembrane pressure (bar).
The BSA rejections of PVDF/O-MWNTs membranes were carried out with a 500×10-6BSA aqueous solution in phosphate buffer solution (PBS,pH = 7.0)[4].The permeation device mentioned above was used again and whole process lasted for 30 min.The concentrations of permeate and feed solutions were determined by an ultraviolet spectrophotometer (UV3600 Shimadzu,Japan) at 280 nm.The rejection was defined as
(2)
whereCPandCFare the BSA concentration of permeate and feed solution respectively.
2.4.4Crystallization behaviors
The crystallization behaviors of PVDF/O-MWNTs membranes were also investigated by attenuated total reflection flourier transformed infrared spectroscopy (ATR-FTIR,ElectronCorp Nicolet 380,USA) at the wavenumber range of 400-4000 cm-1and wide-angle X-ray diffraction (WAXD,D/max-II B,Japan) at the angle range of 5-50°.
2.4.5Mechanical properties
The mechanical properties of PVDF/O-MWNTs membranes were evaluated by a material test machine (QJ210A,Shanghai Qingji Instrumentation Sci.& Tech.Co.,Ltd,Shanghai,China).The samples with 0.3 cm in width and 5 cm in length were measured according to Chinese National Standard (GB/T228-2002),and the stretching rate is 50 mm/min.Each membrane was tested at least five times and the average values were adopted.
3Results and discussion
3.1Membrane morphologies
The FESEM images of the cross-sections,top surfaces and bottom surfaces of the resultant PVDF/O-MWNTs membranes are shown in Figs.1-3.From Fig.1,all the membranes present an asymmetric structure with finger-like structures near top surface which are the typical structure of instantaneous precipitation process[19].M-1 and M-2 exhibit spherulite grains near the bottom surface which are different from other membranes.This is the result of PVDF nuclei growth.For M-1 and M-2 membrane,the intrinsic hydrophobic properties of PVDF retard the rate of phase demixing rare without PEG or PVP auxiliary[20].Therefore,PVDF nuclei have enough time to growth.For M-3-M-5,the finger-like structure grows bigger and spherulite structure turns into sponge-like structure near the bottom surface with the increase of O-MWNTs content.From Fig.1,the pore size in the sponge-like structure of M-6 and M-7 is bigger than other membranes.It verifies that PVP can induce the formation of finger-like structure and macrovoids for its high molecular weight[21].
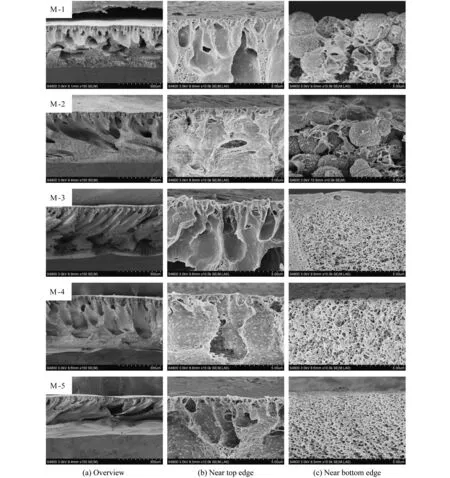
Figure 1 The cross-sectional morphologies of PVDF/O-MWNTs membranes
The top surfaces of PVDF/O-MWNTs membranes are shown in Fig.2.M-1-M-5 show denser top layers while M-6 and M-7 exhibit some mini pores which can be seen from the enlarged images.
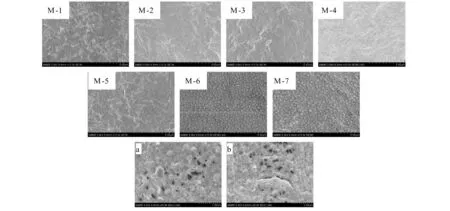
(a)(b):the enlarged imges of M-6 and M-7
The bottom surfaces of PVDF/O-MWNTs membranes are shown in Fig.3.For M-1 and M-5,the structure changes from spherulite to cell with the addition of O-MWNTs.And the pore sizes of M-6 and M-7 are evidently bigger than that of M-5.
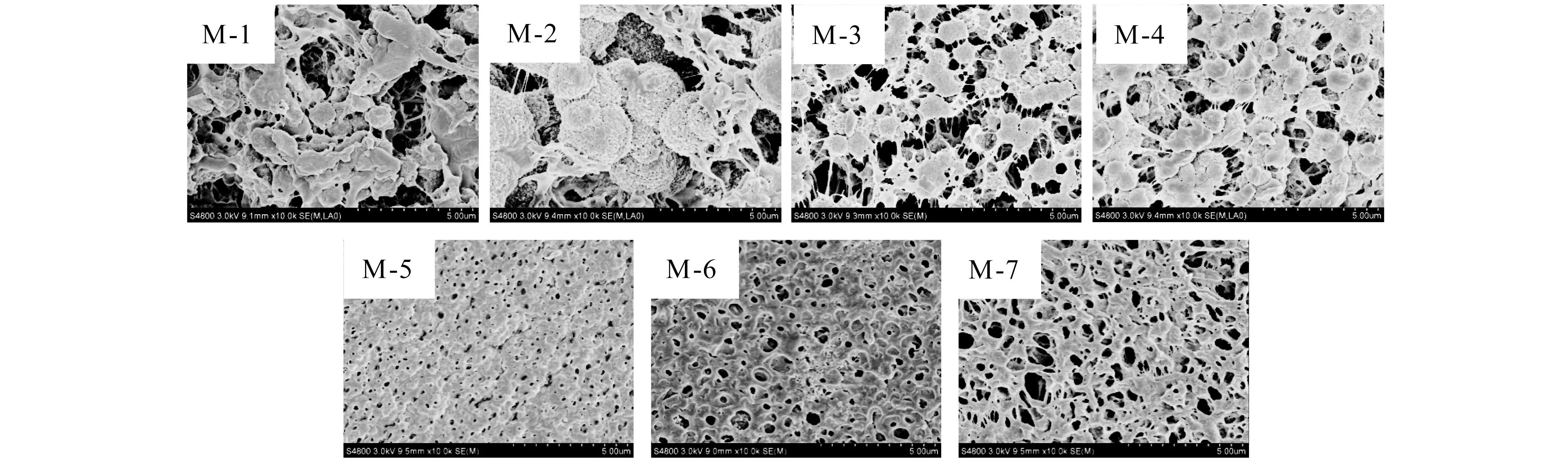
Figure 3 The FESEM images of the bottom surfaces of PVDF/O-MWNTs membranes
3.2Dynamic contact angle
The hydrophilicity of PVDF/O-MWNTs membranes was tested by contact angle measurements and the results are shown in Fig.4 and Table 2.According to Fig.4 and Table 2,the final contact angle increases from 69.7° for M-1 to 82.7° for M-2 with the addition of PEG200.That is because PEG200 can accelerate the exchange rate between solvent and non-solvent.Thus,the top surface of M-2 is denser than that of M-1,which results in the higher contact angle for M-2.Meanwhile,the contact angle increases from 62.5° for M-6 to 64.2° for M-7 which is similar to M-1 and M-2.With the addition of O-MWNTs from 0.0 wt.% to 0.6 wt.% for M-2-M-5,the contact angle decreases from 82.7° to 71.4°,because O-MWNTs can form hydrogen bonds with water molecules on membrane surface.For M-6 and M-7,the macrovoids on the surface benefit for the permeation of water into membranes.So,their contact angles are smaller than those of other membranes.
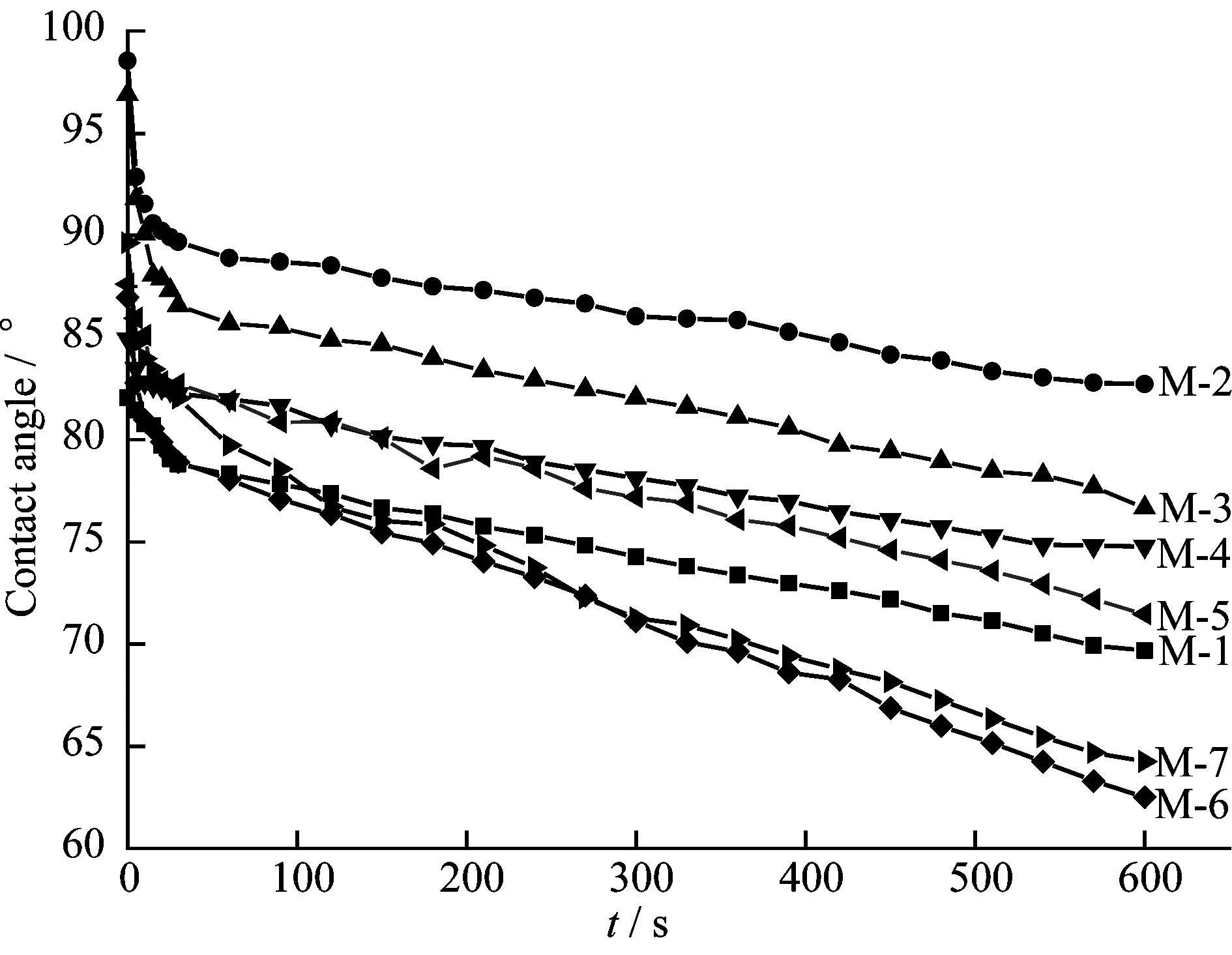
Figure 4 The dynamic contact angles of PVDF/O-MWNTs membranes
3.3Permeation performances
As we all know,many parameters such as porosity,interconnection of cavities,surface pore size and surface hydrophilicity are closely related to the PWFs of membranes[8].The PWFs and BSA rejections of prepared PVDF membranes are shown in Table 2.It can be seen that M-2 shows relatively high BSA rejection and low PWF than M-1 for the addition of PEG 200 which is similar to the difference between M-6 and M-7.For M-2~M-5,the PWFs increase from 73.7±2.7 L·m-2·h-1·bar-1for M-2 to 222.9±12.5 L·M-2·H-1·bar-1for M-5 with the gradual addition of O-MWNTs.Meanwhile,the BSA rejections are also improved with the increase of O-MWNTs in the membranes.The reason is that the higher viscosity of dope solutions with higher O-MWNTs content may result in denser membrane structure and can improve the BSA rejections of membranes.It can be concluded that O-MWNTs can significant by improve the permeation performance of PVDF membranes.Also,the changes of PWFs and BSA rejections are highly highly in accordance with the morphological variations of PVDF/O-MWNTs membranes shown in Figs.1-3.
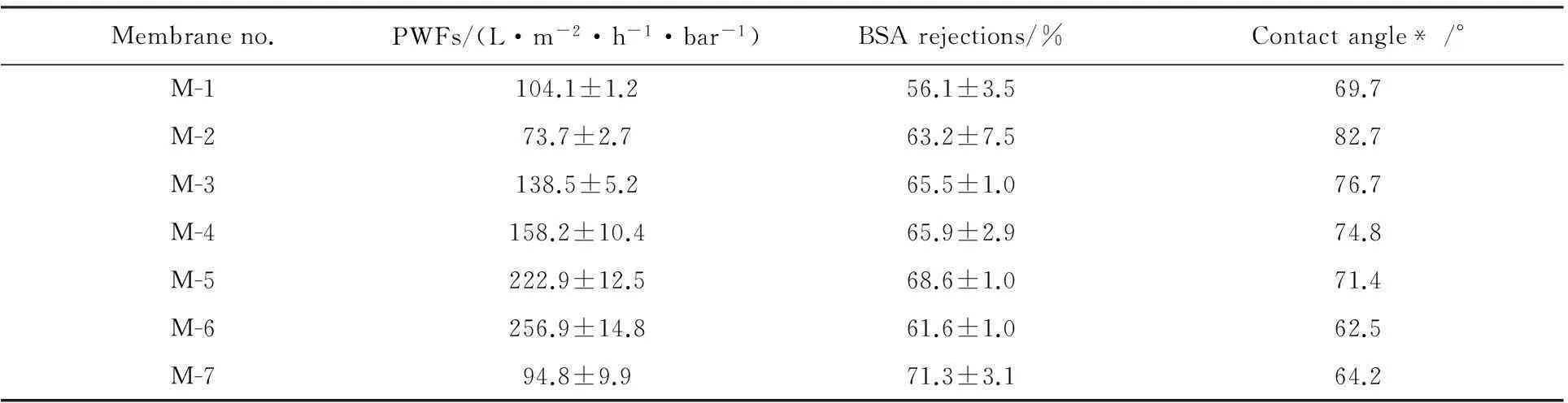
Table 2 The permeation performance and hydrophilicity of PVDF/O-MWNTs membranes
*Note:measured after contacting with water for 3 mins.
3.4Crystallization behaviors
The crystallizat behaviors of PVDF/O-MWNT membranes were detected by ATR-FTIR and WXRD (Figs.5~6).ATR-FTIR spectra of M-5 and M-6 exhibit a peak at 1664 cm-1which is ascribed to the existence of PVP in membrane matrix[22].For each sample,there are well-defined absorption bands at 840,877,1171,1180,1276,and 1403 cm-1,which are the indications of β phase of PVDF crystal[23].Meanwhile,it can be drawn that all PVDF membranes demonstrate similar XRD patterns.The peaks at around 18.6°±0.2°[24],20.6°±0.2°[25]and 36.5°±0.3°[26]are referred to the characteristic diffractions of β phase.
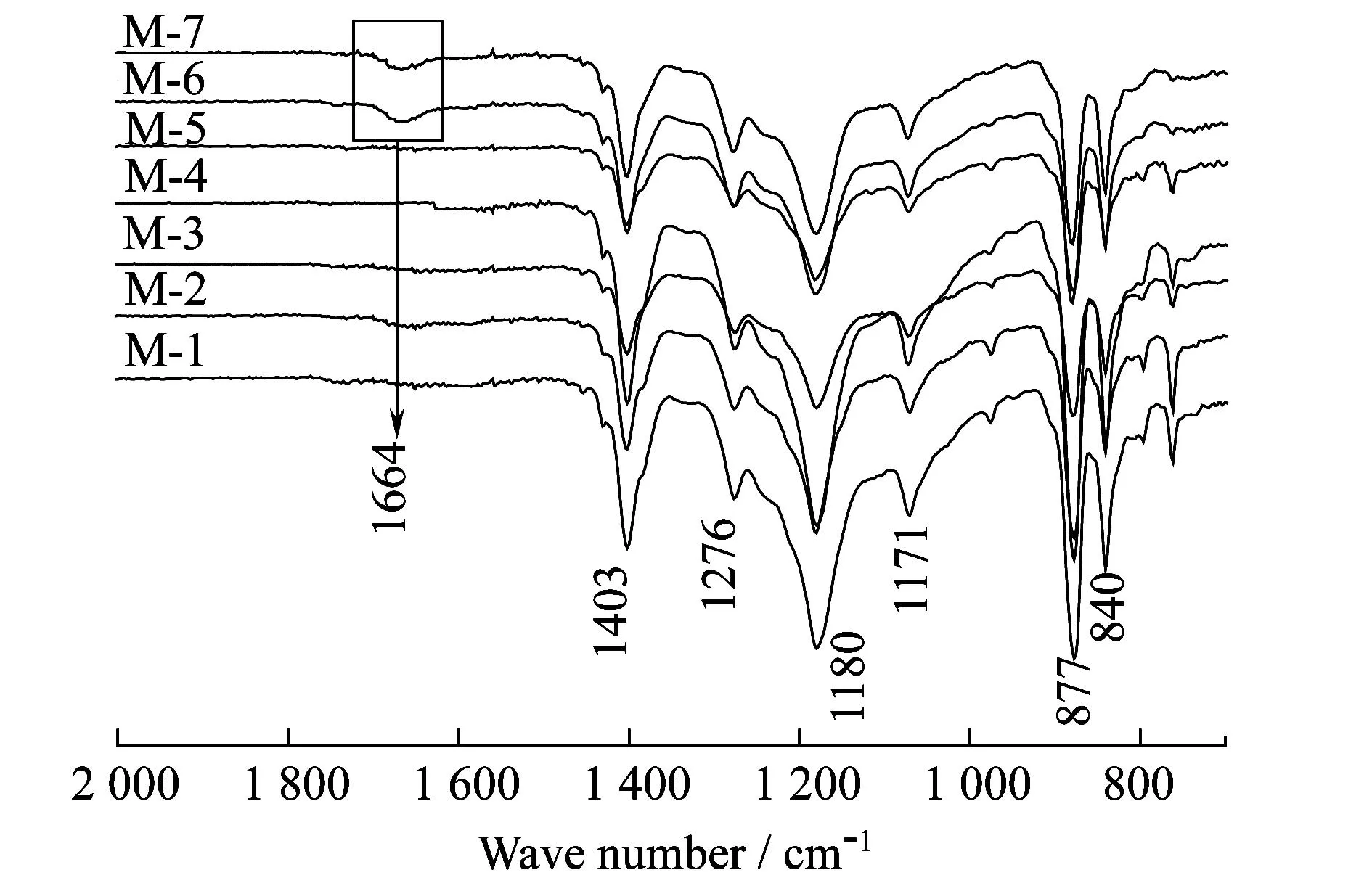
Figure 5 ATR-FTIR spectra of PVDF/O-MWNTs membranes
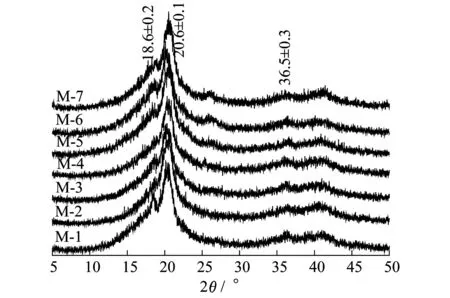
Figure 6 WXRD patterns of PVDF/O-MWNTs membranes
3.5Mechanical properties
The introduced additives have different effects on the mechanical properties of PVDF/O-MWNTs membranes for their critical effects on membrane structures.The mechanical properties of PVDF/O-MWNTs membranes are shown in Table 3.It can be seen that the tensile strength increases from 0.59±0.02 Mpa for M-2 to 1.91±0.05 Mpa for M-5.The results verify that O-MWNTs can improve the mechanical properties of PVDF membranes.It may due to the fillers sufficiently bound to PVDF chains and the decreased thickness of spongy-like structures which are shown in Fig.1.Comparing M-2 to M-1 and M-7 to M-6,we found that the addition of PEG has no promoting effect on mechanical properties of PVDF membranes.
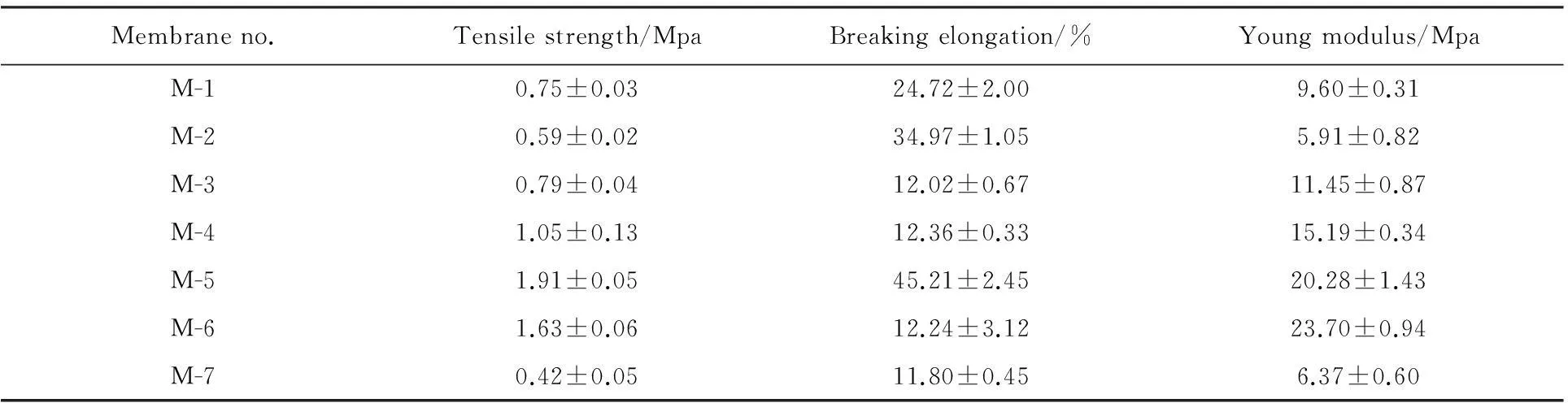
Table 3 The mechanical properties of PVDF/O-MWNTs membranes
4Conclusions
In this work,O-MWNTs accompanied with PEG/PVP additives are used to modify PVDF membranes.The structures,permeation performances,hydrophilicity,crystallization behaviors and mechanism properties of the prepared PVDF membranes are explored.According to the results,the addition of O-MWNTs can enhance the permeation performances,hydrophilicity and mechanical properties of PVDF membranes.The PWF attains 222.9±12.5 L·m-2·h-1·bar-1and BSA rejection is 68.6±1.0% with 0.6 wt.% O-MWNTs in the cast solution.PEG200 additive can enhance the dispersion of O-MWNTs in cast solution,and accelerate the exchange rate between solvent and non-solvent.PVP working as pore-forming agent promotes the growth of pores of PVDF membranes and pure water permeation flux.
References:
[1]Liu F,Hashim N A,Liu Y,et al.Progress in the production and modification of PVDF membranes [J].Journal of Membrane Science,2011,375(1-2):1-27.
[2]Xu Z,Li L,Wu F,et al.The application of the modified PVDF ultrafiltration membranes in further purification of Ginkgo biloba extraction [J].Journal of Membrane Science,2005,255(1-2):125-131.
[3]Lai C Y,Groth A,Gray S,et al.Enhanced abrasion resistant PVDF/nanoclay hollow fibre composite membranes for water treatment [J].Journal of Membrane Science,2014,449:146-157.
[4]Li X,Pang R,Li J,et al.In situ formation of Ag nanoparticles in PVDF ultrafiltration membrane to mitigate organic and bacterial fouling [J].Desalination,2013,324:48-56.
[5]Yan L,Li Y S,Xiang B C.Preparation of poly(vinylidene fluoride)(pvdf) ultrafiltration membrane modified by nano-sized alumina(Al2O3) and its antifouling research [J].Polymer,2005,46(18):7701-7706.
[6]Yan L,Li Y,Xiang C,et al.Effect of nano-sized Al2O3-particle addition on PVDF ultrafiltration membrane performance [J].Journal of Membrane Science,2006,276(1-2):162-167.
[7]Lang W Z,Ji Q,Shen J P,et al.The roles of alkali metal counter-ions of PFSA play in the formation of PVDF/PFSA-M hollow fiber membranes [J].Desalination,2012,292(2012):45-52.
[8]Lang W Z,Xu Z L,Yang H,et al.Preparation and characterization of PVDF-PFSA blend hollow fiber UF membrane [J].Journal of Membrane Science,2007,288(1-2):123-131.
[9]O′bryan G,Yang E L,Zifer T,et al.Nanotube surface functionalization effects in blended multiwalled carbon nanotube/PVDF composites [J].Journal of Applied Polymer Science,2011,120(3):1379-1384.
[10]Zhao Y,Xu Z,Shan M,et al.Effect of graphite oxide and multi-walled carbon nanotubes on the microstructure and performance of PVDF membranes [J].Separation and Purification Technology,2013,03:78-83.
[11]Ma J,Zhao Y,Xu Z,et al.Role of oxygen-containing groups on MWCNTs in enhanced separation and permeability performance for PVDF hybrid ultrafiltration membranes [J].Desalination,2013,320:1-9.
[12]Zinadini S,Zinatizadeh A A,Rahimi M,et al.Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates [J].Journal of Membrane Science,2014,453:292-301.
[13]Lee H J,Oh S J,Choi J Y,et al.In situ synthesis of poly(ethylene terephthalate)(PET) in ethylene glycol containing terephthalic acid and functionalized multiwalled carbon nanotubes(MWNTs) as an approach to MWNT/PET nanocomposites [J].Chemistry of Materials,2005,17:5057-5064.
[14]Gong X,Liu J,Baskaran S,et al.Surfactant-assisted processing of carbon nanotube/polymer composites [J].Chemistry of Materials,2000,12(8):1049-1052.
[15]Majeed S,Fierro D,Buhr K,et al.Multi-walled carbon nanotubes(MWCNTs) mixed polyacrylonitrile(PAN) ultrafiltration membranes [J].Journal of Membrane Science,2012,403-404:101-109.
[16]Gui M M,Yap Y X,Chai S P,et al.Amine-functionalization of multi-walled carbon nanotubes for adsorption of carbon dioxide [J].Asia-Pacific Journal of Chemical Engineering,2013,8(2):262-270.
[17]Zhang X,Lang W Z,Xu H P,et al.Improved performances of PVDF/PFSA/O-MWNTs hollow fiber membranes and the synergism effects of two additives [J].Journal of Membrane Science,2014,469:458-470.
[18]Xu H P,Lang W Z,Yan X,et al.Preparation and characterizations of poly(vinylidene fluoride)/oxidized multi-wall carbon nanotube membranes with bi-continuous structure by thermally induced phase separation method [J].Journal of Membrane Science,2014,467:142-152.
[19]Zhang P,Wang Y,Xu Z,et al.Preparation of poly(vinyl butyral) hollow fiber ultrafiltration membrane via wet-spinning method using PVP as additive [J].Desalination,2011,278(1-3):186-193.
[20]Wongchiphimon S,Wang R,Jiraratananon R,et al.Effect of polyethylene glycol(PEG) as an additive on the fabrication of polyvinylidene fluoride-co-hexafluropropylene(PVDF-HFP) asymmetric microporous hollow fiber membranes [J].Journal of Membrane Science,2011,369(1-2):329-338.
[21]Simones S,Figoli A,Criscuoli A,et al.Preparation of hollow fibre membranes from PVDF/PVP blends and their application in VMD [J].Journal of Membrane Science,2010,364(1-2):219-232.
[22]Lang W Z,Guo Y J,Chu L F.Evolution of the precipitation kinetics,morphologies,permeation performances,and crystallization behaviors of polyvinylidenefluoride(PVDF) hollow fiber membrane by adding different molecular weight polyvinylpyrrolidone(PVP) [J].Polymers for Advanced Technologies,2011,22(12):1720-1730.
[23]Boccaccio A B T,Capannelli G,Piaggio P.Characterization of PVDF membranes by vibrational spectroscopy [J].Journal of Membrane Science,2002,210(2):315-329.
[24]Gu M,Zhang J,Wang X,et al.Crystallization behavior of PVDF in PVDF-DMP system via thermally induced phase separation [J].Joural of Applied Polymer Science,2006,102(4):3714-3719.
[25]Cheng L P.Effect of temperature on the formation of microporous PVDF membranes by precipitation from 1-Octanol/DMF/PVDF and Water/DMF/PVDF systems [J].Macromolecules,1999,32(20):6668-6674.
[26]Yu S,Zheng W,Yu W,et al.Formation mechanism of β phase in PVDF/CNT composite prepared by the sonication method [J].Macromolecules,2009,42(22):8870-8874.
(责任编辑:郁慧)
氧化多壁碳纳米管、PEG及PVP添加剂对PVDF膜性能的影响
徐海朋, 陈兴凡, 张旋, 郎万中
(上海师范大学 生命与环境科学学院,上海 200234)
摘要:通过引入亲水性添加剂是改善膜聚偏氟乙烯(PVDF)膜亲水性和膜结构的一种有效方法.在本研究中,利用氧化碳纳米管(O-MWNTs)和聚乙二醇(PEG)及聚乙烯吡咯烷酮(PVP)修饰PVDF膜.对添加剂对PVDF膜的结构、渗透性能、亲水性、结晶行为等影响进行了详细研究.结果表明:加入O-MWNTs可以提高PVDF膜的渗透通量、亲水性和机械性能.当铸膜液中添加0.6% O-MWNTs和5% PEG 200或者0.6% O-MWNTs和3% PVP时,所制PVDF膜渗透通量分别达到222.9±12.5 L·m-2·h-1·bar-1和 256.9±14.8 L·m-2·h-1·bar-1.添加剂PEG200可以强化O-MWNTs的分散性,并能够促进相转化过程中溶剂与非溶剂间物质交换.PVP作为致孔剂能够促进孔生长和纯水通量提高.
关键词:聚偏氟乙烯膜; 超滤; 多壁碳纳米管
Corresponding author:LANG Wanzhong,College of Life and Environmental Sciences,Shanghai Normal University,No.100,Guilin Rd.,Shanghai 200234,China,E-mail:wzlang@shnu.edu.cn
Received date: 2015-09-19
Foundation item: The research is supported by Science and Technology Commission of Shanghai Municipality (13ZR1429900,14520502900) and International Joint Laboratory on Resource Chemistry (IJLRC).

