中国藤黄属植物中酮类化合物研究进展
王丽萍 付文卫 谭红胜 张 洪 徐宏喜
(1 上海中医药大学中药学院,上海,201203; 2 中药创新药物研发上海高校工程研究中心,上海,201203)
王丽萍1,2付文卫1,2谭红胜1,2张洪1,2徐宏喜1,2
(1 上海中医药大学中药学院,上海,201203; 2 中药创新药物研发上海高校工程研究中心,上海,201203)



1 中国藤黄属植物中酮类化合物的分布

2 藤黄属植物中酮类化合物的结构与分类


表1 中国藤黄属植物中的酮类化合物的分布

表1 中国藤黄属植物中的酮类化合物的分布(续)

表1 中国藤黄属植物中的酮类化合物的分布(续)

表1 中国藤黄属植物中的酮类化合物的分布(续)

表1 中国藤黄属植物中的酮类化合物的分布(续)

表1 中国藤黄属植物中的酮类化合物的分布(续)

表1 中国藤黄属植物中的酮类化合物的分布(续)
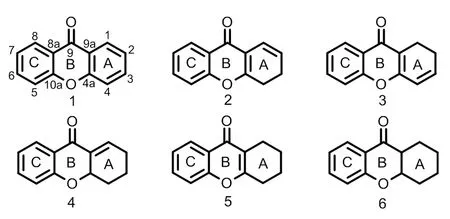
图1 酮类化合物的基本母核
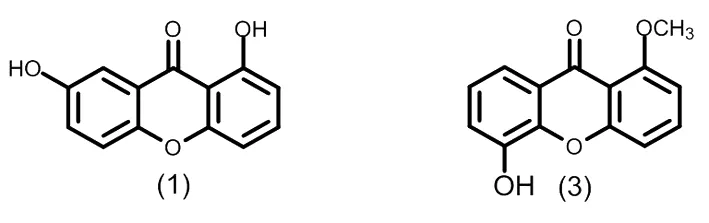
图2 典型的二氧化取代的酮类化合物
图3 典型的三氧化取代的酮类化合物
图4 典型的四氧化取代的酮类化合物
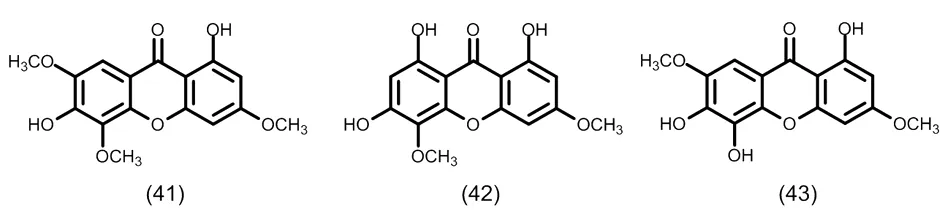
图5 典型的五氧化取代的酮类化合物

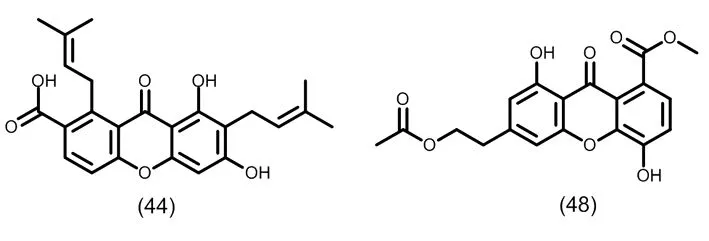
图6 典型的二氧化取代的异戊烯基酮类化合物
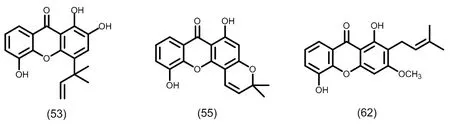
图7 典型的单异戊烯基取代的三氧化酮类化合物
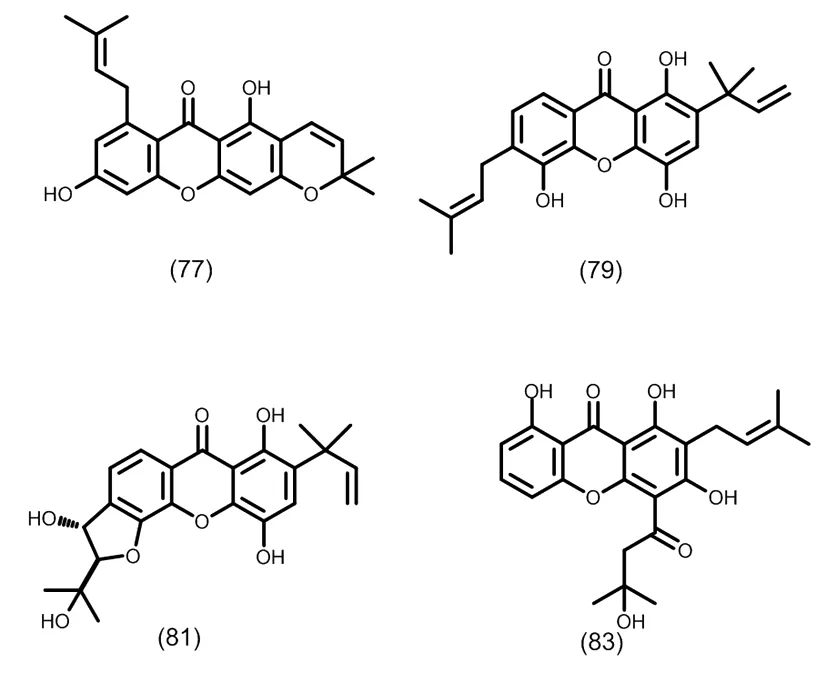
图8 典型的二异戊烯基取代的三氧化酮类化合物
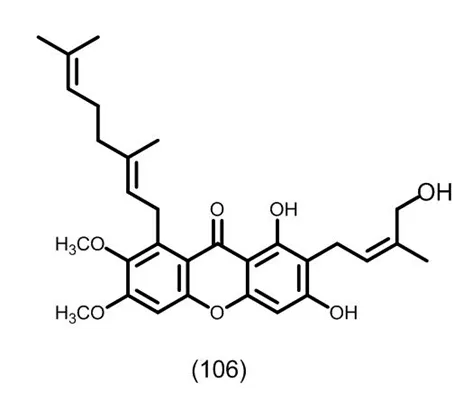
图9 典型的含有C10取代三氧化酮类化合物
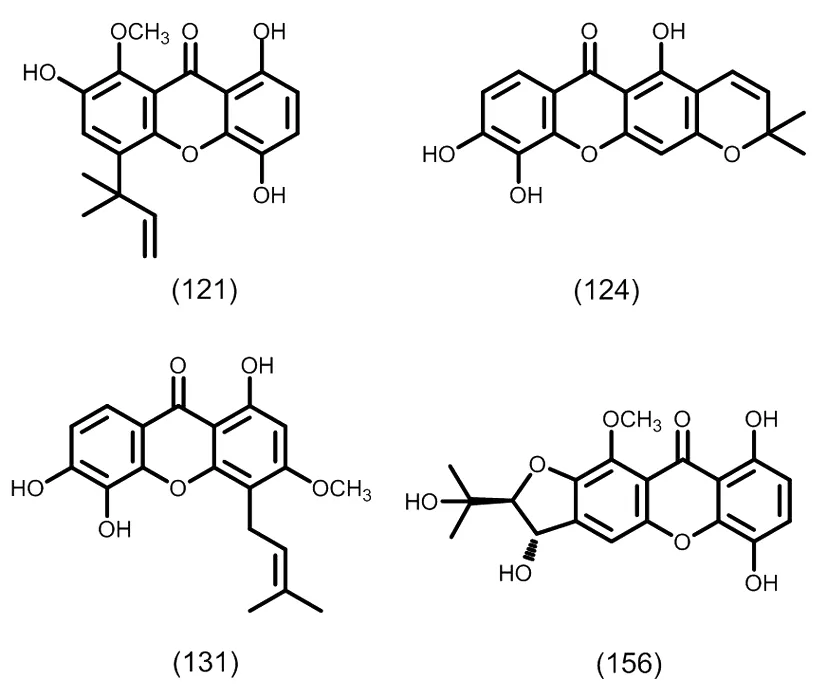
图10 典型的单异戊烯基取代的四氧化酮类化合物
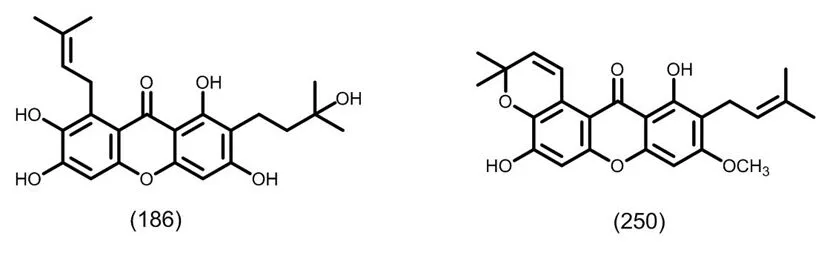
图11 典型的二异戊烯基取代的四氧化酮类化合物
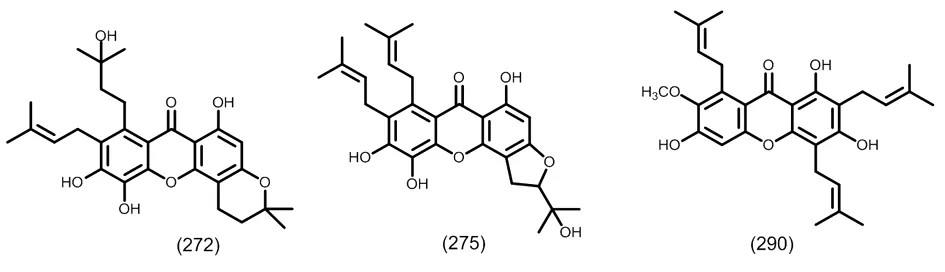
图12 典型的三异戊烯基取代的四氧化酮类化合物
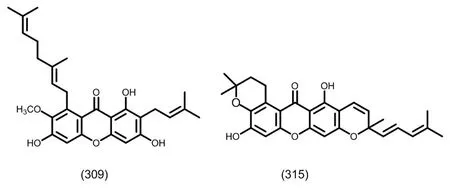
图13 典型的含有C10取代的四氧化酮
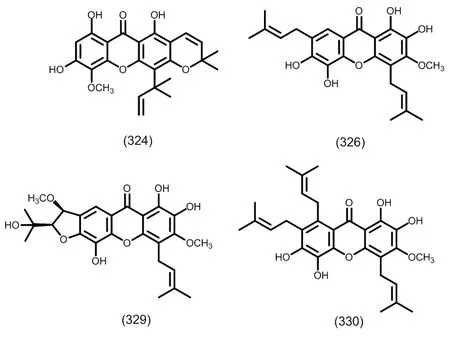
图14 典型的五氧化取代异戊烯基酮类化合物
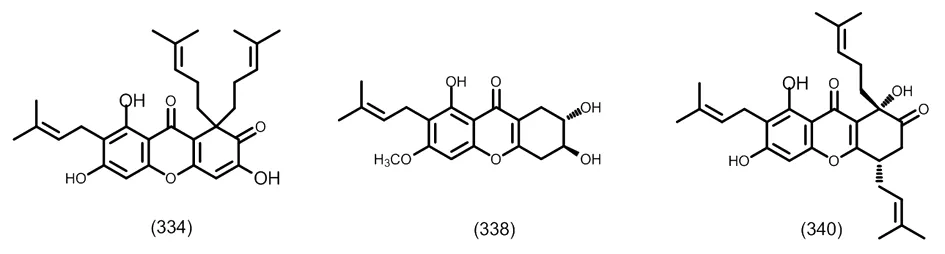
图15 典型的二氢及四氢酮类化合物
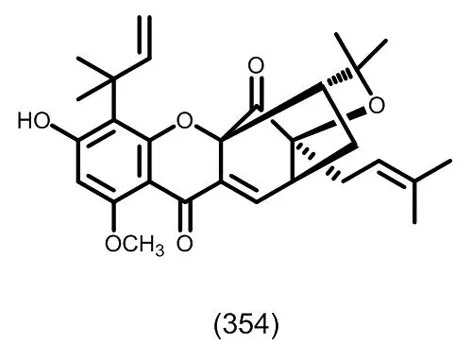
图16 典型的四氢笼状酮类化合物
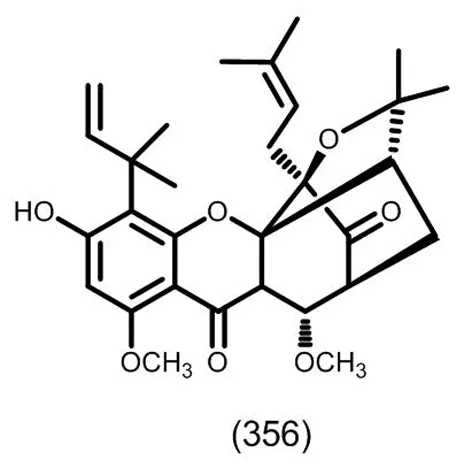
图17 典型的六氢酮类化合物
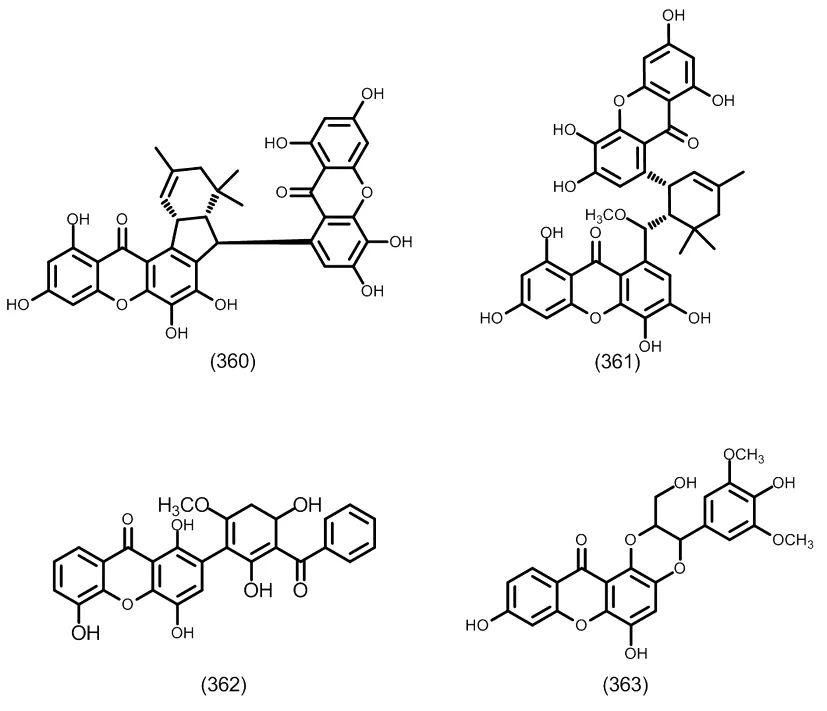
图18 典型的酮二聚体类化合物
3 酮类化合物波谱学特征[111]


3.3核磁共振氢谱[112]
3)甲氧基:甲氧基上的质子通常为3个质子的单峰,其化学位移多在δH3.7~3.8 ppm左右。
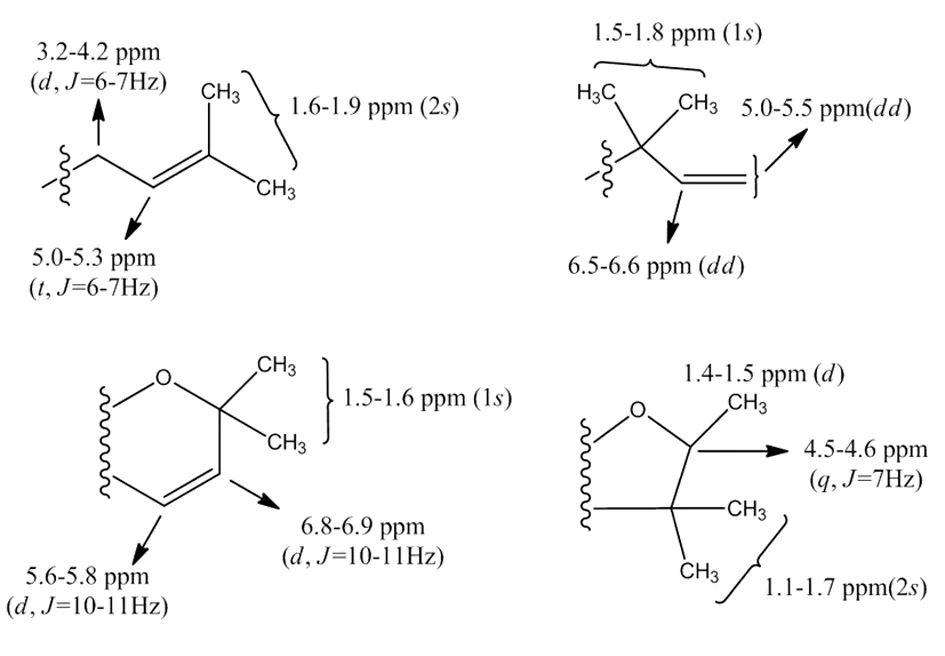
图19 常见异戊烯基上氢的化学位移值


3.4核磁共振碳谱[112]
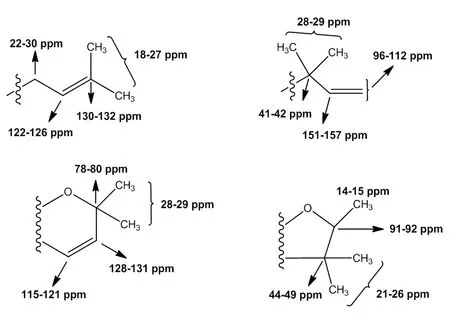
图20 不同类型异戊烯基上碳的化学位移值
4 提取与分离方法[1]




4)乙醇等有机溶剂提取法、HSCCC法分离纯化[115]:取干燥藤黄树脂2 kg,95%乙醇室温浸提5 d(4 L×5),浸提液减压浓缩得浸膏1 428 g。在TBE-1000A高速逆流色谱仪上采用n-hexane-ethyl acetate-methanol-water(7∶3∶8∶2,v/v/v/v)溶剂系统,溶剂系统的有机相中加入0.1 % trifluoroacetic acid,水相中加入0.03 % triethylamine,取3.157 g浸膏以30 mL的上相和10 mL下相的混合溶剂溶解上样,经一次分离,可得到1.134 g的 gambogic acid和180.5 mg的gambogenic acid,将其中的混合物进一步在TBE-300B的高速逆流色谱仪上分离,分别以n-hexane-ethyl acetate-methanol-water(5∶5∶10∶5,v/v/v/v)和n-hexane-methyl tert-butyl ether-acetonitrile-water(8∶2∶6∶4,v/v/v/v)为溶剂系统可以进一步分离得到11.6 mg的isogambogenic acid和10.4 mg的β-morellic acid。
6 结语

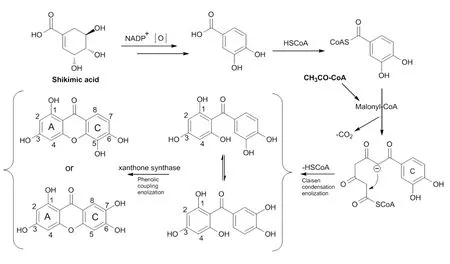
图21 藤黄属植物中酮类化合物可能的生物合成途经
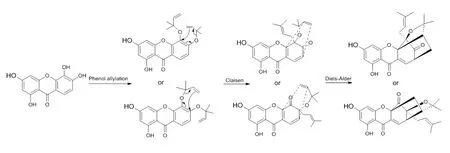
图22 藤黄属植物中笼状酮类化合物可能的生物合成途经
随着现代分析方法的发展和应用,我们课题组基于LC-MS的高效、快速分离分析方法也以用于中国藤黄属植物的化学成分研究,尤其是针对广西藤黄Garciniakwangsiensis及红萼藤黄Garciniarubrisepala等前期研究较少的中国藤黄属植物,以期发现更多结构新颖、活性强、不良反应小的先导化合物。同时,进一步进行结构修饰和优化等方面的研究工作,针对性地开展这些化合物的构-效关系及作用靶点研究,为新药开发奠定基础。
[1]El-Seedi HR,El-Ghorab DM,El-Barbary MA,et al.Naturally occurring xanthones; latest investigations:isolation,structure elucidation and chemosystematic significance[J].Curr Med Chem,2009,16(20):2581-2626.
[2]Vieira LM,Kijjoa A.Naturally-occurring xanthones:recent developments[J].Curr Med Chem,2005,12(21):2413-2446.
[3]El-Seedi HR,El-Barbary MA,El-Ghorab DM,et al.Recent insights into the biosynthesis and biological activities of natural xanthones[J].Curr Med Chem,2010,17(9):854-901.
[5]Baslas RK,Kumar P.Isolation and characterization of biflavanone and xanthones in the fruits ofGarciniaxanthochymus[J].Acta Cienc Indica,[Ser] Chem,1981,7(1-4):31-34.
[6]Chanmahasathien W,Li Y,Satake M,et al.Prenylated xanthones fromGarciniaxanthochymus[J].Chem Pharm Bull,2003,51(11):1332-1334.
[7]Chanmahasathien W,Li Y,Satake M,et al.Prenylated xanthones with NGF-potentiating activity fromGarciniaxanthochymus[J].Phytochemistry,2003,64(5):981-986.
[8]Chen Y,Fan H,Yang GZ,et al.Prenylated xanthones from the bark ofGarciniaxanthochymusand their 1,1-diphenyl-2-picrylhydrazyl(DPPH)radical scavenging activities[J].Molecules,2010,15:7438-7449.
[9]Chen Y,Fan H,Yang GZ,et al.Two Unusual Xanthones from the Bark ofGarciniaxanthochymus[J].Helv Chim Acta,2011,94(4):662-668.
[10]Chen Y,Yang GZ,Zhong FF,et al.Two new prenylated xanthones from the bark ofGarciniaxanthochymus[J].Bull Korean Chem Soc,2010,31(11):3418-3420.
[11]Chen Y,Zhong F,He H,et al.Structure elucidation and NMR spectral assignment of five new xanthones from the bark ofGarciniaxanthochymus[J].Magn Reson Chem,2008,46(12):1180-1184.
[12]Han QB,Yang NY,Tian HL,et al.Xanthones with growth inhibition against HeLa cells fromGarciniaxipshuanbannaensis[J].Phytochemistry,2008,69(11):2187-2192.
[13]Ji F,Li Z,Liu G,et al.Xanthones with antiproliferative effects on prostate cancer cells from the stem bark ofGarciniaxanthochymus[J].Nat Prod Commun,2012,7(1):53-56.
[14]Trisuwan K,Boonyaketgoson S,Rukachaisirikul V,et al.Oxygenated xanthones and biflavanoids from the twigs ofGarciniaxanthochymus[J].Tetrahedron Lett,2014,55(26):3600-3602.
[15]Zhong F,Chen Y,Song F,et al.Three new xanthones fromGarciniaxanthochymus[J].Yaoxue Xuebao,2008,43(9):938-941.
[16]Zhong F,Chen Y,Wang P,et al.Xanthones from the bark ofGarciniaxanthochymusand their 1,1-diphenyl-2-picrylhydrazyl radical-scavenging activity[J].Chin J Chem,2009,27(1):74-80.
[17]Zhong FF,Chen Y,Mei ZN,et al.Xanthones from the bark ofGarciniaXanthochymus[J].Chin Chem Lett,2007,18(7):849-851.
[18]Zhong FF,Chen Y,Yang GZ.Chemical constituents from the bark ofGarciniaxanthochymusand their 1,1-diphenyl-2-picrylhydrazyl(DPPH)radical-scavenging activities[J].Helv Chim Acta,2008,91(9):1695-1703.
[19]Abe F,Nagafuji S,Okabe H,et al.Trypanocidal constituents in plants 2.Xanthones from the stem bark ofGarciniasubelliptica[J].Biol Pharm Bull,2003,26(12):1730-1733.
[20]Fukuyama Y,Kamiyama A,Mima Y,et al.Prenylated xanthones fromGarciniasubelliptica[J].Phytochemistry,1991,30(10):3433-3436.
[21]Fukuyama Y,Minami H,Kinoshita M,et al.Chemical constituents ofGarciniasubellipticaand their biological activities[J].Tennen Yuki Kagobutsu Toronkai Koen Yoshishu,1997(39):577-582.
[22]Fukuyama Y,Mitsunami H,Yoshizawa T,et al.Xanthone derivatives fromGarciniasubellipticaand active oxygen scavengers containing them.JP08193029A[P/OL].1996-07-30.
[23]Iinuma M,Tosa H,Tanaka T,et al.Two xanthones with a 1,1-dimethylallyl group in root bark ofGarciniasubelliptica[J].Phytochemistry,1995,39(4):945-947.
[24]Iinuma M,Tosa H,Tanaka T,et al.three xanthones from root bark ofGarciniasubelliptica[J].Phytochemistry,1995,38(1):247-249.
[25]Iinuma M,Tosa H,Tanaka T,et al.Two new xanthones from the root bark ofGarciniasubelliptica[J].Heterocycles,1995,40(1):279-284.
[26]Iinuma M,Tosa H,Tanaka T,et al.Two xanthones from root bark ofGarciniasubelliptica[J].Phytochemistry,1994,35(5):1355-1360.
[27]Minami H,Hamaguchi K,Kubo M,et al.A benzophenone and a xanthone fromGarciniasubelliptica[J].Phytochemistry,1998,49(6):1783-1785.
[28]Minami H,Kinoshita M,Fukuyama Y,et al.Antioxidant xanthones fromGarciniasubelliptica[J].Phytochemistry,1994,36(2):501-506.
[29]Minami H,Kuwayama A,Yoshizawa T,et al.Novel prenylated xanthones with antioxidant property from the wood ofGarciniasubelliptica[J].Chem Pharm Bull,1996,44(11):2103-2106.
[30]Minami H,Takahashi E,Fukuyama Y,et al.Novel xanthones with superoxide scavenging activity fromGarciniasubelliptica[J].Chem Pharm Bull,1995,43(2):347-349.
[31]Minami H,Takahashi E,Kodama M,et al.Three xanthones fromGarciniasubelliptica[J].Phytochemistry,1996,41(2):629-633.
[32]Jing WY,Jiang C,Ji F,et al.Chemical constituents from the stem barks ofGarciniamultiflora[J].J Asian Nat Prod Res,2013,15(11):1152-1157.
[33]Chiang YM,Kuo YH,Oota S,et al.Xanthones and benzophenones from the stems ofGarciniamultiflora[J].J Nat Prod,2003,66(8):1070-1073.
[34]Chen FC,Lin YM,Hung JC.Phenolic compounds from the heartwood ofGarciniamultiflora[J].Phytochemistry,1975,14(1):300-303.
[35]Xu G,Feng C,Zhou Y,et al.Bioassay and Ultraperformance Liquid Chromatography/Mass Spectrometry Guided Isolation of Apoptosis-Inducing Benzophenones and Xanthone from the Pericarp ofGarciniayunnanensisHu[J].J Agric Food Chem,2008,56(23):11144-11150.
[36]Vo HT,Nguyen N-TT,Maas G,et al.Xanthones from the bark ofGarciniapedunculata[J].Phytochem Lett,2012,5(4):766-769.
[37]Vo HT,Ngo NT,Bui TQ,et al.Geranylated tetraoxygenated xanthones from the pericarp ofGarciniapedunculata[J].Phytochem Lett,2015,13:119-122.
[38]Rao AVR,Sarma MR,Venkataraman K,et al.Benzophenone and xanthone with unusual hydroxylation patterns from the heartwood ofGarciniapedunculata[J].Phytochemistry,1974,13(7):1241-1244.
[39]Na Z,Xu Y.Chemical constituents from twigs ofGarciniaxipshuanbannaensis[J].Zhongguo Zhongyao Zazhi,2009,34(18):2338-2342.
[40]Na Z,Xu YK.A new prenylated xanthone fromGarciniaxipshuanbannaensisY.H.Li[J].Nat Prod Res,2010,24(17):1648-1653.
[41]Zhou Y,Han QB,Song JZ,et al.Characterization of polyprenylated xanthones inGarciniaxipshuanbannaensisusing liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry[J].J Chromatogr A,2008,1206(2):131-139.
[42]Balasubramanian K,Rajagopalan K.Studies of indigenous medicinal plants.Part 1.Novel xanthones fromGarciniamangostana,structures of BR-xanthone-A and BR-xanthone-B[J].Phytochemistry,1988,27(5):1552-1554.
[43]Balunas MJ,Su B,Brueggemeier RW,et al.Xanthones from the Botanical Dietary Supplement Mangosteen(Garciniamangostana)with Aromatase Inhibitory Activity[J].J Nat Prod,2008,71(7):1161-1166.
[44]Bumrungpert A,Kalpravidh RW,Chitchumroonchokchai C,et al.Xanthones from mangosteen prevent lipopolysaccharide-mediated inflammation and insulin resistance in primary cultures of human adipocytes[J].J Nutr,2009,139(6):1185-1191.
[45]Bumrungpert A,Kalpravidh RW,Chuang CC,et al.Xanthones from mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media[J].J Nutr,2010,140(4):842-847.
[46]Chae HS,Kim EY,Han L,et al.Xanthones with pancreatic lipase inhibitory activity from the pericarps ofGarciniamangostanaL.(Guttiferae)[J].Eur J Lipid Sci Technol,2016,:Ahead of Print.
[47]Chin YW,Jung HA,Chai H,et al.Xanthones with quinone reductase-inducing activity from the fruits ofGarciniamangostana(Mangosteen)[J].Phytochemistry,2008,69(3):754-758.
[48]Ee GCL,Daud S,Taufiq-Yap YH,et al.Xanthones fromGarciniamangostana(Guttiferae)[J].Nat Prod Res,2006,20(12):1067-1073.
[49]Govindachari TR,Kalyanaaman PS,Muthukumaraswamy N,et al.Isolation of three new xanthones fromGarciniamangostana[J].Indian J Chem,1971,9(5):505-506.
[50]Mahabusarakam W,Wiriyachitra P,Phongpaichit S.Antimicrobial activities of chemical constituents fromGarciniamangostanaLinn[J].J Sci Soc Thailand,1986,12(4):239-243.
[51]Nilar,Harrison LJ.Xanthones from the heartwood ofGarciniamangostana[J].Phytochemistry,2002,60(5):541-548.
[52]Nilar,Nguyen L-HD,Venkatraman G,et al.Xanthones and benzophenones from Garcinia griffithii andGarciniamangostana[J].Phytochemistry(Elsevier),2005,66(14):1718-1723.
[53]Parveen M,Ud-din Khan N.Two xanthones fromGarciniamangostana[J].Phytochemistry,1988,27(11):3694-3696.
[54]Sen AK,Sarkar KK,Majumder PC,et al.Isolation of three new minor xanthones fromGarciniamangostanaLinn[J].Indian J Chem,Sect B,1980,19B(11):1008.
[55]Suksamrarn S,Suwannapoch N,Ratananukul P,et al.Xanthones from the green fruit hulls ofGarciniamangostana[J].J Nat Prod,2002,65(5):761-763.
[56]Wang JJ,Sanderson BJ,Zhang W.Cytotoxic effect of xanthones from pericarp of the tropical fruit mangosteen(GarciniamangostanaLinn.)on human melanoma cells[J].Food Chem Toxicol,2011,49(9):2385-2391.
[57]Fan QF,Na Z,Hu HB,et al.Chemical constituents fromGarciniabracteataand ultra performance liquid chromatography/mass spectrometry guided isolation of tautomers[J].Tianran Chanwu Yanjiu Yu Kaifa,2012,24(8):1055-1059,1074.
[58]Hu Q,Niu D,Li X,et al.New xanthones fromGarciniabracteataand their cytotoxicities[J].Heterocycles,2013,87(5):1127-1132.
[59]Na Z,Hu HB,Fan QF.A novel caged-prenylxanthone fromGarciniabracteata[J].Chin Chem Lett,2010,21(4):443-445.
[60]Na Z,Hu HB,Fan QF.Three New Caged Prenylxanthones fromGarciniabracteata[J].Helv Chim Acta,2010,93(5):958-963.
[61]Na Z,Hu HB,Xu YK.Cytotoxic caged xanthones from the fruits ofGarciniabracteata[J].Chem Nat Compd,2013,49(3):505-506.
[62]Niu SL,Li ZL,Ji F,et al.Xanthones from the stem bark ofGarciniabracteatawith growth inhibitory effects against HL-60 cells[J].Phytochemistry,2012,77:280-286.
[63]Thoison O,Cuong DD,Gramain A,et al.Further rearranged prenylxanthones and benzophenones fromGarciniabracteata[J].Tetrahedron,2005,61(35):8529-8535.
[64]Thoison O,Fahy J,Dumontet V,et al.Cytotoxic Prenylxanthones fromGarciniabracteata[J].J Nat Prod,2000,63(4):441-446.
[65]Fan Q,Na Z,Hu H,et al.Chemical constituents from stem barks ofGarciniapaucinervis[J].Zhongcaoyao,2012,43(3):436-439.
[66]Li DH,Li CX,Jia CC,et al.Xanthones fromGarciniapaucinerviswith in vitro anti-proliferative activity against HL-60 cells[J].Arch Pharm Res,2016,39(2):172-177.
[67]Wu YP,Zhao W,Xia ZY,et al.Three novel xanthones fromGarciniapaucinervisand their anti-TMV activity.Molecules,2013,18:9663-9669.
[68]Lu Y,Cai S,Nie J,et al.The natural compound nujiangexanthone A suppresses mast cell activation and allergic asthma.Biochem Pharmacol,2016,100:61-72.
[69]Tang ZY,Xia ZX,Qiao SP,et al.Four new cytotoxic xanthones fromGarcinianujiangensis[J].Fitoterapia,2015,102:109-114.
[70]Xia ZX,Zhang DD,Liang S,et al.Bioassay-Guided Isolation of Prenylated Xanthones and Polycyclic Acylphloroglucinols from the Leaves ofGarcinianujiangensis[J].J Nat Prod,2012,75(8):1459-1464.
[71]Auranwiwat C,Trisuwan K,Saiai A,et al.Antibacterial tetraoxygenated xanthones from the immature fruits ofGarciniacowa[J].Fitoterapia,2014,98:179-183.
[72]Kaennakam S,Siripong P,Tip-pyang S.Kaennacowanols A-C,three new xanthones and their cytotoxicity from the roots ofGarciniacowa[J].Fitoterapia,2015,102:171-176.
[73]Lee HH,Chan HK.1,3,6-Trihydroxy-7-methoxy-8-(3,7-dimethyl-2,6-octadienyl)xanthone fromGarciniacowa[J].Phytochemistry,1977,16(12):2038-2040.
[74]Lihitwitayawuid K,Phadungcharoen T,Mahidol C,et al.7-O-Methylgarcinone E fromGarciniacowa[J].Phytochemistry,1997,45(6):1299-1301.
[75]Likhitwitayawuid K,Phadungcharoen T,Krungkrai J.Antimalarial xanthones fromGarciniacowa[J].Planta Med,1998,64(1):70-72.
[76]Mahabusarakam W,Chairerk P,Taylor WC.Xanthones fromGarciniacowaRoxb.latex[J].Phytochemistry,2005,66(10):1148-1153.
[77]Na PP,Thongtheeraparp W,Wiriyachitra P,et al.Xanthones ofGarciniacowa[J].Planta Med,1994,60(4):365-368.
[78]Na Z,Song Q,Hu H.A new prenylated xanthone from latex ofGarciniacowaRoxb[J].Rec Nat Prod,2013,7(3):220-224.
[79]Panthong K,Hutadilok-Towatana N,Panthong A.Cowaxanthone F,a new tetraoxygenated xanthone,and other anti-inflammatory and antioxidant compounds fromGarciniacowa[J].Can J Chem,2009,87(11):1636-1640.
[80]Panthong K,Pongcharoen W,Phongpaichit S,et al.Tetraoxygenated xanthones from the fruits ofGarciniacowa[J].Phytochemistry,2006,67(10):999-1004.
[81]Ritthiwigrom T,Laphookhieo S,Pyne SG.Chemical constituents and biological activities ofGarciniacowaRoxb[J].Maejo Int J Sci Technol,2013,7(2):212-231.
[82]Shen J,Yang JS.Two new xanthones from the stems ofGarciniacowa[J].Chem Pharm Bull,2006,54(1):126-128.
[83]Siridechakorn I,Phakhodee W,Ritthiwigrom T,et al.Antibacterial dihydrobenzopyran and xanthone derivatives fromGarciniacowastem barks[J].Fitoterapia,2012,83(8):1430-1434.
[84]Sriyatep T,Siridechakorn I,Maneerat W,et al.Bioactive Prenylated Xanthones from the Young Fruits and Flowers ofGarciniacowa[J].J Nat Prod,2015,78(2):265-271.
[85]Trisuwan K,Ritthiwigrom T.Benzophenone and xanthone derivatives from the inflorescences ofGarciniacowa[J].Arch Pharmacal Res,2012,35(10):1733-1738.
[86]Wahyuni FS,Byrne LT,Dachriyanus,et al.A new ring-reduced tetraprenyltoluquinone and a prenylated xanthone fromGarciniacowa[J].Aust J Chem,2004,57(3):223-226.
[87]Wahyuni FS,Shaari K,Lajis NH,et al.Cytotoxic Properties and Complete Nuclear Magnetic Resonance Assignment of Isolated Xanthones from the Root ofGarciniacowaRoxb[J].Pharmacogn Mag,2016,12(Suppl 1):S52-56.
[88]Wahyuni F S,Shaari K,Stanslas J,et al,Dachriyanus.Cytotoxic xanthones from the stem bark ofGarciniacowaRoxb[J].J Chem Pharm Res,2015,7(1):227-236.
[89]Xia Z,Zhang H,Xu D,Lao Y,et al.Xanthones from the leaves ofGarciniacowainduce cell cycle arrest,apoptosis,and autophagy in cancer cells[J].Molecules,2015,20(6):11387-11399.
[90]Fu WM,Zhang JF,Wang H,et al.Heat shock protein 27 mediates the effect of 1,3,5-trihydroxy-13,13-dimethyl-2H-pyran[7,6-b] xanthone on mitochondrial apoptosis in hepatocellular carcinoma[J].J Proteomics,2012,75(15):4833-4843.
[91]Shan WG,Lin TS,Yu HN,et al.Polyprenylated xanthones and benzophenones from the bark ofGarciniaoblongifolia[J].Helv Chim Acta,2012,95(8):1442-1448.
[92]Shi JM,Huang HJ,Qiu SX,et al.Griffipavixanthone fromGarciniaoblongifoliachamp induces cell apoptosis in human non-small-cell lung cancer H520 cells in vitro[J].Molecules,2014,19(2):1422-1431.
[93]Xu H,Zhang H,Lao Y,et al.Anti-cervical cancer compound griffipavixanthone and method of use thereof.US20150038569A1[P/OL].2015-05-02.
[94]Xu H,Zhang H,Xi Z,et al.Polyisoprenylated tetracyclic xanthone with anticancer activity.US20140194498A1[P/OL].2014-10-07.
[95]Zhang DD,Xu JW,Zhang H,et al.Anti-Inflammatory Effect of 1,3,5,7-Tetrahydroxy-8-isoprenylxanthone Isolated from Twigs ofGarciniaesculentaon Stimulated Macrophage[J].Mediators Inflamm,2015,2015:350564.
[96]Zhang H,Zhang DD,Lao YZ,et al.Cytotoxic and anti-inflammatory prenylated benzoylphloroglucinols and xanthones from the twigs ofGarciniaesculenta[J].J Nat Prod,2014,77(7):1700-1707.
[97]Ding Z,Lao Y,Zhang H,et al.Griffipavixanthone,a dimeric xanthone extracted from edible plants,inhibits tumor metastasis and proliferation via downregulation of the RAF pathway in esophageal cancer[J].Oncotarget,2016,7(2):1826-1837.
[98]Zhu LL,Fu WW,Shao YN,et al.Xanthine oxidase inhibitors fromGarciniaesculentatwigs[J].Planta Med,2014,80(18):1721-1726.
[99]Guo YE,Wang LL,Li ZL,et al.Triterpenes and xanthones from the stem bark ofGarciniatetralata[J].J Asian Nat Prod Res,2011,13(5):440-443.
[100]Na Z,Xu Y.Chemical constituents ofGarciniatetralata[J].Zhongcaoyao,2010,41(3):367-370.
[101]Wang L,Li Z,Hua H,et al.Chemical constituents from barks ofGarciniatetralata[J].Zhongguo Zhongyao Zazhi,2008,33(20):2350-2352.
[102]Han QB,Tian HL,Yang NY,et al.Polyprenylated xanthones fromGarcinialancilimbashowing apoptotic effects against HeLa-C3 cells[J].Chem Biodiversity,2008,5(12):2710-2717.
[103]Sun Y,Li D,Jia C,et al.Three new xanthones from the leaves ofGarcinialancilimba[J].J Nat Med,2016,70(2):173-178.
[104]Yang NY,Han QB,Cao XW,et al.Two new xanthones isolated from the stem bark ofGarcinialancilimba[J].Chem Pharm Bull,2007,55(6):950-952.
[105]Chen JJ,Chen IS,Duh CY.Cytotoxic xanthones and biphenyls from the root ofGarcinialinii[J].Planta Med,2004,70(12):1195-1200.
[106]Chen JJ,Peng CF,Huang HY,et al.Benzopyrans,biphenyls and xanthones from the root ofGarcinialiniiand their activity against Mycobacterium tuberculosis[J].Planta Med,2006,72(5):473-477.[107]Masters K S,Brase S.Xanthones from fungi,lichens,and bacteria:the natural products and their synthesis[J].Chem Rev,2012,112(7):3717-3776.
[108]Negi JS,Bisht VK,Singh P,et al.Naturally Occurring Xanthones:Chemistry and Biology[J].Journal of Applied Chemistry,2013,2013:9.
[109]Tanaka N,Takaishi Y.Xanthones fromHypericumchinense[J].Phytochemistry,2006,67(19):2146-2151.

[111]Wolfender JL,Urbain A,Hostettmann K.Profiling,isolation,chemical characterisation and distribution of gentianaceae constituents[M].:Springer Berlin Heidelberg,2015.
[112]Silva A,Pinto D.Structure Elucidation of Xanthone Derivatives:Studies of Nuclear Magnetic Resonance Spectroscopy[J].Curr Med Chem,2005,12(21):2481-2497.
[113]Han QB,Xu HX.Caged Garcinia xanthones:development since 1937[J].Curr Med Chem 2009,16,3775-3796.
[114]Tao SJ,Guan SH,Wang W,et al.Cytotoxic polyprenylated xanthones from the resin ofGarciniahanburyi[J].J Nat Prod 2009,72,117-124.
[115]Xu M,Fu W,Zhang B,et al.Combinative application of pH-zone-refining and conventional high-speed counter-current chromatography for preparative separation of caged polyprenylated xanthones from gamboge[J].J Sep Sci 2016,39,559-565.
[116]Anantachoke N,Tuchinda P,Kuhakarn C,et al.Prenylated caged xanthones:chemistry and biology[J].Pharm Biol,2012,50(1):78-91.
(2016-07-05收稿责任编辑:洪志强)
Chemistry of Xanthones Isolated from Garcinia Species in China
Wang Liping1,2,Fu Wenwei1,2,Tan Hongsheng1,2,Zhang Hong1,2,Xu Hongxi1,2
(1SchoolofPharmacy,ShanghaiUniversityofTraditionalChineseMedicine,Shanghai201203,China; 2EngineeringResearchCenterofShanghaiCollegesforTCMNewDrugDiscovery,Shanghai201203,China)
Xanthones are one of the biggest classes of natural compounds in Garcinia species with various biological activities and are subdivided according to the degree of oxygenation and different substituted positions.This review focuses on the distribution,isolation,structural classification,spectral characteristics and biosynthesis pathway of xanthones isolated from Garcinia species in China.
Garcinia species; Xanthone; Structural classification; Spectral characteristics; Biosynthesis pathway
国家自然科学基金重点项目(编号:81130069);国家自然科学基金面上项目(编号:81173485)
王丽萍(1991.04—),女,硕士研究生,研究方向:中药活性成分研究,E-mail:maxine_wlp0411@163.com
徐宏喜(1961.07—),男,博士,教授,院长,研究方向:中药活性成分及药理作用机制研究,E-mail:xuhongxi88@gmail.com
R284.1;R284.2
A doi:10.3969/j.issn.1673-7202.2016.07.004

