Energetic Abilities of Solid Composite Propellants Based on 3,4,5-Trinitropyrazole and Ammonium Dinitramide
LEMPERT David B., CHUKANOV Nikita, SHU Yuan-jie
(1.Institute of Problems of Chemical Physics, Russian Academy of Sciences, Moscow 142432, Russia;2. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
Energetic Abilities of Solid Composite Propellants Based on 3,4,5-Trinitropyrazole and Ammonium Dinitramide
LEMPERT David B.1, CHUKANOV Nikita1, SHU Yuan-jie2
(1.Institute of Problems of Chemical Physics, Russian Academy of Sciences, Moscow 142432, Russia;2. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
Abstract:The investigation aims at the expansion of the basis of formulations of solid composite propellants by introducing new compositions with lower sensitivity to mechanic impact and improved thermal stability. The formulations based on trinitropyrazole (TNP) contains a binder (a hydrocarbon or active one), aluminum and inorganic oxidizer ADN. The results show that a binary formulation TNP + active binder (18%-19%)(volume fraction) with no metal is well designed which would achieve high specific impulse (atPc∶Pa=40∶1) of 248 s, high density of 1.80 g/cm3and combustion temperatureTcabout 3450 K. In terms of energy, metal-free compositions with TNP lose a bit to those with HMX, only if HMX fraction in formulation is higher than 45%-50%.
Keywords:solid composite propellants; specific impulse; 3,4,5- trinitropyrazole; TNP; binder; ammonium dinitramide; ADN
Biography:LEMPERT David B.(1942-), male, Ph.D, professor. Researcher field: Aerospace propultion. E-mail:lempert@icp.ac.ru
Introduction
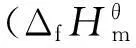
However, experimental value of impact sensitivity of TNP is unknown, but it is possible to be evaluated from the correlation with the value of so-called maximal explosion heat (Qmax)[3].Qmaxmay be simply calculated if one supposes that during the explosion all hydrogen is oxidized into water, when the residual oxygen oxidizes carbon atoms into CO2, and the residual carbon forms soot. It is shown that the impact sensitivity correlates rather well with the critical detonation diameter (Pcr). The latter correlates withQmaxaccording to the linear lawPcr=A+B·Qmax[3]. The smaller isQmax, the smaller is the impact sensitivity. Consequently, TNP withQmax= 371kJ/kg is less sensitive than HMX withQmax= 388kJ/kg.
In this work, the energetic abilities of solid composite propellants based on TNP have been studied and the optimal ratios of compounds to maximize the specific impulse and some other ballistic characteristics have been found.
1Computational
The calculations were carried out using the code TERRA[4]. The equations (1)-(3) list the relation of specific impulseIspand effective impulsesIef(n) atPc∶Pa=40∶1. This makes it possible to compare the compositions with different values ofIspand densitiesρwith regard to different stages of multistage rocket complexes[5]:
for the first stage
Ief(1) =Isp+ 100·(ρ- 1.9)
(1)
for the second stage
Ief(2) =Isp+ 50·(ρ- 1.8)
(2)
for the third stage
Ief(3) =Isp+ 25·(ρ- 1.7)
(3)
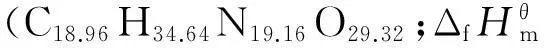

2Results and Discussions
2.1Energetic characteristics of composition based on HCB

(4)
(5)
(6)
Compositions based on HCB have too low energetic characteristics (Table 1, Fig.1), which is rather expected, because of low oxygen content in TNP.
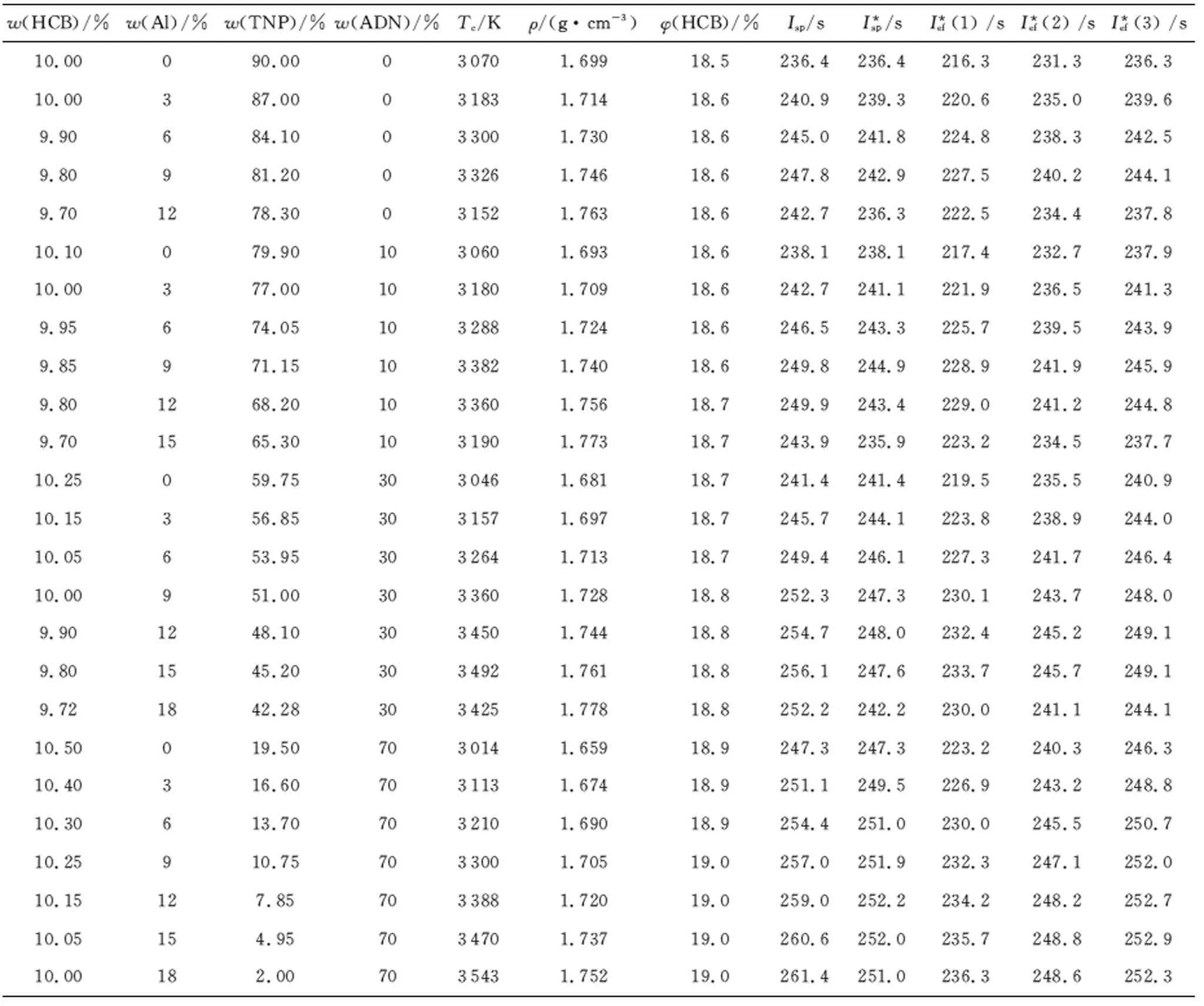
Table 1 Energetic characteristic of compositions based on HCB
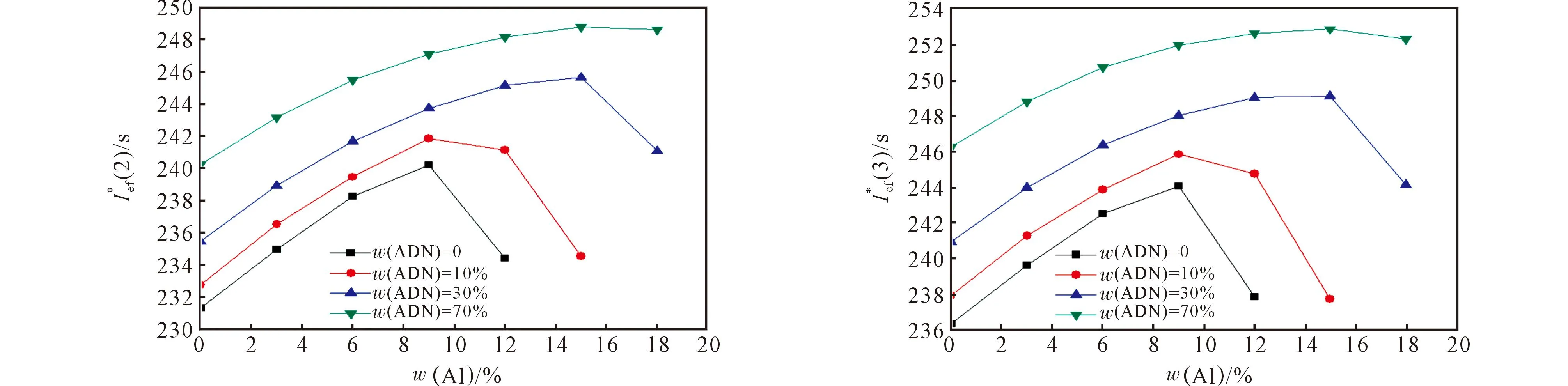
Fig.1 The values of(2) and(3) for the compositions based on HCB


2.2Energetic characteristics of composition based on AB
The situation changes if one uses an active binder AB instead of hydrocarbon binder (Table 2).
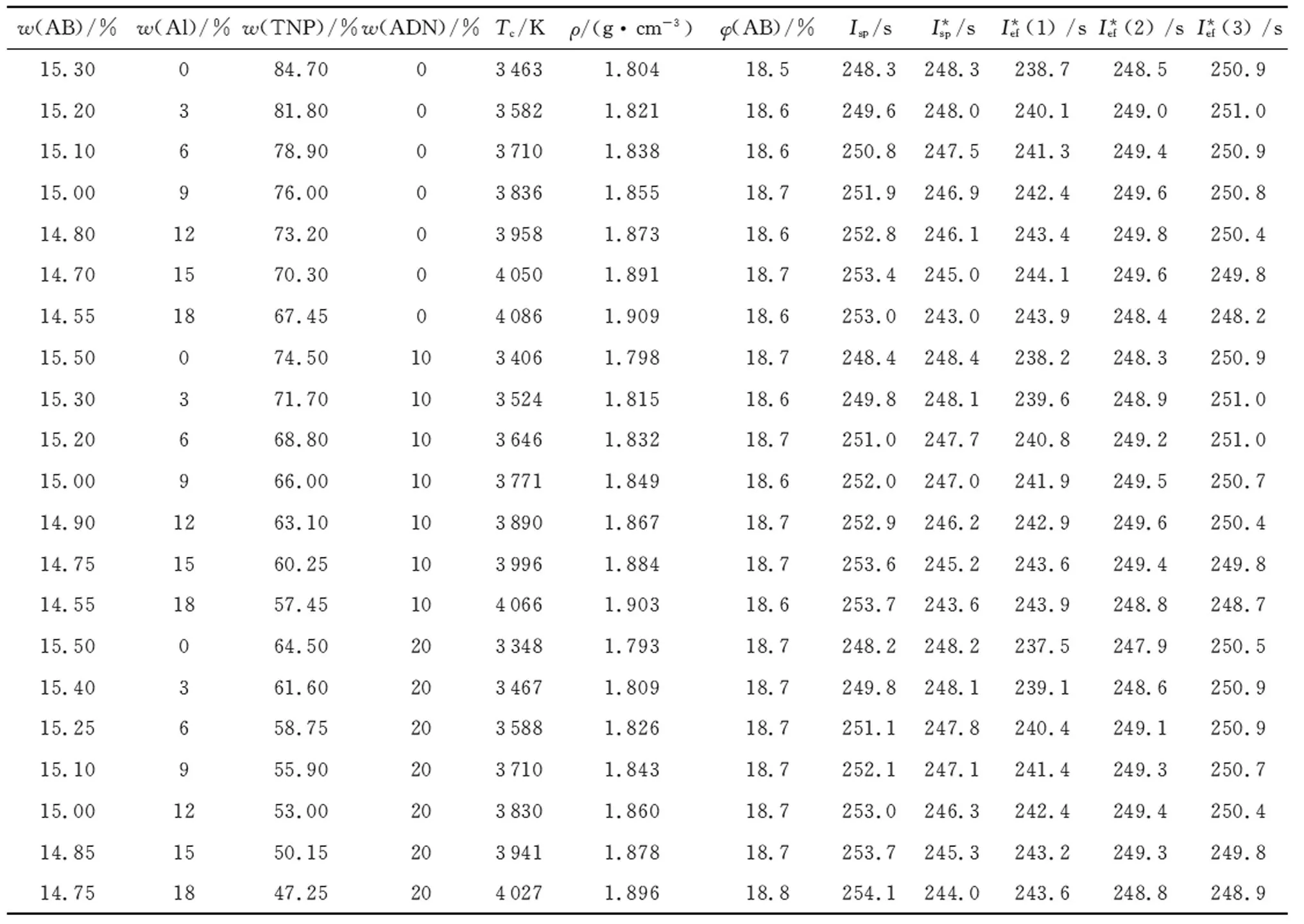
Table 2 Energetic characteristic of compositions based on AB

Attention should be paid to the fact that the TNP molecule contains one hydrogen atom with acid properties, pH=2.35[2]or even 0.05[9-10]. In order to estimate acid-base properties of TNP in crystalline state, its infrared spectrum has been obtained using a conventional technique of pelletization with KBr (Fig.3). The wavenumber of the band of N-H-stretching vibrations of solid TNP is 3145cm-1, which corresponds to a weak-acidic NH group. It can be expected that TNP will exhibit acidic properties and a tendency to form salts only in the presence of compounds or solvents with basic amine groups. Hydrocarbons and compounds with functional groups such as -NO2, -ONO2, -NNO2(e.g. usual active binders) would not react with TNP at ambient temperature.

Fig. 3 Infrared spectrum of TNP
3Conclusions
(1) A binary propellant based on TNP and active binder makes it possible to design a metal-free composition with the specific impulse value equal to 248.3s (atPc∶Pa=40∶1) at density of 1.805g/cm3, which is considerably higher than most of other oxidizers could provide for metal-free propellants.
(2) Using the formulaPcr=A+B·Qmax[3], one may expect that TNP is less sensitive to impact than HMX.
(3) Based on TNP infrared spectroscopic data, one can suppose that the presence of a weak-acidic NH group in TNP molecule would not result in essential problems in compatibility of TNP with other components.
Acknowledge
The investigation was supported by the Ministry of Education and Science of the Russian Federation by agreement of 10.11.2015 № 14.613.21.0043, a unique identifier RFMEFI61315X0043.
References:
[1]Konkova T S, Miroshnichenko E A, Matyushin Yu N, et al. Energies of salt formation of heterocyclic compounds [J]. Combustion and Explosion, 2015, 8(2): 175-185.
[2]Hervé G, Roussel C, Graindorge H. Selective preparation of 3,4,5-trinitro-1H-pyrazole: a stable all-carbon-nitrated arene [J]. Angewandte Chemie International Edition, 2010, 49(18): 3177-3181.
[3]Pepekin V I, Korsunskii B L, Denisaev A A. Initiation of solid explosives by mechanical impact [J]. Combustion, Explosion and Shock Waves, 2008, 44(5): 586-590.
[4]Lempert D, Nechiporeeko G, Manelis G. Influence of heat release value and gaseous combustion products content on energetic parameters of solid composite propellants .Theory and practice of energetic materials[C]∥Proceedings of the 2009 International Autumn Seminar on Propellants, Explosives and Pyrotechnics. Kunming: PEP, 2009, VIII: 234-243.
[5]Pavlovets G, Tsutsuran V. Physicochemical properties of powders and propellants [J]. Russian Ministry of Defense Publishing House, 2009: 408.
[6]Lempert D, Nechiporenko G, Manelis G. The outlook for the use of pseudopolymorphic solvates in energetic materials[J]. Central European Journal of Energetic Materials, 2014, 11(2): 219-235.
[7]Nedelko V V, Korsounskii B L, Chukanov N V, et al. Thermal decomposition of 1,3,3-trinitroazetidine in gas, solution and melt[C]∥Proceedings of the 37th International Annual Conference. Karlsruhe: ICT, 2006. 154/1-154/12.
[8]Lempert D, Nechiporenko G, Manelis G. The ways for development of environmentally safe solid composite propellants [J]. Progress in Propulsion Physics, 2009, 63-80.
[9]Dalinger I L, Vatsadze I A, Shkineva T K, et al. The specific reactivity of 3,4,5-trinitro-1H-pyrazole[J]. Mendeleev Communications, 2010, 20(5): 253-254.
[10]Dalinger I L, Vatsadze I A, Shkineva T K, et al. Synthesis and comparison of the reactivity of 3,4,5-1H-trinitropyrazole and its N-methyl derivative [J]. Journal of Heterocyclic Chemistry, 2013, 50(4): 911-924.
DOI:10.14077/j.issn.1007-7812.2016.03.003
Received date:2016-02-02;Revised date:2016-05-12
CLC number:TJ55;V512
Document Code:AArticle ID:1007-7812(2016)03-0017-04
Foundation:Ministry of Education and Science of the Russian Federation (14.613.21.0043)

