Characterization and adsorption behaviors of a novel synthesized mesoporous silica coated carbon composite☆
Sai Wang ,Songsong Xu ,Chengbao Liu ,Feng Chen ,Dongtian Wang ,Shouqing Liu ,Zhigang Chen *,Zhengying Wu ,*
1 Jiangsu Key Laboratory for Environment Functional Materials,School of Chemistry,Biology and Material Engineering,Suzhou University of Science and Technology,Suzhou 215009,China
2 Department of Clinical Oncology,the Second Affiliated Hospital of Soochow University,Suzhou 215004,China
1.Introduction
Most of the organic dyes and pigments used in textile,paper and plastic industrial fields are toxic and carcinogenic even at low concentrations[1].The sedyesdis charged into water are difficult to decompose because they are very stable to light and oxidation reactions[2,3].So it has great significance to remove dyes from waste waters for reducing water pollution and protecting our living environments.Many methods such as physical,chemical and biological methods have been investigated to remove dye materials from waste water[4-9].Among those proposed techniques,adsorption has been proved to be a very efficient method for water decontamination and carbon materials are the most widely used adsorbents because of their diversities[6-11].
Expanded graphite(EG)is a w ell know n carbon material which was invented by Carburet Company in 1968[12].Similar to the other carbon materials,EG is widely used in gasketing,adsorption,electromagnetic interference shielding,vibration damping and other fields due to its excellent properties such as compatibility,spring-back, flexibility,heat conducting,anti-acid,anti-base,and so on[12,13].Different from the other carbon materials,EG normally has worm-or accordion-like morphology and possesses lots of micrometer-ranged interconnected pores,which are constructed from the opened graphite flakes[14].As a result,EG is very useful in the adsorption of large molecular organic pollutants,especially heavy oil[15].How ever,the large pores in EG also limit its application in capture of small organic molecules[16,17].
Herein in this work,a mesoporous silica layer was grow n onto the pores of EG to generate mesopores inside the EG particles via a facile hydrothermal method.A novel hierarchical mesoporous silicacarbon composite(denoted SEG)containing both of mesopores and micrometer-sized pores was thus obtained.This silica-carbon composite was used as adsorbent to remove dyes from wastewater and it presents much higher adsorption capacity to the organic methylene blue(MB)than EG in the batch tests.
2.Experimental
2.1.Material synthesis
Expanded graphite(EG)was prepared according to the reported method[18].Mesoporous silica-carbon composite was synthesized by atraditional hydrothermal method.In a typical synthesis,1 g of Pluronic P123 and 2.42 g of AlCl3·6H2O were dissolved in 37.5 g of deionized water.0.125 g of EG was then added into the solution and the mixture was stirred for 24 h at room temperature.After that,2.08 g of tetraethylorthosilicate(TEOS)was introduced(rSiO2/C=0.96).The resulting mixture was stirred for 24 h at 318 K and then hydrothermal treated at 373 K for another 24 h under static conditions.The assynthesized sample was filtered,washed,dried and calcined at 823 K for 5 h to remove the template.The finally obtained composite was denoted SEG.To study the effect of the silica content in SEG,different amounts of EG were introduced into the solution for adjusting the
2.2.Characterization
XRD patterns of the EG and SEG were recorded on a Bruker D8 Advance diffractometer with Cu Kαradiation.N2physisorption isotherms at 77 K were measured using a Belsorp II system,in which the samples were outgassed at 473 K for 4 h prior to testing.The Brunauer-Emmett-Teller(BET)surface area was calculated with the relative pressure ranging from 0.04 to 0.20,w hile the total pore volume was derived from the amount adsorbed at the relative pressure of about 0.99.The pore size distributions were calculated by the Barrett-Joyner-Halenda(BJH)method according to the adsorption branches.SEM imageswere taken with a Hitachi S-4800 scanning electron microscope.TEM was performed on a JEM-2100 electron microscope.FT-IR spectra of pow dered samples suspended in KBr pallets were recorded on a Bruker Vertex 70 spectrometer.
2.3.Adsorption performance test
The stock solution of methylene blue(MB)was prepared in distilled water.Batch tests were typically carried out by adding 50 mg of EG or SEG samples into set of 100 ml plastic flasks containing 50 ml of MB solutions with different initial concentrations(20-120 mg·L-1).The mixtures were shaken for desired duration at room temperature with the speed of 50 r·min-1.For the study of kinetic adsorption behavior,the mixtures were shaken at appropriate time of 5,10,15,30,60,90 and 120 min respectively.For obtaining the adsorption isotherm,experiment was carried out for 2 h to obtain equilibrium.After that,the filtrate was collected and analyzed using a UV-2450 spectrophotometer at maximum wavelength around 665 nm.
The amount of dye adsorbed onto SEG sample was calculated from the mass balance equation as:

w here Qe(mg·g-1)is the amount of MB adsorbed per gram of SEG at equilibrium;C0(mg·L-1)and Ce(mg·L-1)are the initial and equilibrium liquid-phase concentration of dye;V(L)is the volume of the solution and M(g)is mass of the dry adsorbent.
3.Results and Discussion
3.1.Structure and morphology of the mesoporous SEG composite
Fig.1a show s the low-and wide-angle XRD patterns of the SEG composite(rSiO2/C=0.96).SEG possesses one diffraction peak at 2θ of 0.89°(Fig.1a,insert),which is a reflection of the presence of mesopores in the composite.Wide-angle XRD patterns reveal that EG has typical diffraction peaks at 2θ of 26.52°and 54.6°with corresponding dspacings of 0.3358 nm and 0.1679 nm,which can be indexed to the hexagonal graphite(002)and(004)crystal faces(JCPDS No.41-1487)[14,19].The same(002)and(004)diffraction peaks of the SEG composite shift to a bit low er values and appear wider,which is due to some disorientations of the graphite sheets when EG was modified by the silicacoating[14].According to the data calculated from the(002)diffraction peaks(Table 1),there is a certain increase of 0.0003 nm in the interlayer spaces of EG sample.Moreover,the intensities of the tw o EG characteristic peaks are dramatically declined in SEG composite because of the coated amorphous silica in the pores of EG.
SEM images of SEG clearly demonstrate that the silicalayer is steadily and uniformly grown on the surface of the graphite slices in SEG composite(Fig.1b).Moreover,the grow n silica on EG is not a single layer but multiple layers.Small irregular silica particles can be observed on the surface of the SEG sample.The thickness of the silica-covered carbon layer in SEG is nearly 50-75 nm(Fig.1b,insert).TEM results show the presence of both long-range ordered and less-ordered mesopores in SEG composite(Fig.1c-1e).Both twisting worm-hole like pores(Fig.1d)and straight channels(Fig.1e)are visible in the TEM images of SEG.The main pore size of the SEG composite observed from TEM images is around 7.05 nm.In addition,TEM image also con firms that the mesoporous silica layer is stably and regularly grow n on the surface of graphite slice in SEG(Fig.1c).
In order to investigate the formation process of such interesting silica-carbon composite,SEG composites with different SiO2/C molar ratio(rSiO2/C=0.96,0.48,0.24 and 0.16)were carefully synthesized and examined by SEM.It is show n that EG is composed of thin smooth graphite slices and these graphite slices are curved and loosely connected to each other(Fig.2a).The thin graphite flakes of EG became thicker w hen they were modified by silica coatings(Fig.2b-2e).Furthermore,the thickness of the silica layer decreases according to the reduction of the rSiO2/Cin the initial reaction solution(Fig.2b-2e).The amount of the silica coating in SEG plays very important role in improving the adsorption property of the SEG composite.N2physisorption results will give more information about the SEG composites synthesized with different rSiO2/C.
Fig.3 presents the N2adsorption-desorption isotherms and corresponding BJH pore size distributions of EGand SEG composites with different SiO2coatings.EG has a type II isotherm according to the IUPAC classification,which is typical for powders with large pores[20].The hysteresis loop of EG is identified as type B of de Boer classification,associating with slits shaped pores[20].The N2adsorption-desorption isotherms of SEG composites are type IV with H3 hysteresis loops(Fig.3a,curve a-d),which are obviously different from that of EG.All SEG composites show a one-step capillary condensation,which indicates uniform mesopores,while a two-step capillary evaporation is observed in the desorption branches,which are typical characteristics for plugged mesoporous silica materials[21].The special tw o-step branched desorption isotherm is inconspicuous in sample with rSiO2/Cof 0.16 but obvious in other SEG composites.The plugs/constrictions in the SEG composites are related to the aluminum species and the weak acidities of the original reaction mixtures[21].During the synthesis of mesoporous silica,the hydrolysis and condensation of siliceous source can be slow ed under w eak acidic conditions near the isoelectric point of silica.As a result,the transition process of micelles'‘sphere-torod’will take longer and plugs and/or constrictions are easily formed in the final mesopores if large amounts of salts existed in the reaction system[21].In present study,the presence of plenty aluminum chloride leads to the generation of weak acidic reaction condition and the formation of constrictions/plugs in the mesopores.Furthermore,a distinctive limitless adsorption of SEG at high relative pressures can be observed,indicating the presence of macropores.
According to the BJH analysis,the SEG composite with the highest SiO2content(rSiO2/C=0.96)has a much narrower pore size distribution(PSD)curve than the other SEG composites(Fig.3b).All SEG composites have the same PSD centered at 7.05 nm,which is approximately consistent with the above-mentioned TEM results.

Fig.1.XRD patterns(a),SEM images(b)and TEM images(c-e)of the mesoporous silica coated SEG composite(r SiO2/C=0.96).

Table 1 Crystalline parameters of EG and SEG calculated from the XRD patterns
Table 2 gives the physisorption results of expanded graphite(EG)and the mesoporous silica-carbon SEG composites with different amounts of silica coatings.EG has a very small surface area of about 20 m2·g-1and pore volume of 0.11 cm3·g-1.BET surface and pore volume of EG are dramatically increased when the mesoporous silica layer was coated onto the interconnected pores of EG.Consequently,the SEG composite with rSiO2/Cof 0.16 has surface area of 366 m2·g-1and pore volume of 0.37 cm3·g-1,respectively,which are much higher than those of EG(Table 2).It should be pointed that the BET surface area,pore volume and micropore surface area of the SEG composite raise according to the increase in the SiO2amount,demonstrating that mesoporous silica makes chief contributions to the high surface area and large pore volume of the composites.Moreover,all SEG samples have considerable micropore surface of 186-432 m2·g-1,indicating the presence of large amounts of micropores in the SEG composites(Table 2).The primary pore size of SEG does not change with the increase of rSiO2/C,con firming that the mesopore of the SEG samples is mainly generated from the silica coatings.
The structural changes in SEG composites were also illustrated by FT-IR results(Fig.4).EG presents tw o absorption bands centered at 3452 and 1623 cm-1,which are related to the water molecules.Four new bands at ca.460,800,955 and 1075 cm-1appear in the spectra of SEG composites,which are assigned to Si-O-Si bending,Si-O-Si symmetric stretching,terminal Si-OH groups and Si-O-Si asymmetric stretching,respectively[22].It is noticeable that all SEG composites display similar FT-IRspectra with typical patterns for mesoporoussilica.The intensities of these bands corresponding to silica enhance a bit according to the increase in silica amount,indicating the successful formation of silica-carbon composites.This is also consistent with the SEM results w e mentioned above.
3.2.Adsorption performance of the mesoporous SEG composites
Fig.5 presents the adsorption isotherms of MB on EG and SEG composites.The adsorbed amounts of MB on all SEG composites are much higher than that on EG,which suggest that the coated mesoporoussilica in SEG can greatly improve the adsorption capacity of the material.The adsorption data for MB onto EG and SEG samples were analyzed by Langmuir and Freundlich equations to investigate the relationship between the adsorbate molecules and adsorbent in solutions[23].The Langmuir adsorption model is based on the assumption that adsorption takes place on homogeneous surface,while the Freundlich model is an empirical equation employed to describe heterogeneous system.Both isotherm models are represented as following equations:
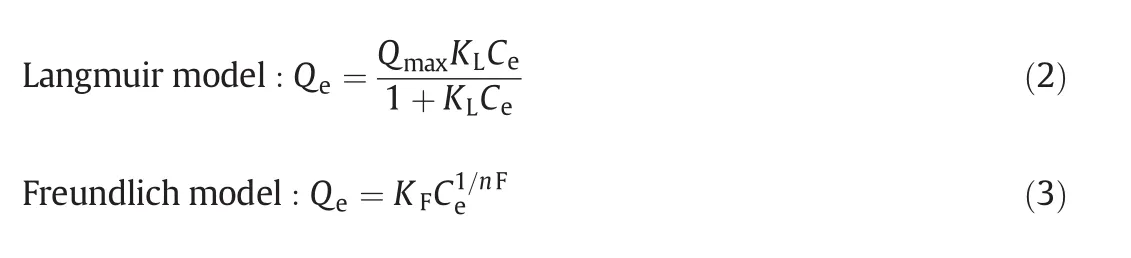
w here Qmax(mg·g-1)represents the saturated(or maximum)adsorption capacity of adsorbent,KL(L·mg-1)is the Langmuir binding constant.KF[mg·g-1·(L·mg-1)1/n]and 1/nFare Freundlich constants,indicator of adsorption capacity and adsorption intensity,respectively.The values of Qmax,KL,KF,1/nFand the linear regression correlations for Langmuir(RL2)and Freundlich(RF2)isotherms are given in Table 3.
It is clear that isotherm of MB adsorption on EG can be fitted well by two isotherm models(Table 3).How ever,the Langmuir model with the higher correlation coefficients is more suitable than the Langmuir model to describe the adsorption isotherms of SEG composites(RL2>0.93,RF2=0.614-0.793).It implies that the surfaces of SEG composites are more homogeneous than heterogeneous,and the accessible sorption sites of SEG are probably identical and energetically equivalent to the MB molecules during the adsorption[21].The Langmuir constant KLand calculated monolayer capacity Qmaxof the SEG samples are also listed in Table 3.All mesoporous SEG composites show much larger KLvalues than the parent EG,mirroring the improved adsorption intensity(Table 3).Similar to the experimental results,the calculated Qmaxenhances according to the increase in the rSiO2/Cof SEG and the sample with the highest silica content(rSiO2/C=0.96)has the highest Qmaxthan the other adsorbents.

Fig.2.SEM images of EG(a)and SEG composites synthesized with different r SiO2/C:0.96(b),0.84(c),0.24(d)and 0.16(e).

Fig.3.N2 adsorption-desorption isotherms(a)and pore size distributions(b)of EG and the mesoporous silica coated SEG composites synthesized with different r SiO2/C:0.16(a),0.24(b),0.48(c)and 0.96(d).Curves are offset for clarify.

Table 2 N2 physisorption results of EG and SEG composites
If compare the saturated adsorption capacity(Qmax)of EG(12.4 mg·g-1)w ith those of SEG composites(43.8-59.7 mg·g-1),it is reasonable to conclude that the silica layers in SEG contributes most to the adsorption abilities of the composites,for the sample with relative low silica content(rSiO2/C=0.16)still has a much higher Qmax(43.8 mg·g-1)than EG.Moreover,the adsorption capacity of SEGvaries nonlinearly according to the silica content in SEG.The Qmaxof SEGcomposite alters from 43.8,48.9,51.8 to 59.7 mg·g-1when silicacontent in SEG changes from rSiO2/C=0.16,0.24,0.48 to 0.96.The adsorption amount of MB by SEG(0.96)is about 1.4 times than that by SEG(0.16)while silica content in SEG(0.96)is 5 times higher than that in SEG(0.16).Actually,besides the mesoporous silica layer,the micrometer pores of EG also play important roles for improving the adsorption property of the hierarchical SEG composites.For those four SEG composites with different silica contents,probably the surface areas affect their adsorption capacities strongly.SEG(0.96)w ith the largest surface area(609 m2·g-1)has the highest Qmax(59.7 mg·g-1)while SEG(0.16)with the smallest surface area(366 m2·g-1)presents the low est Qmax(43.8 mg·g-1).Similarly,Qmaxfor SEG(0.24)and SEG(0.48)(which have the surface areas of 427 and 483 m2·g-1)are 48.9 and 51.8 mg·g-1,respectively.

Fig.4.FI-IR spectra of EG and the silica coated SEG composites synthesized with different r SiO2/C:0.16(a),0.24(b),0.48(c)and 0.96(d).
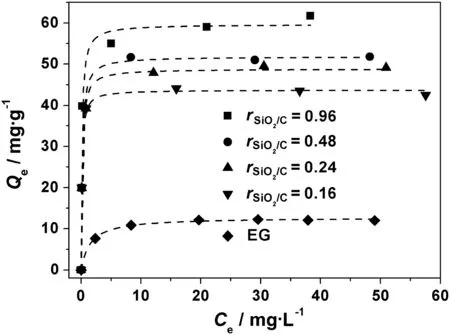
Fig.5.Adsorption isotherms of MB on EG and the SEG composites syn the sized with different r SiO2/C.
The kinetic behavior of MB adsorption on SEG composites was carried out in order to evaluate the effectiveness of the adsorbents and to gain insight into the underlying mechanisms.An extremely rapid uptake of MB during the initial stage(w ithin 5 min)of the adsorption process is observed when using EG as adsorbent.The adsorption equilibrium is established at about 60 min and there is no significant change in the absorbed MB amount after 2 h.The kinetic behavior of MB adsorption on SEG composites is similar to that on EG and there are very fast uptakes of MB during the first 5 min.After 5 min,the adsorption rates slow down as the sorption proceeded and the equilibrium were reached within 60 min(Fig.6).Adsorption kinetics of MB on EG and SEG were examined using a versatile pseudo-second-order model[24],which gives a linear form as follow:

w here k(g·g-1·min-1)is the rate constant of pseudo-second-order adsorption;Qt(g·mg-1)is the amount of MB adsorbed at time t(min).Rate parameters,k and Qe,can be directly calculated from the intercept and slope of the plot of(t/Qt)against time t.Fig.7 show s plots of pseudo-second-order kinetic model for adsorption of MB on EG and SEG composites.The kinetic parameters and linear coefficients R2obtained by fitting are listed in Table 4.
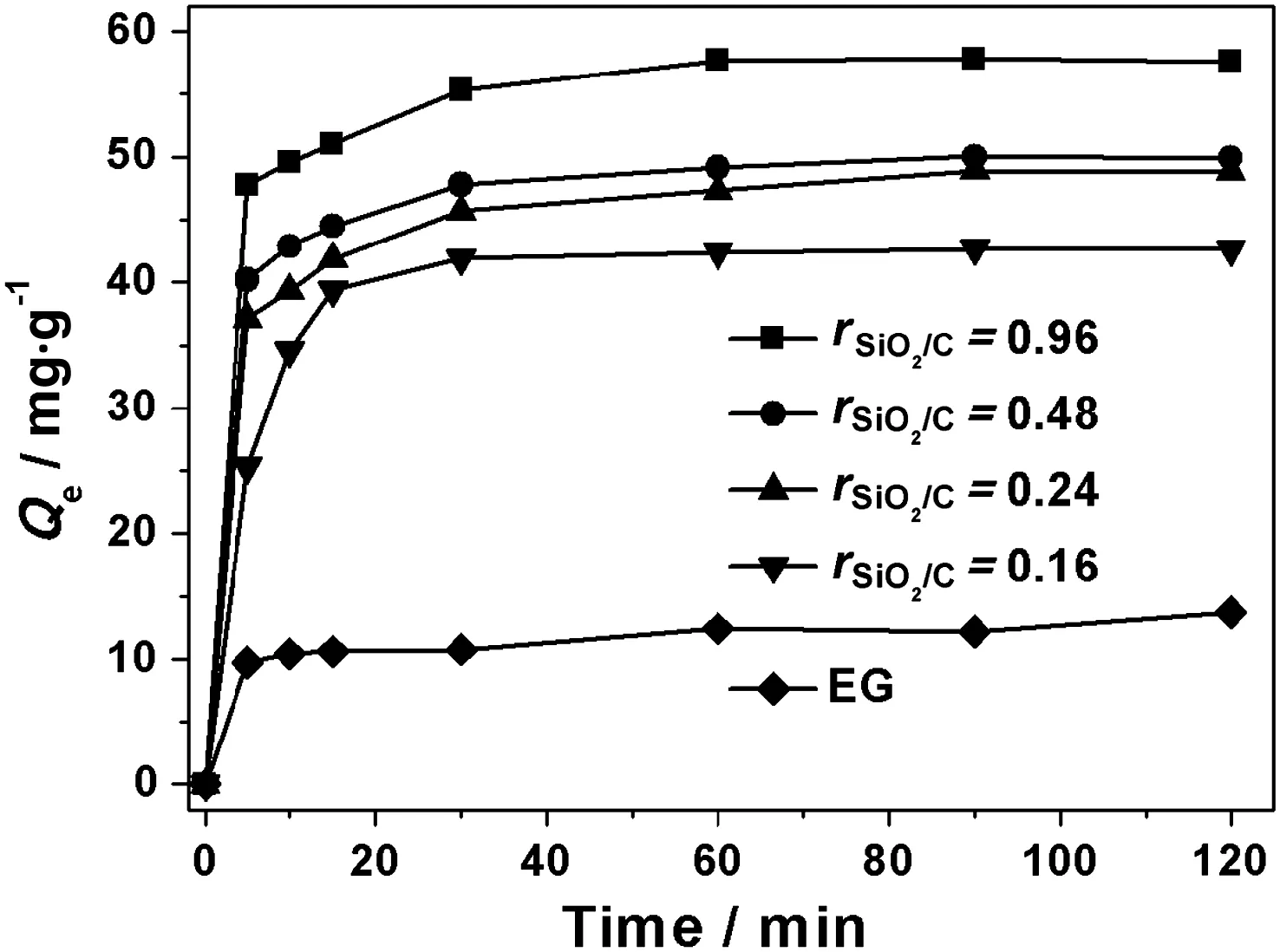
Fig.6.Kinetic study of MBabsorbs on EGand the SEG composites.(Adsorption conditions:initial MB concentration=80 mg·L-1;adsorbent dose=1 g·L-1).
It can be observed from Table 4 that the calculated Qevalues are identical to those obtained from experiments and the R2values for SEG are all greater than 0.999,suggesting the adsorption of MB onto SEG composites can be represented w ell by the pseudo-second-order model.The kinetic constants(k)over SEG composites are similar to that over EG.Among all silica-carbon composites,SEG with rSiO2/Cof 0.48 show s a bit faster adsorption rate than the others w hen the calculated kinetic constant(k)is compared.The adsorption data were also analyzed using Lagergren's pseudo- first-order kinetic model,but the values of correlation coefficient(R2)are low for most of the adsorption data,indicating that the adsorption process may not be fitted to the Lagergren's equation.
4.Conclusions
In this study,we successfully synthesized ahierarchical silica-carbon composite(SEG)by coating mesoporous silica layer onto expanded graphite(EG).The mesoporous silica can be stably grow n onto the surface of the graphite slices and the thickness of the silica increases with the increase in the SiO2/C ratio.The obtained SEG composite possesses both w ell-ordered hexagonal and less-ordered wormhole like mesostructures.The main pore size of SEG is 7.05 nm,which is generated from the silica coatings.

Fig.7.Liner plots for the pseudo-second-order kinetics of MB adsorption onto EG and the SEG composites.
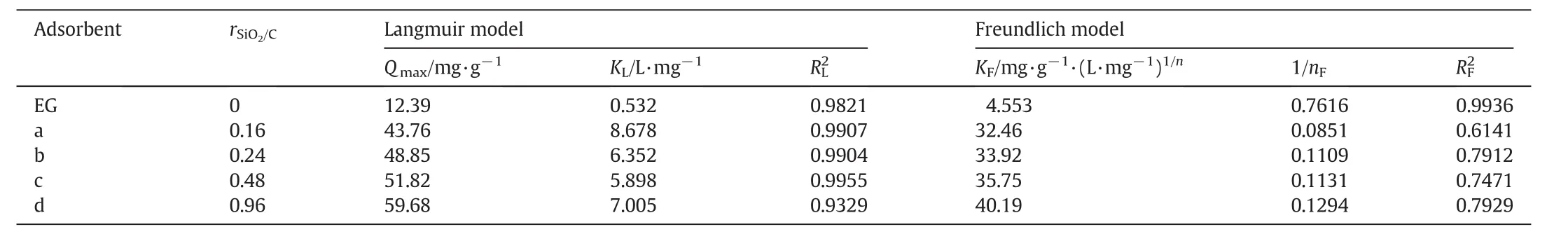
Table 3 Langmuir and Freundlich parameters for the adsorption of MB onto EG and SEG composites
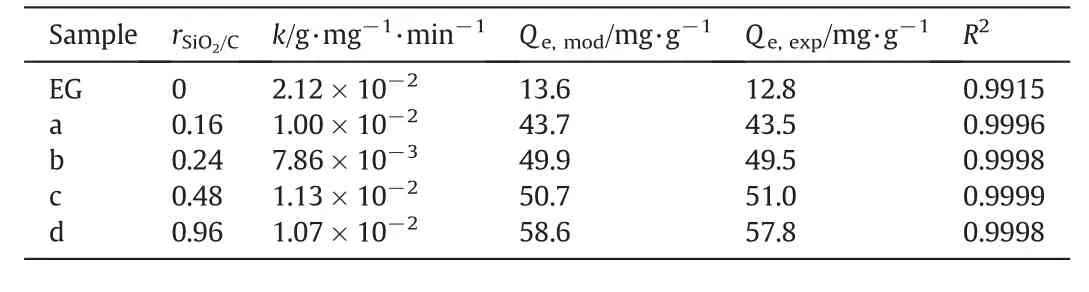
Table 4 Kinetic parameters calculated by the pseudo-second-order model for MB adsorption onto EG and the SEG composites
The novel SEG composites have much higher adsorption capacities to methylene blue(MB)than the pure carbonized EG.The MB removal percentage over the SEG composites increased according to the SiO2/C ratio.The sample with the highest silica content(rSiO2/C=0.96)can adsorb about 60 mg·g-1MB from the aqueous solution,which is 4-times larger than EG(~12 mg·g-1).The adsorption isotherm of MB over SEG composites could be fitted by Langmuir model and SEG composites also show rapid uptakes of MB in the kinetic studies.These resultant new silica-carbon composites may provide interesting possibilities for further application in the fast wastewater purification field.
Acknowledgments
The authors acknow ledge Prof.Yi Meng Wang in East China Normal University and Prof.Lin-Bing Sun in Nanjing Industrial University for the XRD and N2adsorption measurements.
[1]G.Crini,Non-conventional low-cost adsorbents for dye removal:A review,Bioresour.Technol.97(2006)1061-1085.
[2]M.J.Iqbal,M.N.Ashiq,Adsorption of dyes from aqueous solutions on activated charcoal,J.Hazard.Mater.B139(2007)57-66.
[3]E.Haque,J.W.Jun,S.H.Jhung,Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framew ork material,iron terephthalate(MOF-235),J.Hazard.Mater.185(2011)507-511.
[4]Sh.Sohrabnezhad,A.Pourahmad,E.Radaee,Photocatalytic degradation of basic blue 9 by CoS nanoparticles supported on AlMCM-41 material as a catalyst,J.Hazard.Mater.170(2009)184-190.
[5]L.Pereira,A.V.Coelho,C.A.Viegas,M.M.Correia dos Santos,M.P.Robalo,L.O.Martins,Enzymatic biotrans formation of the azo dye Sudan Orange G with bacterial CotA-laccase,J.Biotechnol.139(2009)68-77.
[6]D.Mohan,K.P.Singh,G.Singh,K.Kumar,Removal of dyes from wastewater using lf yash,a low-cost adsorbent,Ind.Eng.Chem.Res.41(2002)3688-3695.
[7]X.Yuan,S.-P.Zhuo,W.Xing,H.-Y.Cui,X.-D.Dai,X.-M.Liu,Z.-F.Yan,Aqueous dye adsorption on ordered mesoporous carbons,J.Colloid Interface Sci.310(2007)83-89.
[8]T.Wu,X.Cai,S.Tan,H.Li,J.Liu,W.Yang,Adsorption characteristics of acrylonitrile,p-toluenesulfonic acid,1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions,Chem.Eng.J.173(2011)144-149.
[9]S.R.Sandeman,V.M.Gun'ko,O.M.Bakalinska,C.A.Howell,Y.Zheng,M.T.Kartel,G.J.Phillips,S.V.Mikhalovsky,Adsorption of anionic and cationic dyes by activated carbons,PVA hydrogels,and PVA/AC composite,J.Colloid Interface Sci.358(2011)582-592.
[10]Y.Yin,P.Tan,X.Q.Liu,J.Zhu,L.B.Sun,Constructing con fined space in silica nanopores:An ideal platform for the formation and dispersion of cuprous sites,J.Mater.Chem.A 2(2014)3399-3406.
[11]Y.Yin,D.M.Xue,X.Q.Liu,G.Xu,P.Ye,M.Y.Wu,L.B.Sun,Unusual ceria dispersion formed in con fined space:A stable and reusable adsorbent for aromatic sulfur capture,Chem.Commun.48(2012)9495-9497.
[12]J.H.Li,L.L.Feng,Z.X.Jia,Preparation of sulfur-free expanded graphite with 320 μm mesh of flake graphite,Mater.Lett.60(2006)3927-3930.
[13]A.Celzard,J.F.Marêché,G.Furdin,Surface area of compressed expanded graphite,Carbon 40(2002)2713-2718.
[14]L.Zhong,X.Zhang,Y.Luan,G.Wang,Y.Feng,D.Feng,Preparation and thermal properties of porous heterogeneous composite phase change materials based on molten salts/expanded graphite,Sol.Energy 107(2014)63-73.
[15]Y.P.Zheng,H.N.Wang,F.Y.Kang,L.N.Wang,M.Inagaki,Sorption capacity of exfoliated graphite for oils-sorption in and among worm-like particles,Carbon 42(2004)2603-2607.
[16]W.Gao,L.B.Alemany,L.Ci,P.M.Ajayan,New insights into the structure and reduction of graphite oxide,Nat.Chem.1(2009)403-408.
[17]C.Liu,Z.Chen,X.Cheng,Z.Wang,X.Duan,Preparation and structure analysis of expanded graphite-based composites made by phosphoric acid activation,J.Porous.Mater.4(2010)425-428.
[18]A.D.Lueking,L.Pan,D.L.Narayanan,C.E.B.Clifford,Effect of expanded graphite lattice in exfoliated graphite nano fibers on hydrogen storage,J.Phys.Chem.B 109(2005)12710-12717.
[19]A.Yasmin,J.J.Luo,I.M.Daniel,Processing of expanded graphite reinforced polymer nanocomposites,Compos.Sci.Technol.66(2006)1182-1189.
[20]J.H.Han,K.W.Cho,K.H.Lee,H.Kim,Porous graphite matrix for chemical heat pumps,Carbon 36(1998)1801-1810.
[21]Z.Y.Wu,H.J.Wang,T.T.Zhuang,L.B.Sun,Y.M.Wang,J.H.Zhu,Multiple functionalization of mesoporous silica in one-pot:direct synthesis of aluminumcontaining plugged SBA-15 from aqueous nitrate solutions,Adv.Funct.Mater.18(2008)82-94.
[22]A.H.Karim,A.A.Jalil,S.Triwahyonoa,N.H.N.Kamarudinc,A.Ripin,Influence of multi-walled carbon nanotubes on textural and adsorption characteristics of in situ synthesized mesostructured silica,J.Colloid Interface Sci.421(2014)93-102.
[23]Z.Wu,Q.Lu,W.H.Fu,S.Wang,C.Liu,N.Xu,D.Wang,Y.M.Wang,Z.Chen,Fabrication of mesoporous Al-SBA-15 as a methylene blue capturer via a spontaneous in filtration route,New J.Chem.39(2015)985-993.
[24]L.L.Pérez,S.Perdriau,G.ten Brink,B.J.Kooi,H.J.Heeres,I.Melián-Cabrera,Stabilization of self-assembled alumina mesophases,Chem.Mater.25(2013)848-855.
 Chinese Journal of Chemical Engineering2016年1期
Chinese Journal of Chemical Engineering2016年1期
- Chinese Journal of Chemical Engineering的其它文章
- Scoping biology-inspired chemical engineering☆
- Review on the nanoparticle fluidization science and technology☆
- Multi-functional forward osmosis draw solutes for seawater desalination☆
- Bio-inspired enantioseparation for chiral compounds☆
- Process engineering in electrochemical energy devices innovation☆
- In-situ design and construction of lithium-ion battery electrodeson metal substrates with enhanced performances:A brief review☆
