实用饲料中补充铜对斑点叉尾生长和体色的影响
万祖德 李小勤 高启平 帅 柯 陈佳楠 徐怀兵 冷向军,3,4,5*
(1.上海海洋大学水产与生命学院,上海201306;2.通威股份有限公司,成都610041;
3.上海海洋大学农业部淡水水产种质资源重点实验室,上海201306;4.上海市水产养殖
工程技术研究中心,上海201306;5.水产动物遗传育种中心
上海市协同创新中心,上海201306)
万祖德1李小勤1高启平2帅柯2陈佳楠1徐怀兵1冷向军1,3,4,5*
(1.上海海洋大学水产与生命学院,上海201306;2.通威股份有限公司,成都610041;
3.上海海洋大学农业部淡水水产种质资源重点实验室,上海201306;4.上海市水产养殖
工程技术研究中心,上海201306;5.水产动物遗传育种中心
上海市协同创新中心,上海201306)
摘要:在实用饲料(含铜11.1 mg/kg)中分别添加0(对照)、5、10、20和40 mg/kg铜[以五水硫酸铜(CuSO4·5H2O)形式],制成5种试验饲料,投喂平均体重为(98.1±0.5) g的斑点叉尾42 d,研究实用饲料中补充铜对斑点叉尾生长和体色的影响。每种饲料设3个重复,每个重复放养20尾鱼。结果表明:与对照组相比,饲料中添加10 mg/kg铜显著提高了鱼体的增重率(P<0.05),显著降低了饲料系数(P<0.05);饲料铜添加量进一步增加到40 mg/kg,鱼体的增重率则较添加量为10 mg/kg时显著降低(P<0.05),同时饲料系数显著升高(P<0.05)。肝脏和骨骼铜含量随着饲料中铜添加量的增加而上升,其中20、40 mg/kg铜添加组的肝脏铜含量显著高于对照组和5 mg/kg铜添加组(P<0.05),40 mg/kg铜添加组的骨骼铜含量也显著高于对照组(P<0.05),而肌肉铜含量保持基本不变(P>0.05)。饲料中添加0~40 mg/kg铜对斑点叉尾背部皮肤、肌肉色度值、总叶黄素含量及背部皮肤酪氨酸酶活性均未产生显著影响(P>0.05)。各组血清天门冬氨酸氨基转移酶(AST)、丙氨酸氨基转移酶(ALT)活性和总胆红素(T-Bil)含量以及肌肉水分、粗蛋白质、粗脂肪和粗灰分含量无显著差异(P>0.05)。10 mg/kg铜添加组具有最高的血清铜锌-超氧化物歧化酶(Cu,Zn-SOD)活性,显著高于其他各组(P<0.05),而其他各组间则无显著差异(P>0.05)。综上,在本试验条件下,斑点叉尾实用饲料中铜的添加量建议为10 mg/kg(饲料铜含量实测值为20.2 mg/kg)。
关键词:斑点叉尾;铜;生长;体色


1材料与方法
1.1试验设计和试验饲料
以鱼粉、豆粕、菜籽粕、棉籽粕、次粉、麦麸等为主要原料配制粗蛋白质水平为31%的实用型基础饲料,在基础饲料中分别添加0(对照)、5、10、20和40 mg/kg铜[以五水硫酸铜(CuSO4·5H2O)形式],共制成5种试验饲料,试验饲料铜含量实测值分别为11.1、16.0、20.2、28.9和44.2 mg/kg。饲料原料经粉碎过40目筛,充分混合混匀后,用膨化机(SLP-45,中国水产科学研究院渔业机械仪器研究所研制)制粒[制粒温度为(110±5) ℃]形成直径2 mm的浮性膨化饲料,晾干后置于4 ℃冰箱内保存备用。基础饲料组成及营养水平见表1。
1.2试验用鱼及饲养管理

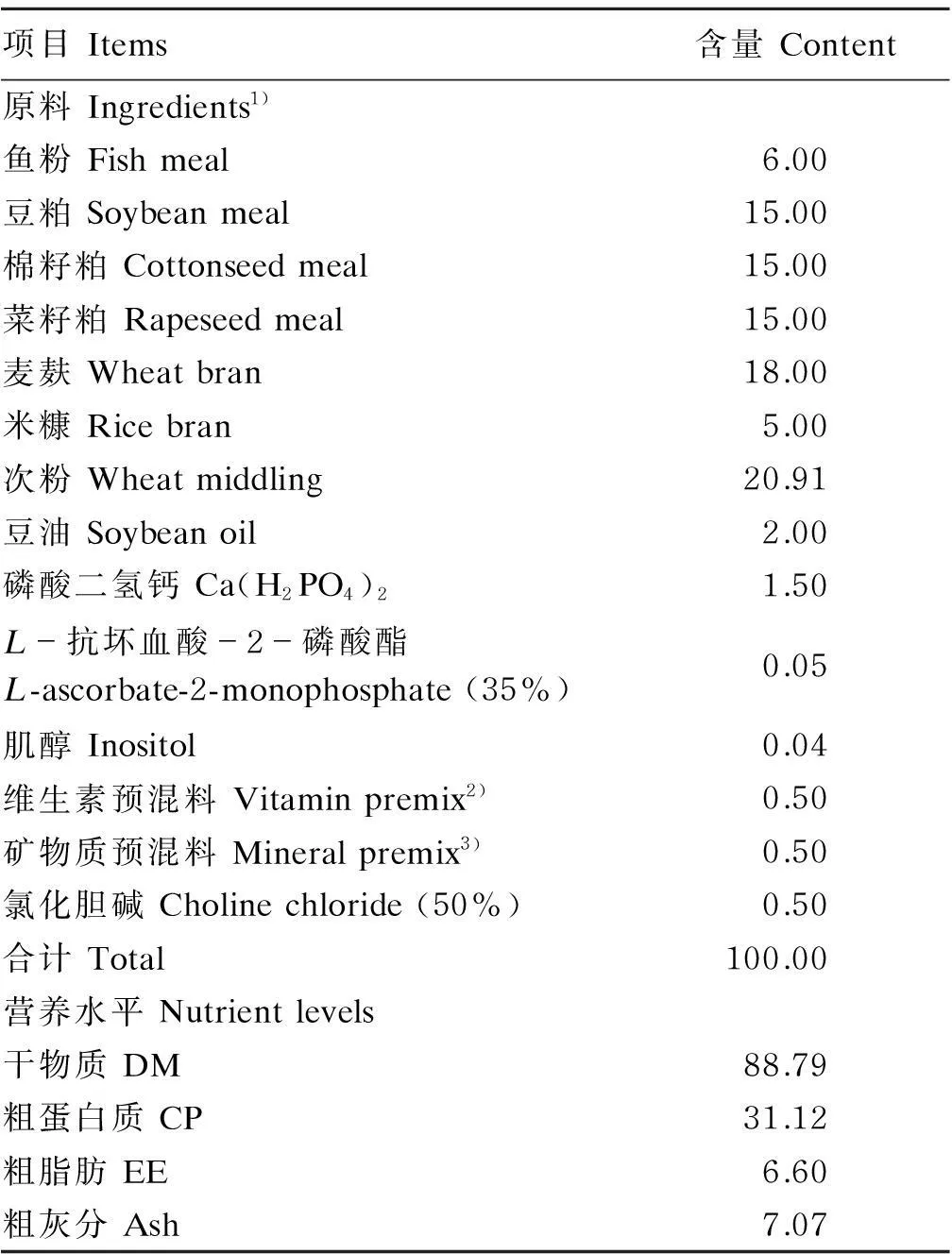
表1 基础饲料组成及营养水平(风干基础)
1)配方中的饲料原料购自上海农好饲料有限公司,其中鱼粉(秘鲁)、豆粕、菜籽粕和棉籽粕的粗蛋白质含量分别为67.6%、46.5%、35.6%和45.0%。The ingredients in formula were purchased from theShanghaiNonghaoFeed Co., Ltd., and the crude protein content of fish meal (Peru), soybean meal, rapeseed meal and cottonseed meal was 67.6%, 46.5%, 35.6% and 45.0%, respectively.
2)维生素预混料为每千克饲料提供The vitamin premix provided the following per kg of the diet:VA 6 000 IU,VD 2 000 IU,VE 50 IU,VK 5 mg,VB115 mg,VB215 mg,VB330 mg,VB535 mg,VB66 mg,生物素 biotin 0.2 mg,叶酸 folic acid 3 mg,VB120.03 mg。
3)矿物质预混料为每千克饲料提供The mineral premix provided the following per kg of the diet:Ca(IO3)20.04 g,CoCl2·6H2O 0.01 g,FeSO4·H2O 0.446 g,ZnSO4·H2O 0.232 g,MnSO4·H2O 0.063 g,Na2SeO3·5H2O 0.01 g,MgSO4·7H2O 0.645 g。
1.3测定指标和方法
1.3.1生长与形体指标计算
养殖试验结束后,鱼体饥饿24 h,统计每口网箱内的鱼尾数并称总重,计算增重率(WGR)、饲料系数(FCR)、存活率(SR);每个网箱随机取出3尾鱼,测量鱼体长和体重,解剖后称量其内脏重、肝脏重,计算肝体指数(HSI)、脏体指数(VSI)及肥满度(CF)。
增重率(%)=100×[(终末体重(g)-
初始体重(g)]/初始体重(g);
饲料系数=总投喂量(g)/[终末体重(g)-
初始体重(g)];
存活率(%)=100×终末鱼总数(尾)/
初始鱼总数(尾);
肝体指数(%)=100×肝脏重(g)/体重(g);
脏体指数(%)=100×内脏重(g)/体重(g);
肥满度(g/cm3)=100×体重(g)/
体长(cm)3。
1.3.2饲料、肌肉常规成分分析
养殖试验结束后,每口网箱随机取3尾鱼,取背鳍下方侧线以上的背部肌肉,置于-20 ℃冰箱中冻存,用于肌肉常规成分分析。饲料、肌肉样品水分含量的测定采用105 ℃烘干失水法(GB/T 5009.3—2003);粗蛋白质含量的测定采用凯氏定氮法(GB/T 5009.5—2003);粗脂肪含量的测定参照Folch等[12]的氯仿甲醇抽提法;粗灰分含量测定采用马福炉灰化法(GB/T 5009.4—2003)。
1.3.3皮肤、肌肉色度值测定
养殖试验结束后,随机从每口网箱取3尾鱼,用吸水纸将鱼体表面水分吸干,将色差计(WSC-S型色差计,上海精密科学仪器有限公司物理光学仪器厂)的探头紧贴鱼体侧线以上的背部皮肤,测量背部皮肤色度值,之后剥去皮肤,将探头紧贴在侧线以上的背部肌肉上,测量肌肉色度值,记录亮度(L*)、红绿度(a*)、黄蓝度(b*)值。
1.3.4皮肤、肌肉总叶黄素含量测定
上述皮肤、肌肉测定色度值后,各取2~3 g,参考Quackenbush等[13]的分析法进行总叶黄素含量的测定,具体方法如下:将样品剪碎,装入25 mL棕色容量瓶中,加入7.5 mL提取液(正己烷∶丙酮∶无水乙醇∶甲苯=10∶7∶6∶7),塞上塞子旋转振摇1 min,加入1 mL 40%氢氧化钾-甲醇溶液,旋转摇匀1 min,于55.5 ℃水浴加热20 min(注意冷却容量瓶颈部以防止溶剂损失),冷却样品,放置暗处1 h,加入7.5 mL正己烷,旋转振摇1 min,以10%硫酸钠溶液定容至25 mL,猛烈振摇1 min,于暗处放置1 h后,将上层液用分光光度计于474 nm处测定其吸光度值,根据标准曲线计算其总叶黄素含量。
1.3.5背部皮肤酪氨酸酶活性测定
取1 g左右背部皮肤,按1∶5比例用67 mmol/L pH 6.8的磷酸缓冲液匀浆(冰水浴),在4 ℃下离心25 min (8 000 r/min),取上清液参照丁玉庭等[14]的方法测定其中酪氨酸酶活性,具体方法如下:取3 mg/mLL-多巴0.5 mL,加入28 ℃预热的2 mL上述上清液,总反应体积为2.5 mL,混合后立即室温下用分光光度计于475 nm处测定其吸光度值(OD0),10 min后再次测定吸光度值(OD10)。
酪氨酸酶活性按照下述公式计算:
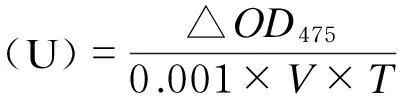
式中:△OD475为2次测定吸光度值的差值,即△OD475=OD10-OD0;V为样品体积;T为2次测定间隔时间。
1.3.6血清生化指标测定
养殖试验结束后,每口网箱随机取3尾鱼,尾静脉取血,离心(3 000 r/min,15 min),取血清于-80 ℃冷冻保存。分别测定血清天门冬氨酸氨基转移酶(AST)、丙氨酸氨基转移酶(ALT)活性及总胆红素(T-Bil)含量,均采用迈瑞BS-200全自动生化分析仪测定。血清Cu,Zn-SOD活性采用南京建成生物工程研究所提供的试剂盒进行测定。
1.3.7组织(肌肉、肝脏、脊椎骨)铜含量测定
组织铜含量的测定参考张韵华[15]的方法,采用原子吸收法,具体方法如下:准确称取样品1 g,置于坩埚中,先在电炉上炭化至不再冒烟为止,然后移入550 ℃的马福炉中灰化6 h;取出,冷却至室温,加入硝酸与高氯酸的混合酸(4∶1)10 mL;放置5 h以上;然后在电炉上小心加热,使灰化样品溶解(黑色炭粒消失),直到溶液接近蒸干为止;用1%的盐酸溶液溶解析出的晶体,再转移到25 mL的容量瓶定容、待测。以不加样品,用同样方法获得的试液作为空白对照。采用TAS-900原子吸收分光光度计(北京普析通用公司)测定铜含量。
1.4数据分析
试验结果采用SPSS 17.0统计软件进行处理分析,数据以平均值±标准差(mean±SD)表示,采用单因素方差分析(one-way ANOVA),用Duncan氏法进行多重比较,P<0.05为差异显著。
2结果
2.1生长与形体指标
表2 饲料中铜添加量对斑点叉尾生长与形体指标的影响
Table 2 Effects of dietary copper supplemental level on growth and morphological in
dices of channel catfish
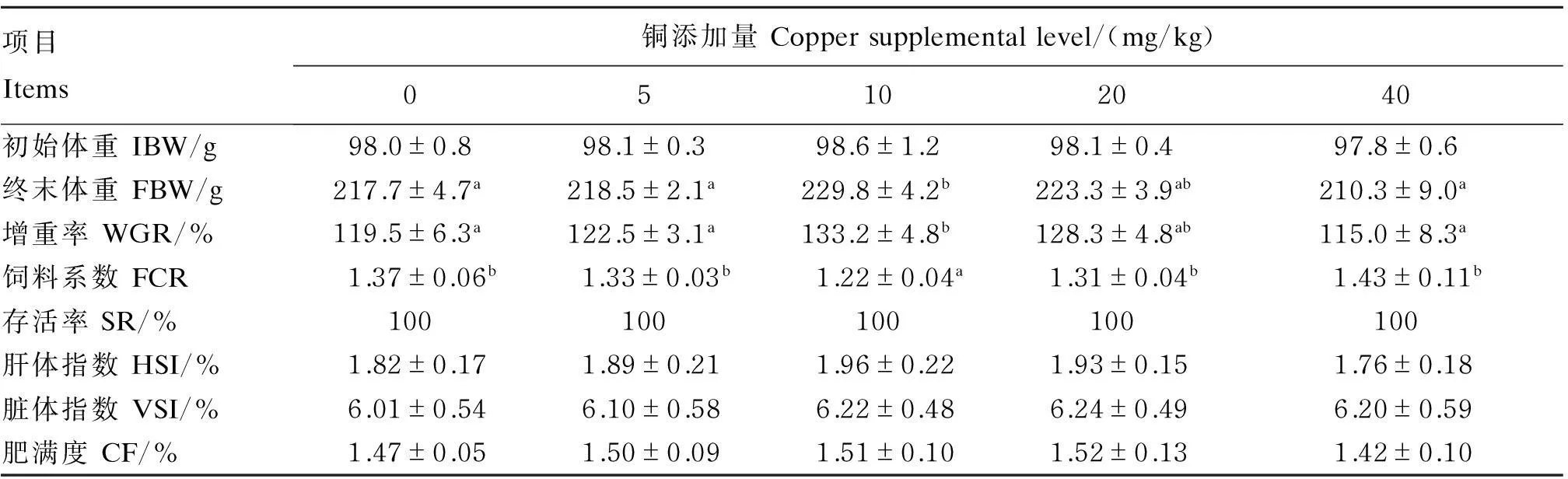
表2 饲料中铜添加量对斑点叉尾生长与形体指标的影响
项目Items铜添加量Coppersupplementallevel/(mg/kg)05102040初始体重IBW/g98.0±0.898.1±0.398.6±1.298.1±0.497.8±0.6终末体重FBW/g217.7±4.7a218.5±2.1a229.8±4.2b223.3±3.9ab210.3±9.0a增重率WGR/%119.5±6.3a122.5±3.1a133.2±4.8b128.3±4.8ab115.0±8.3a饲料系数FCR1.37±0.06b1.33±0.03b1.22±0.04a1.31±0.04b1.43±0.11b存活率SR/%100100100100100肝体指数HSI/%1.82±0.171.89±0.211.96±0.221.93±0.151.76±0.18脏体指数VSI/%6.01±0.546.10±0.586.22±0.486.24±0.496.20±0.59肥满度CF/%1.47±0.051.50±0.091.51±0.101.52±0.131.42±0.10
同行数据肩标无字母或相同字母表示差异不显著(P>0.05),不同小写字母表示差异显著(P<0.05)。下表同。
In the same row, values with no letter or the same letter superscripts mean no significant difference (P>0.05), while with different small letter superscripts mean significant difference (P<0.05). The same as below.
2.2肌肉常规成分
由表3可见,肌肉水分、粗蛋白质、粗脂肪及粗灰分含量在各组间无显著差异(P>0.05)。
2.3背部皮肤、肌肉色度值和总叶黄素含量及背部皮肤酪氨酸酶活性
由表4可见,各组背部皮肤、肌肉的色度值和总叶黄素含量及背部皮肤酪氨酸酶活性均无显著差异(P>0.05)。
2.4血清生化指标
由表5可见,各组血清AST、ALT活性和T-Bil含量无显著差异(P>0.05);10 mg/kg铜添加组具有最高的血清Cu,Zn-SOD活性,与其他各组差异显著(P<0.05),而其他组间则无显著差异(P>0.05)。
表3 饲料铜添加量对斑点叉尾肌肉常规成分的影响(鲜重基础)
Table 3 Effects of dietary copper supplemental level on muscle conventional
components of channel catfish (wet weight basis) %
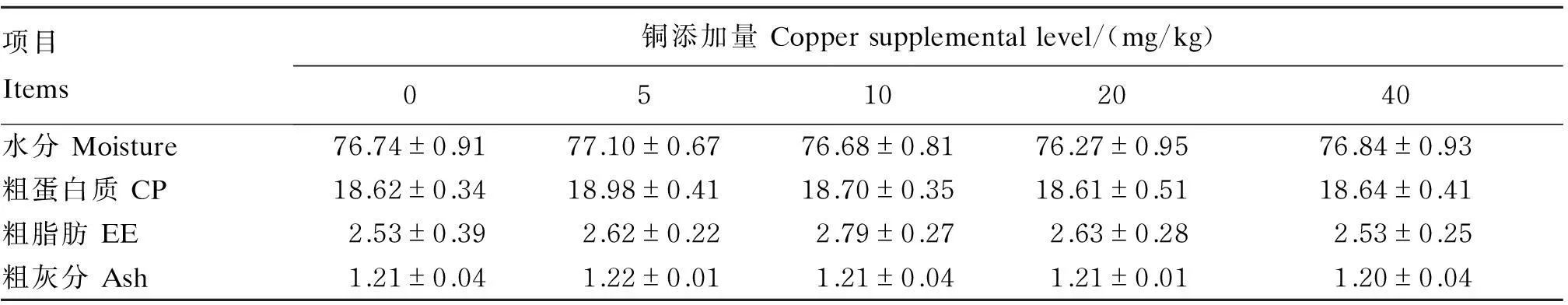
表3 饲料铜添加量对斑点叉尾肌肉常规成分的影响(鲜重基础)
项目Items铜添加量Coppersupplementallevel/(mg/kg)05102040水分Moisture76.74±0.9177.10±0.6776.68±0.8176.27±0.9576.84±0.93粗蛋白质CP18.62±0.3418.98±0.4118.70±0.3518.61±0.5118.64±0.41粗脂肪EE2.53±0.392.62±0.222.79±0.272.63±0.282.53±0.25粗灰分Ash1.21±0.041.22±0.011.21±0.041.21±0.011.20±0.04
表4 饲料中铜添加量对斑点叉尾背部皮肤、肌肉色度值和总叶黄素含量及背部皮肤酪氨酸酶活性的影响
Table 4 Effects of dietary copper supplemental level on chroma values and total xanthophylls
content of dorsal skin, muscle and tyrosinase activity of dorsal skin of channel catfish

表4 饲料中铜添加量对斑点叉尾背部皮肤、肌肉色度值和总叶黄素含量及背部皮肤酪氨酸酶活性的影响
项目Items铜添加量Coppersupplementallevel/(mg/kg)05102040背部皮肤Dorsalskin亮度L*20.48±2.6019.35±1.9421.38±2.2621.28±1.9921.43±1.66红绿度a*3.53±1.203.10±1.312.67±0.712.50±0.832.11±0.63黄蓝度b*2.48±0.732.02±1.092.72±1.212.44±0.502.02±0.91总叶黄素Totalxanthophylls2.75±0.052.81±0.573.02±0.982.86±0.462.86±0.18酪氨酸酶Tyrosinase0.99±0.271.10±0.081.14±0.061.03±0.131.14±0.10背部肌肉Dorsalmuscle亮度L*50.76±2.6850.72±2.8151.68±2.5951.61±2.7349.37±2.44红绿度a*3.59±0.894.54±0.964.22±0.933.96±1.013.42±1.13黄蓝度b*3.50±0.994.08±1.484.49±0.683.91±1.164.13±0.98总叶黄素Totalxanthophylls/(mg/kg)1.06±0.261.18±0.331.18±0.351.24±0.111.24±0.28
表5 饲料中铜添加量对斑点叉尾血清生化指标的影响
Table 5 Effects of dietary copper supplemental level on serum biochemical indices of channel catfish
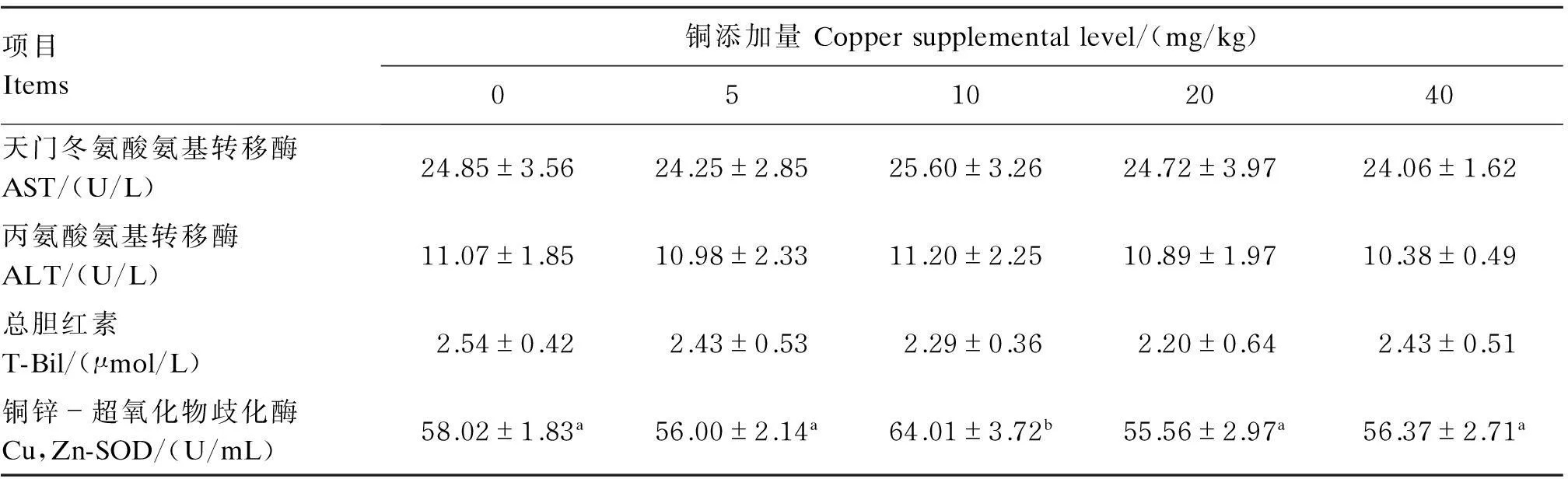
表5 饲料中铜添加量对斑点叉尾血清生化指标的影响
项目Items铜添加量Coppersupplementallevel/(mg/kg)05102040天门冬氨酸氨基转移酶AST/(U/L)24.85±3.5624.25±2.8525.60±3.2624.72±3.9724.06±1.62丙氨酸氨基转移酶ALT/(U/L)11.07±1.8510.98±2.3311.20±2.2510.89±1.9710.38±0.49总胆红素T-Bil/(μmol/L)2.54±0.422.43±0.532.29±0.362.20±0.642.43±0.51铜锌-超氧化物歧化酶Cu,Zn-SOD/(U/mL)58.02±1.83a56.00±2.14a64.01±3.72b55.56±2.97a56.37±2.71a
2.5组织铜含量
由表6可见,各组肌肉铜含量无显著差异(P>0.05);肝脏、骨骼铜含量随饲料铜添加量的增加而增加,其中20、40 mg/kg铜添加组的肝脏铜含量显著高于对照组和5 mg/kg铜添加组(P<0.05),40 mg/kg铜添加组的骨骼铜含量也显著高于对照组(P<0.05)。
表6 饲料中铜添加量对斑点叉尾组织铜含量的影响
Table 6 Effects of dietary copper supplemental level on tissue copper content of channel catfish mg/kg
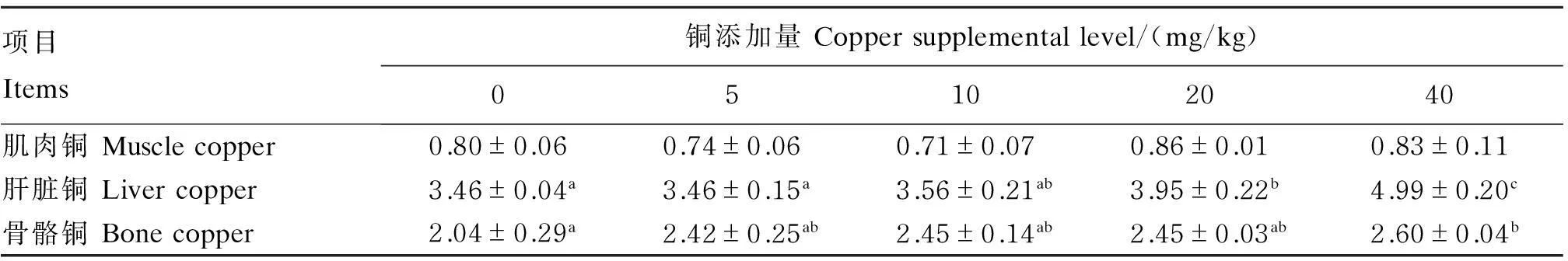
表6 饲料中铜添加量对斑点叉尾组织铜含量的影响
项目Items铜添加量Coppersupplementallevel/(mg/kg)05102040肌肉铜Musclecopper0.80±0.060.74±0.060.71±0.070.86±0.010.83±0.11肝脏铜Livercopper3.46±0.04a3.46±0.15a3.56±0.21ab3.95±0.22b4.99±0.20c骨骼铜Bonecopper2.04±0.29a2.42±0.25ab2.45±0.14ab2.45±0.03ab2.60±0.04b
3讨论






Lorentze等[30]认为,肝脏中铜的蓄积量是评价机体铜状况最敏感的指标。分别用含铜5、35、700 mg/kg饲料饲喂大西洋鲑4周,发现肝脏铜含量显著升高[22];在鲤鱼的研究中,肝脏铜含量随饲料铜含量(0~1 000 mg/kg)的增加而增加,而肌肉铜含量只有在饲料铜含量达到500 mg/kg后才显著升高[29];De Boeck等[5]在鲤鱼上也有类似报道;此外,乔永刚[31]对军曹鱼的研究发现,铜在骨骼中的沉积随饲料铜含量的增加而增加。本试验中,肝脏、骨骼铜含量随饲料铜添加量的增加而增加,但肌肉铜含量则保持基本稳定。
4结论

参考文献:
[1]姜云霞.微量元素铜的研究进展及其对动物健康的影响[J].微量元素与健康研究,2007,24(5):58-61.
[2]NRC.Nutrient requirements of fish and shrimp[S].Washington,D.C.:National Academy Press,2011.
[3]OGINO C,YANG G Y.Requirements of carp and rainbow trout for dietary manganese and copper[J].Bulletin of the Japanese Society of Scientific Fisheries,1980,46(4):455-458.
[4]LIN Y H,SHIE Y Y,SHIAU S Y.Dietary copper requirements of juvenile grouper,Epinephelusmalabaricus[J].Aquaculture,2008,274(1):161-165.
[5]DE BOECK G,VLAEMINCK A,BLUST R.Effects of sublethal copper exposure on copper accumulation,food consumption,growth,energy stores,and nucleic acid content in common carp[J].Archives of Environmental Contamination and Toxicology,1997,33(4):415-422.
[6]POPPE,T T,HÅSTEIN T,FRØSLIE A,et al.Nutritional aspects of haemorrhagic syndrome (‘Hitra disease’) in farmed Atlantic salmon,Salmosalar[J].Diseases of Aquatic Organisms,1986,1(3):152-162.
[7]SHAW B J,HANDY R D.Dietary copper exposure and recovery in Nile tilapia,Oreochromisniloticus[J].Aquatic Toxicology,2006,76(2):111-121.
[8]BERNTSSEN M H G,LUNDEBYE A K,MAAGE A.Effects of elevated dietary copper concentrations on growth,feed utilisation and nutritional status of Atlantic salmon (SalmosalarL.) fry[J].Aquaculture,1999,174(1/2):167-181.
[9]LANNO R P,SLINGER S J,HILTON J W.Maximum tolerable and toxicity levels of dietary copper in rainbow trout (SalmogairdneriRichardson)[J].Aquaculture,1985,49(3/4):257-268.
[10]黄斌,别立洁.铜(Cu2+)对麦穗鱼苗的急性毒性与非生物因子的相关性研究[J].淡水渔业,2006,36(2):34-38.
[11]HANDY R D.The effect of acute exposure to dietary Cd and Cu on organ toxicant concentrations in rainbow trout,Oncorhynchusmykiss[J].Aquatic Toxicology,1993,27(1/2):1-14.
[12]FOLCH J,LEES M,SLOANE STANLEY G H.A simple method for the isolation and purification of total lipides from animal tissues[J].The Journal of Biological Chemistry,1957,226(1):497-509.
[13]QUACKENBUSH F W,MILLER S L.Composition and analysis of the carotenoids in marigold petals[J].Journal-Association of Official Analytical Chemists,1972,55(3):617-621.
[14]丁玉庭,杨更生.外加因子对黑豚皮酪氨酸酶活性的影响[J].食品科学,1999,20(4):12-14.
[15]张韵华.原子吸收法测定重金属的预处理方法讨论[J].云南环境科学,2004,23(增刊):213-214.
[16]GATLIN D M,WILSON R P.Dietary copper requirement of fingerling channel catfish[J].Aquaculture,1986,54(4):277-285.
[17]FANG Y Z,YANG S,WU G Y.Free radicals,antioxidants,and nutrition[J].Nutrition,2002,18(10):872-879.
[18]WANG W F,MAI K S,ZHANG W B,et al.Effects of dietary copper on survival,growth and immune response of juvenile abalone,HaliotisdiscushannaiIno[J].Aquaculture,2009,297(1/2/3/4):122-127.

[20]MURAI T,ANDREWS J W,SMITH R G,Jr.Effects of dietary copper on channel catfish[J].Aquaculture,1981,22:353-357.
[21]LANNO R P,SLINGER S J,HILTON J W.Effect of ascorbic acid on dietary copper toxicity in rainbow trout (SalmogairdneriRichardson)[J].Aquaculture,1985,49(3/4):269-287.
[22]BERNTSSEN M H G,HYLLAND K,BONGA S E W,et al.Toxic levels of dietary copper in Atlantic salmon (SalmosalarL.) parr[J].Aquatic Toxicology,1999,46(2):87-99.
[23]BAKER R T M,HANDY R D,DAVIES S J,et al.Chronic dietary exposure to copper affects growth,tissue lipid peroxidation,and metal composition of the grey mullet,Chelonlabrosus[J].Marine Environmental Research,1998,45(4/5):357-365.
[24]唐精,叶元土.四种微量元素对胡子鲶体表色素含量的影响[J].饲料工业,2007,28(24):27-30.
[25]诸葛燕,叶元土,高艳玲,等.七种淡水鱼类色素含量和酪氨酸酶活力的比较研究[J].上海海洋大学学报,2007,16(5):431-436.
[26]许兰娇,万根,黎观红,等.饲粮铜添加水平对9~12周龄泰和乌骨鸡生产性能及组织黑色素含量的影响[J].动物营养学报,2014,26(4):1061-1067.
[27]罗育春,陈大兰.肝硬化患者血清谷丙转氨酶、谷草转氨酶和总胆红素与肝纤维化标志物的相关性研究[J].实用预防医学,2011,18(8):1542-1544.
[28]KIM S G,KANG J C.Effect of dietary copper exposure on accumulation,growth and hematological parameters of the juvenile rockfish,Sebastesschlegeli[J].Marine Environmental Research,2004,58(1):65-68.
[29]AL-AKEL A S,AL-BALAWI H F A,AL-MISNED F,et al.Effects of dietary copper exposure on accumulation,growth,and hematological parameters inCyprinuscarpio[J].Toxicological and Environmental Chemistry,2010,92(10):1865-1878.
[30]LORENTZEN M,MAAGE A,JULSHAMN K.Supplementing copper to a fish meal based diet fed to Atlantic salmon parr affects liver copper and selenium concentrations[J].Aquaculture Nutrition,1998,4(1):67-72.
[31]乔永刚.军曹鱼微量元素锌、铁、铜营养生理的研究[D].博士学位论文.青岛:中国海洋大学,2007:69-81.
(责任编辑菅景颖)
Effects of Practical Diet with Copper on Growth and Body Color of Channel Catfish
WAN Zude1LI Xiaoqin1GAO Qiping2SHUAI Ke2CHEN Jianan1XU Huaibing1LENG Xiangjun1,3,4,5*
(1. College of Fisheries and Life Science, Shanghai Ocean University, Shanghai 201306, China; 2. Tongwei Co., Ltd., Chengdu 610041, China; 3. Key Laboratory of Freshwater Fishery Germplasm Resources, Ministry of Aquaculture, Shanghai 201306, China; 4. Shanghai Engineering Research Center of Aquaculture, Shanghai 201306, China; 5. Shanghai Collaborative Innovation Collaborative Innovation Center for Aquatic Animal Genetics and Breeding, Shanghai 201306, China)
Abstract:In the present study, copper (as the form of CuSO4·5H2O) with the levels of 0 (control), 5, 10, 15 or 20 mg/kg were supplemented in a practical diet which contained 11.1 mg/kg copper to formulate five experimental diets to evaluate the effects of practical diet with copper on growth and body color of channel catfish (Ictalurus punctatus). The five experimental diets were fed to channel catfish with the average body weight of (98.1±0.5) g for 42 d. Each diet had 3 replicates, and each replicate had 20 fish. The results showed that weight gain rate was significantly increased and feed conversion ratio was significantly decreased by the supplementation of 10 mg/kg copper when compared with control group (P<0.05), but with the copper supplemental level increased to 40 mg/kg, the weight gain rate was significantly decreased and the feed conversion ratio was significantly increased compared with 10 mg/kg group (P<0.05). The copper content in liver and bone was increased with the increase of dietary copper supplemental level, the liver copper content in 20 and 40 mg/kg groups was significantly higher than that in control group and 5 mg/kg group (P<0.05), and the bone copper content in 40 mg/kg group was significantly higher than that in control group (P<0.05), while the muscle copper content showed no significant difference among all groups (P>0.05). The chroma values and total xanthophylls content of dorsal skin, muscle and tyrosinase activity of dorsal skin of channel catfish were not significantly affected by 0 to 40 mg/kg copper supplementation (P>0.05). Serum aspartate aminotransferase (AST), alannine aminotransferase (ALT) activities and total bilirubin (T-Bil) content, and muscle moisture, crude protein, ether extract and ash contents were not significantly different among all groups (P>0.05). The activity of serum copper, zinc-superoxide dismutase in 10 mg/kg group was the highest, and significantly higher than that in other groups (P<0.05), but no significant difference was found among other groups (P>0.05). Base on results above, the optimal copper supplementation in the practical diet is suggested to be 10 mg/kg for channel catfish under this experimental condition, and the measured value of dietary copper content is 20.2 mg/kg.[Chinese Journal of Animal Nutrition, 2016, 28(1):265-273]
Key words:channel catfish; copper; growth; body color
*Corresponding author, professor, E-mail: xjleng@shou.edu.cn
中图分类号:S963
文献标识码:A
文章编号:1006-267X(2016)01-0265-09
作者简介:万祖德(1991—),男,湖北武汉人,硕士研究生,研究方向为水产动物营养与饲料。E-mail: wanzude1991@163.com*通信作者:冷向军,教授,博士生导师,E-mail: xjleng@shou.edu.cn
基金项目:通威集团产学研合作项目(TW2014A004)
收稿日期:2015-07-16
doi:10.3969/j.issn.1006-267x.2016.01.034

