基于三萘苯的溶液可加工螺旋形寡聚物的合成及其在蓝光有机电致发光器件中的应用
初增泽 王 丹 吴红伟 邹德春,*
(1沈阳师范大学化学化工学院,沈阳110034;2北京大学化学与分子工程学院,北京分子科学国家实验室,北京100871)
基于三萘苯的溶液可加工螺旋形寡聚物的合成及其在蓝光有机电致发光器件中的应用
初增泽1王丹2吴红伟2邹德春2,*
(1沈阳师范大学化学化工学院,沈阳110034;2北京大学化学与分子工程学院,北京分子科学国家实验室,北京100871)
近年来,人们在有机电致发光材料和器件结构方面取得了巨大的进步。然而由于蓝光材料具带隙宽的内禀属性,在发光效率、色纯度和稳定性上仍然面临巨大挑战。本文将螺旋形三萘苯共轭体系引入电致发光材料领域,它独特的螺旋形分子结构和易于化学修饰的特点有利于抑制聚集体和基激缔合物的形成。通过SiCl4催化的环三缩合反应和Suzuki偶联反应,我们设计合成了以三萘基苯为核心,萘、蒽和三苯胺为取代基团的系列螺旋形蓝光寡聚物,并系统地研究了它们的热学、光物理和电化学性质。研究发现,萘和三苯胺取代的寡聚物1,3,5-三(3-(1-甲氧基萘-2-基)-4-甲氧基萘-1-基)苯(TNNB)和1,3,5-三(3-(4-(N,N-二苯胺基)苯基)-4-甲氧基萘-1-基)苯(TPANB)具有最好的热稳定性。在溶液中,这两种材料都具有深蓝发射,发射峰分别为382和415 nm;在薄膜中,TNNB的发射峰仅有1 nm的红移,而TPANB甚至产生了6 nm的蓝移。以这些寡聚物为发光材料,通过旋涂法制备的有机电致发光器件结果表明,基于TNNB的器件获得了最大亮度达到5273 cd∙m-2,色坐标(0.17,0.11)的纯蓝光器件。
三萘苯;环三缩合反应;蓝光;螺旋形;有机电致发光二极管
1 Introduction
In the past decade,tremendous progress has been made in the exploration of the organic light-emitting materials for flat-panel displays and white lighting resources1-3.In particular,for full-color displays,the deep-blue emitter having a Commission Internationale de L′Eclairage(CIE)y coordinate value<0.15 has attracted significant attention because it not only is one of the three primary RGB colors(red,green and blue),but also can produce other colors via energy cascading to lower energy emitting materials4,5.However,blue-emitting materials still remain challenges in the efficiency,color purity and stability due to their intrinsic wide band-gap6-9.These problems have become the main obstacles for the commercial reality of the large-area full-color displays.
There are two types of blue-emitting materials:fluorescent and phosphorescent materials.From a practical and industrial view,the deep-blue emission and reliability are two main challenges for blue phosphorescent materials4,10,11.Therefore,in recent years,a series of blue fluorescent emitters,host-dopant systems and nondoped compounds,have been developed for efficient blue organic light-emitting diode(OLED)applications.Compared to hostdopant systems,non-doped materials have greater benefits.On the one hand,for host-dopant systems,the phase separation and concentration quenching are two main problems4,which usually lead to inferior device efficiency,low operation stability and/or poor spectra purity;moreover,it is difficult to control the doping concentration of blue-emitting dopant in host via vacuum codeposition or large-area solution deposition processes12.On the other hand,obviously,non-doped emitters have great advantages of fabricating simplification and low cost.Nevertheless,for nondoped systems,excimers and aggregations derived from intermolecular π-π interactions,which often result in poor color purity and low efficiency,have to be resolved urgently13.
In this study,we employed trinaphthylbenzene(TNB)as the central moiety for the construction of the blue light-emitting materials for non-doped OLED.TNB has features of nonplanar propeller-shaped structures,and its highly twisted skeleton is beneficial for suppressing aggregates and excimers,which can also decreases the effective conjugation length of the π-π systems to enlarge energy band gaps for blue emission.Meanwhile,the naphthalene moiety has multiple reactive sites,which will be good for easy chemical modification14-18.Furthermore,well-defined oligomers have the advantages both of small organic molecules and polymers:uniform molecular structures,excellent chemical purity,and controllable conjugated length19-21.In addition,conjugated oligomers can also be deposited using solution-based techniques such as spin-coating or inject printing for large-area manufacturing.We designed and synthesized a series of welldefined 3D π-conjugated oligomers based on TNB.We systematic investigated the correlation of the thermal,optical and electrochemical properties with their chemical structures,and further explored their applications as blue-emitters in the OLEDs.
2 Experimental
2.1Instrument
1H and13C NMR spectra were recorded on a Mercury Plus 300 MHz(Varian,USA)or a Bruker ARX 400 MHz spectrometer (Bruker,Switzerland).Mass spectra were recorded on a BIFLEX III MALDI-TOF MS spectrometer(Bruker Daltonics Inc.,USA). Elemental analyses were carried out on a Vario EL III elemental analyzer(Elementar Analysensysteme GmbH,Germany).Thermal gravity analyses(TGA)were conducted on a TAInstrument Q600 analyzer(Thermal Analysis,USA)under a rate of 20°C∙min-1in nitrogen.Differential scanning calorimetry(DSC)analyses were performed on a TA Instrument Q100 analyzer(Thermal Analysis, USA)with a rate of 10°C∙min-1in nitrogen.UV-Vis spectra were measured on a Jasco V-500 spectrophotometer(Jasco,Japan),and photoluminescence(PL)spectra were obtained with a Jasco FP-6200 spectrofluorometer(Jasco,Japan).Cyclic voltammetry(CV) was carried out on a Model 283 potentiostat/galvanostat(Princeton Applied Research,USA)using tetrabutylammonium perchlorate(n-Bu4NClO4)in freshly distilled acetonitrile as a supporting electrolyte.
2.2Materials and synthetic procedures
Tetrahydronfuran(THF)was distilled from sodium under argon and acetonitrile was distilled over P2O5.All other commercially available chemicals are analytical reagents(AR)and were used as received unless otherwise stated.
2.2.12-Bromo-1-methoxynaphthalene(1)
1-Naphthol(4.33 g,30.0 mmol)was dissolved in 150 mL CS2, and then NBS(5.35 g,30.0 mmol)was added to the solution under vigorously stirring.The mixture was stirred for 1 h at room temperature.The product was filtered and concentrated underreduced pressure,and the residue was purified by column chromatography on silica gel eluted with petroleum/EtOAc(25:1,V/ V)to produce 2-bromo-1-naphthol(4.27 g,19.2 mmol)with a yield of 64%.To a mixture of 2-bromo-1-naphthol(4.07 g,18.0 mmol)and K2CO3(5.05 g,36.5 mmol)in 50 mL DMF was treated with 2.5 mL methyl iodide.After vigorously stirring at room temperature overnight,product was poured into water,extracted with dichloromethane and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel eluted with petroleum/ethyl acetate(10:1,V/V)to afford 1 as yellow oil(4.08 g,17.2 mmol)in 96%yield.1H NMR(300 MHz,CDCl3)δ:8.16-8.13(m,1H),7.86-7.83(m,1H),7.61-7.50(m,4H),4.01(s,3H);13C NMR(75 MHz,CDCl3)δ:153.10, 133.93,130.04,128.97,128.00,126.71,126.50,125.23,122.02, 112.63,61.40.
2.2.21-(3-Bromo-4-methoxynaphthalen-1-yl) ethanone(2)
Compound 2 was obtained as a pale yellow solid with a yield of 80%according to the procedure described for 8.1H NMR(300 MHz,CDCl3)δ:8.81-8.77(m,1H),8.21-8.18(m,1H),8.09(s, 1H),7.68-7.58(m,2H),4.05(s,3H),2.73(s,3H);13C NMR(75 MHz,CDCl3)δ:199.55,156.89,133.55,132.77,131.08,129.60, 128.56,127.31,126.63,122.28,110.75,61.65,29.69.
2.2.31,3,5-Tris(3-bromo-4-methoxynaphthalen-1-yl) benzene(3)
Compound 3 was obtained as a white solid with a yield of 57% according to a similar procedure described for TNB1.1H NMR (300 MHz,CDCl3)δ:8.25-8.22(m,3H),8.10(d,J=7.8 Hz,3H), 7.73(s,3H),7.69(s,3H),7.62-7.52(m,6H),4.07(s,9H);13C NMR(75 MHz,CDCl3)δ:152.91,139.62,136.97,132.00,131.07, 130.82,129.31,126.91,126.85,126.34,122.53,112.14,61.54. 2.2.4(1-Methoxynaphthalen-2-yl)boronic acid(4)
Asolution of compound 1(1.43 g,6 mmol)in dry THF(10 mL) was added dropwise to magnesium turnings(0.18 g,7.2 mmol) under Ar.The mixture was heated to 50°C and stirred for 1 h. Next,the mixture was cooled to-78°C and a solution of trimethylborate(1.30 mL,12 mmol)in THF was added.The reaction mixture was slowly warmed to room temperature and stirred overnight.Aqueous 1 mol∙L-1HCl solution(30 mL)was added to the mixture at 0°C and extracted with ethyl ether.The organic phase was separated,dried over MgSO4.The solvent was evaporated and the resulting product was wash with hexane to afford 4 as a white solid(0.71 g,3.5 mmol)in 59%yield.1H NMR(300 MHz,CDCl3)δ:8.14-8.10(m,1H),7.91-7.86(m,2H),7.68(d, J=8.4 Hz,1H),7.58-7.54(m,2H),6.44(s,2H),4.05(s,3H);13C NMR(75 MHz,CDCl3)δ:163.51,137.15,130.96,128.27,127.34, 126.74,126.05,124.31,122.24,63.71.
2.2.54-(Diphenylamino)phenyl bromide(5)
A mixture of diphenylamine(1.71 g,10 mmol),p-bromoiodobenzene(3.51 g,12.5 mmol),CuI(0.19 g),1,10-phenanthroline(0.20 g),KOH(5.0 g)and 50 mL toluene was rigorously stirred and heated to reflux for 24 h under argon,and the generated water was removed using a Dean-Stark trap.The reaction mixture was cooled down to room temperature and filtered over a pad of silica gel,and the filtrate was concentrated under reduced pressure.The resulting product was purified by column chromatography on silica gel eluted with petroleum to obtain a white solid 2.21 g in 68%yield.1H NMR(300 MHz,CDCl3)δ: 7.33(d,J=8.7 Hz,2H),7.29-7.24(m,4H),7.09-7.01(m,6H), 6.95(d,J=8.7 Hz,2H);13C NMR(75 MHz,CDCl3)δ:147.33, 146.98,132.11,129.34,125.09,124.37,123.19,114.72.
2.2.64-(Diphenylamino)phenylboronic acid(6)
Compound 6 was obtained as a white solid with a yield of 40% according to the procedure described for 4.1H NMR(300 MHz, CDCl3)δ:8.02(d,J=8.4 Hz,2H),7.33-7.26(m,4H),7.17(d, J=7.5 Hz,4H),7.12-7.07(m,4H);13C NMR(75 MHz,CDCl3) δ:151.66,147.15,136.70,129.38,125.41,123.78,120.98.
2.2.79-(4,4,5,5-Tetramethyl-[1,3,2]dioxaborolan-2-yl) anthracene(7)
To a solution of 9-bromoanthracene(2.57 g,10.0 mmol)in dry THF 30 mLat-78°C under argon atmosphere was added n-BuLi (7.4 mL,11.0 mmol,1.6 mol∙L-1in hexane).After stirring for 1h at-78°C,trimethylborate(2.2 mL)was added,and the reaction mixture was gradually warmed to room temperature and stirred overnight.Aqueous 2 mol∙L-1HCl solution(50 mL)was added to the mixture at 0°C and extracted with CH2Cl2.The organic phase was separated,dried over MgSO4.The solvent was evaporated and the resulting product was washed with hexane/CH2Cl2(15:1,V/V)to afford 9-anthracene boronic acid as a yellow solid (1.23 g,5.5 mmol)in 60%yield.A solution of the resulting 9-anthracene boronic acid and 1.43 g pinacol in 50 mL toluene was rigorously stirred under argon and refluxed for 5 h.After the solvent was removed,the crude product was purified by column chromatography on silica gel eluted with petroleum/ethyl acetate (25:1,V/V)to obtain a yellow solid(1.38 g,4.5 mmol)in 82% yield.1H NMR(300 MHz,CDCl3)δ:8.49-8.44(m,3H),8.01-7.98(m,2H),7.52-7.43(m,4H),1.58(s,12H).
2.2.81-(4-Methoxynaphthalen-1-yl)ethanone(8)
To a mixture of compound 1(3.47 g,22.0 mmol),nitrobenzene (1.43 g,11.6 mmol)and aluminum chloride(4.10 g,30.8 mmol) in 100 mL dichloromethane was dropwisely added acetyl chloride (1.64 mL,23.1 mmol)with vigorous stirring at 0-5°C.The reaction mixture was stirred at room temperature overnight,and then poured into ice water and extracted with dichloromethane.The organic layer was washed with an aqueous solution of NaOH (10%)and water,dried over MgSO4and concentrated.The product was purified by column chromatography on silica gel eluted with petroleum/ethyl acetate(10:1,V/V)to afford 2 as yellow oil(4.05 g,20.2 mmol)with a yield of 91%.1H NMR(300 MHz,CDCl3) δ:9.03(d,J=8.4 Hz,1H),8.33(d,J=8.4 Hz,1H),8.04(d,J= 8.4 Hz,1H),7.67-7.61(m,1H),7.56-7.50(m,1H),6.80(d,J= 8.4 Hz,1H),4.07(s,3H),2.72(s,3H);13C NMR(75 MHz,acetone-d6)δ:199.93,159.15,131.90,128.64,127.15,126.16,125.70, 122.00,101.92,55.70,29.24.
2.2.91,3,5-Tris(4-methoxynaphthalen-1-yl)benzene (TNB1)
To a solution of compound 2(1.91 g,9.5 mmol)in dry ethanol (130 mL)at 0°C under argon atmosphere,SiCl4(31 mL,270 mmol)was added slowly by syringe.After stirring for 24 h at room temperature,the reaction mixture was poured into ice water and filtered.The crude solid was dissolved in chloroform and washed with water.The organic layer was dried over MgSO4and concentrated under reduced pressure.The crude product was purified by column chromatography on silica gel eluted with petroleum/dichloromethane(2:1,V/V)to afford a yellow solid (0.72 g,1.3 mmol)in 42%yield.1H NMR(300 MHz,CDCl3)δ: 8.88-8.34(m,3H),8.21-8.18(m,3H),7.68(s,3H),7.54-7.48 (m,9H),6.91(d,J=7.8 Hz,3H),4.05(s,9H);13C NMR(100 MHz,CDCl3)δ:154.99,140.70,132.40,132.37,130.61,127.19, 126.64,125.79,125.69,125.15,122.24,103.46,55.56.
2.2.101,3,5-Tris(3-(1-methoxynaphthalen-2-yl)-4-methoxynaphthalen-1-yl)benzene(TNNB)
To a solution of tris(2-bromo-1-methoxynaphthalen-4-yl)benzene(0.39 g,0.5 mmol)and methoxynaphthalene-2-boronic acid (0.61 g,3.0 mmol)in 45 ml toluene/ethanol(2:1,V/V)was added a aqueous solution of 2 mol∙L-1Na2CO315 mL.After the mixture was purged with argon for 30 min,Pd(PPh3)4(0.13 g)was added. The mixture was heated to 78°C and vigorously stirred for 48 h. The reaction mixture was extracted with chloroform and washed with water.The organic layer was dried over MgSO4and concentrated under reduced pressure.The crude product was purified by column chromatography on silica gel eluted with petroleum/ dichloromethane(12:1,V/V)to afford a white solid 0.42 g in 83% yield.1H NMR(300 MHz,CDCl3)δ:8.39-8.36(m,3H),8.28-8.23(m,6H),7.90-7.87(m,3H),7.81(s,3H),7.76(s,3H),7.70-7.62(m,6H),7.60-7.52(m,12H),3.66(s,9H),3.64(s,9H);13C NMR(75 MHz,CDCl3)δ:153.58,153.21,140.51,135.53,134.53, 132.49,130.87,130.47,129.25,128.68,128.32,127.78,126.62, 126.54,126.33,126.21,126.00,123.51,122.99,122.61,61.47, 61.34;MALDI-TOF MS(m/z):Calcd.for C72H54O6,Exact mass: 1014.39,Mol.Wt.:1015.2;Found:1037.7([M+Na]+),1014.7 (M+);Anal.Calcd.(%)for C72H54O6,C,85.18;H,5.36;Found(%): C,84.54;H,5.44.
2.2.111,3,5-Tris(3-(4-(N,N-diphenylamino)phenyl)-4-methoxynaphthalen-1-yl)benzene(TPANB)
TPANB was obtained with yields of 75%according to a similar procedure described for TNNB.1H NMR(300 MHz,CDCl3)δ: 8.34(d,J=8.4 Hz,3H),8.19(d,J=8.4 Hz,3H),7.65(s,3H), 7.62(d,J=8.4 Hz,6H),7.57(td,J=7.8 Hz,1.5Hz,3H),7.49(td, J=7.7,1.5 Hz,3H),7.29-7.24(m,12H),7.16-7.14(m,18H), 7.03(t,J=7.4 Hz,6H),3.72(s,9H);13C NMR(100 MHz,CDCl3) δ:152.67,147.66,146.91,140.59,135.83,132.31,131.95,130.75, 130.12,129.95,129.23,129.14,128.92,128.74,126.36,126.11, 124.40,123.45,122.88,122.80,61.11;MALDI-TOF MS(m/z): Calcd.for C93H69N3O3,Exact mass:1275.53,Mol.Wt.:1276.56; Found:1276.7(M+);Anal.Calcd.(%)for C93H69N3O3,C,87.50;H, 5.45;N,3.29;Found(%):C,87.40;H,5.40;N,3.05.
2.2.121,3,5-Tris(3-(anthracen-9-yl)-4-methoxynaphthalen-1-yl)benzene(TANB)
TANB was obtained with yields of 10%according to a similar procedure described for TNNB.1H NMR(300 MHz,CDCl3)δ: 8.51(s,3H),8.36(dd,J=8.1 Hz,0.9Hz,3H),8.28(d,J=7.8 Hz, 3H),8.03(d,J=8.1 Hz,6H),7.80(s,3H),7.73(d,J=8.4 Hz, 6H),7.63-7.53(m,6H),7.47-7.40(m,9H),7.32-7.25(m,6H), 3.29(s,9H);13C NMR(100 MHz,CDCl3)δ:154.12,140.32, 135.46,133.25,132.74,131.69,131.39,130.94,130.33,128.57, 128.44,127.02,126.81,126.75,126.27,126.11,125.77,125.63, 125.10,123.05,61.42;MALDI-TOF MS(m/z):Calcd.for C81H54O3,Exact mass:1074.41,Mol.Wt.:1075.29;Found:1074.6 (M+).Anal.Calcd.(%)for C81H54O3,C,90.47;H,5.06;Found(%): C,90.32;H,5.18.
2.3Device fabrication and measurement
The etched ITO(20 Ω∙□-1)glass substrateswere rinsed in turn with detergent,deionized water,acetone,and ethanol,and then treated with UV-ozone.Poly(ethlyenedioxythiophene):poly(styrenesulfonicacid)(PEDOT:PSS,Bayer)was spin-coated on the surface ofthe ITO substrate and dried at 120°C for 2 h.Oligomers were spin-coated on the top of the PEDOT:PSS from chloroform solution(10 mg∙mL-1).Next,a thin film of TPBI(1,3,5-tris(N-phenylbenzimidazol-2-yl)benzene)(20 nm)was prepared by thermal sublimation under vacuum as the hole-blocking layer. Finally,a LiF layer(1 nm),and an Al layer(80 nm)were deposited successively by vacuum evaporation.The electroluminescence(EL)spectra were recorded by a fiberoptic spectrometer S2000(Ocean Optics,USA).The voltage-current density-luminance curve was measured using a Keithley 238 source measure unit(Keithley,USA)and a power meter 1835-C(Newport,USA) calibrated with a luminance meter LS-110(Minolta,Japan).All measurements were performed under an ambient atmosphere at room temperature.
3 Results and discussion
3.1Synthesis
We prepared these propeller-shaped oligomers by convergent synthetic routes to introduce aryl units into the meta-position of methoxyl group of the trinaphthylbenzene backbone,as illustrated in Schemes 1 and 2.The methoxyl groups at the TNB core will greatly increase the steric hindrance of their meta-substituted aryl moieties,and therefore this molecular design can obtain a more highly twisted non-planar 3D molecule.As shown in Scheme 1, compound 1 was prepared by bromination of the starting material, naphthol,with N-bromosuccinimide and subsequent hydroxylmethylation with methyl iodide.Next,compound 1 was converted to compound 2 through a Friedel-Craft acylation reaction22,23.The key intermediate 3 was synthesized via a SiCl4-catalyzed cyclocondensation reaction of three molecules of compound 2 with a moderate yield of 57%,according to similar literature methods24,25. The boronic or boronate precursors 4,6,and 7 were synthesized via Grignard reaction or lithium-halogen exchange reaction followed by treatment with trimethylborate.
As comparison,the prototypical molecule,TNB1,was also prepared through a three-step synthetic route similarly as that ofcompound 3,as shown in Scheme 2.The three propeller-shaped oligomers,TNNB,TPANB,and TANB,were synthesized by Pdcatalyzed Suzuki coupling reactions of the key precursor 3 with boronic or boronate compounds 4,6,and 7,respectively.Both TNNB and TPANB have good yields of around 80%.However, TANB was obtained in a very low product yield of 10%,which is attributed to the high steric hindrance of the bulky anthracene moiety;actually,the one-and two-substituted side products were also isolated in the yields of 28%and 34%,respectively.These oligomers were characterized by1H and13C NMR,MALDI-TOF mass spectroscopy,and elemental analysis.
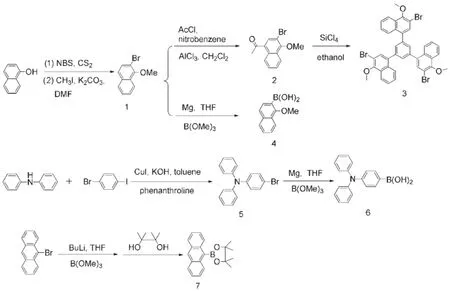
Scheme 1 Synthetic routes to compound 3 and monomers
3.2Thermal properties
We investigated the thermal properties of these propellershaped oligomers by thermogravimetric analysis(TGA)and differential scanning calorimetry(DSC)in a nitrogen atmosphere. As shown in Fig.1,TNNB and TPANB exhibit relatively high decomposition temperatures(Td,5%weight loss)at 373 and 425°C,respectively,whereas the Tdvalue of TANB is only 273°C.The two compounds exhibit high glass transition temperatures(Tg)at 147°C for TNNB and at 157°C for TPANB, indicating their propeller-shaped structure and bulky aryl moieties are both beneficial for the amorphous stability.However,the Tgvalue of TANB was not obtained due to its very low Tdvalue.We presume that the low thermal stability of TANB is probably due to the huge intramolecular strain arising from its highly twisted 3D skeleton.
3.3Optical properties
The absorption and PL spectra of the oligomers in a chloroform solution(10-6mol∙L-1)and in thin films are illustrated in Fig.2. In dilute solutions,similar to that of TNB1(Fig.2(a)),the UV-Vis spectra of the three TNB-based oligomers show an absorption band from 300 nm to 350 nm(Fig.2(b-d)),which belongs to theπ-π*transitions of the trinaphthyl central moiety.Notably,for TANB,the three peaks located in long wavelength of 340-405 nm can be assigned to the characteristic absorption peaks of anthracene26.Normalized PL spectra of TNB1,TNNB,and TPANB in solution show deep blue emission color with single emission peaks at 376,382,and 415 nm,respectively,whereas TANB exhibits a double-peak emission located at 407 and 425 nm.The remarkably red-shift fromTNB1 to TPANB and TANB can be attributed to the strong electron-donating property of triphenyl amine group in TPANB,and the large π-conjugated system of anthracene moiety in TANB.In the solid state,the shapes of the absorption spectra of the four oligomers are similar to those in solution,and their absorption λmaxshows a red-shift of 2-5 nm.Among them,TPANB exhibits the smallest red-shift of 2 nm,which indicates that in the aggregates the highly twisted conformation of TPANB prevented strong molecular packing in the ground state.These oligomers exhibit wide optical band gap(Eg)of 3.46,3.14,and 3.00 eV for TNNB,TPANB,and TANB,respectively,as estimated from the onset position of the absorption band in film(Table 1).The decrease of the band gaps implies the increasing of conjugation length from TNNB to TANB due to the different aryl-substitution groups.As shown in Fig.2(b,c),in comparison with those in solution,the emission spectrum in film of TNNB shows only a very slight red-shift of 1 nm,whereas TPANB even displays a noticeable blue-shift of 6 nm in solid state.These results demonstrate their special propeller-like 3D geometries resulted in weak intermolecular interaction.Especially for the blue-shift of TPANB,we presume that this observation may be attributed to the non-planar triphenylamine groups combined with its twisted TNB core.Compared with the planar rigid naphthalene moiety,previousexperiment and theoretical calculation show that TPA has a propeller-like structure27-29:the three phenyl rings exhibit a dihedral angle of about 44°with respect to the plane consisting of the center N atom and three adjacent carbon atoms.In solution,the phenyl rings on TPA can intramolecularly rotate more freely due to the solvation effect and less restriction,leading to molecular planarization and thus longer effective conjugation length.But in solid state,the constrained intramolecular rotations intensified the distortion conformation of TPA moiety30,resulting in a shorter effective conjugation length;meanwhile,the combination of the propeller-shape structures of TPA and TNB can provide great steric hindrance to dramatically decrease the intermolecular interactions and π-π stacking effect.The two aspects both contribute to the blue-shift of TPANB in film.As shown in Fig.2(d), TANB film exhibits a very broad PLemission and its two emitting peaks red-shift 8 and 17 nm,relatively.Unlike methoxyl substituted naphthalene or triphenylamine moieties,the anthracene has a larger planar structure and a more extended π-conjugation system,which presumably lead to stronger π-π stacking interactions,resulting in a larger red-shift.
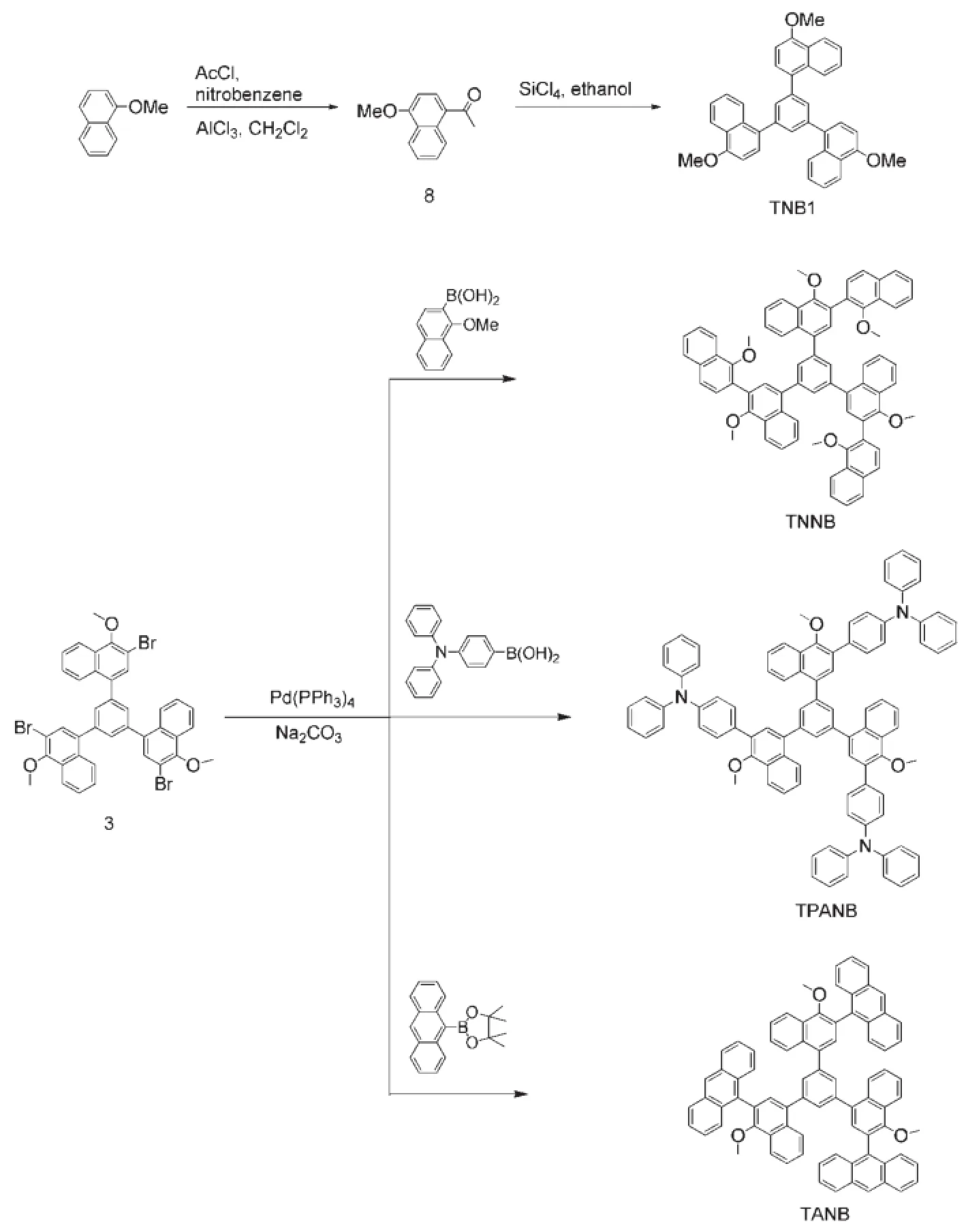
Scheme 2 Molecular structures and synthetic routes of the propeller-shaped oligomers
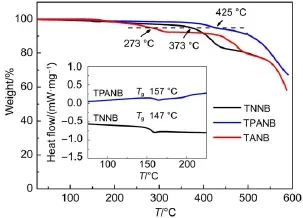
Fig.1 TGAcurvesandDSCscans(inset)measuredfortheoligomers
3.4Electrochemical properties
The electrochemical behaviors of the three TNB derivatives are investigated by cyclic voltammetry(CV)in anhydrous acetonitrile solution with 0.1 mol∙L-1n-Bu4NClO4as a supporting electrolyte. The potentials were recorded relative to an Ag/AgCl reference electrode using ferrocene(Fc)as the internal standard.Their cyclic voltammograms are shown in Fig.3.The three oligomers exhibit irreversible oxidation processes:the oxidation peak potentials at 1.38 and 1.42 V for TNNB and TPANB,respectively;for TANB, it also has a similar shoulder peak at around 1.36 V.These oxidation potentials can be ascribed to the oxidation of the naphthalene moieties on the core skeleton.In addition,TPANB has another quasi-reversible oxidation at 1.03 V,which are attributed to the oxidation of the triphenylamine group;TANB exhibits an irreversible oxidation at a higher potential of 1.66 V corresponding to the oxidation of the anthracene moiety.The highest occupied molecular orbital(HOMO)energy levels are calculated on the basis of the onset potentialsof oxidation process following the equation of.TNNB and TANB exhibit similarly deep HOMO levels for-5.64 and-5.65 eV,respectively.However,TPANB shows a high-lying HOMO level at-5.11 eV which is higher than that of the hole-injecting material,PEDOT:PSS(-5.20 eV),indicating that the electron-rich triphenylamine moieties can significantly promote TPANB′s HOMO level.The lowest unoccupied molecular orbital(LUMO) energy levels of TNNB,TPANB,and TANB can be further calculated from subtracting the optical bandgap from the HOMO level at 2.18,1.97,and 2.65 eV,respectively.The above data are also summarized in Table 1.
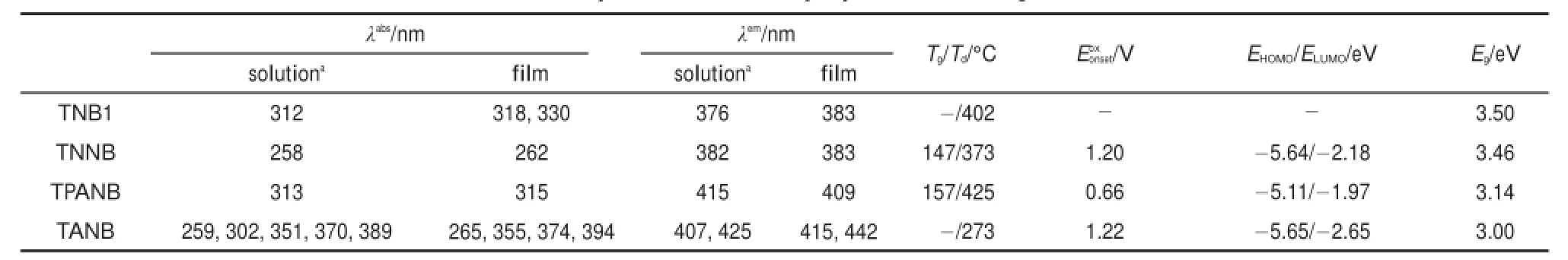
Table 1 Optical and thermal properties of the oligomers
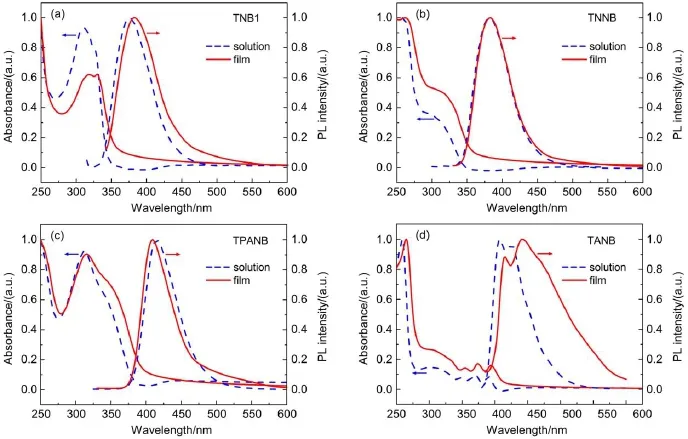
Fig.2 Absorption and photoluminescence spectra of the oligomers in CHCl3and film
3.5Electroluminescence properties
According to the wide band gaps and the blue-emitting fluorescence properties of these propeller-like oligomers,we investigated their EL properties using the oligomers as emitters.The devices were fabricated with a structure of ITO/PEDOT:PSS(40 nm)/oligomer/TPBI(20 nm)/LiF(1 nm)/Al(80 nm)by spincoating from the solutions of the oligomers.Fig.4 shows the EL spectra of the devices.TNNB and TPANB exhibit deep blue emissions with main peaks at 405 and 429 nm with full widths at half-emission maximum(FWHM)of about 75 nm and 48 nm;their CIE color coordinates exhibit at(0.17,0.11)and(0.17,0.08), respectively.Their low coordinate y values(<0.15)and narrow emission spectra demonstrate that the propeller-shaped 3D skeleton can not only effectively shield the intermolecular closepacking and thus depress the excimer formation,but also efficiently shorten the π-π conjugative length resulting in blue emission.However,the device based on TANB shows very poor EL property and its EL spectrum exhibits a weak and broad skyblue emission with two rough peaks at 440 and 488 nm,respectively.In fact,except for EL spectrum,we did not obtain other device properties for TANB-based device.Its poor device property is probably due to the inferior thermal property of TANB for Tdonly at 273°C,which could lead to the degradation of the active layer during device preparation and/or device operation.
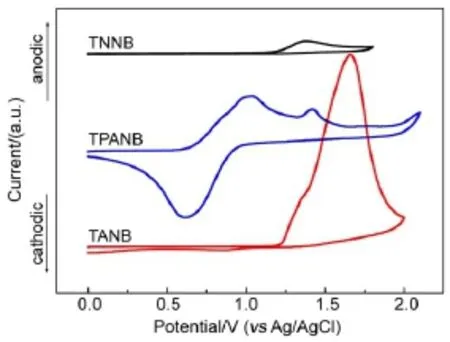
Fig.3 Cyclic voltammograms of the oligomers at a scanning rate of 50 mV∙s-1
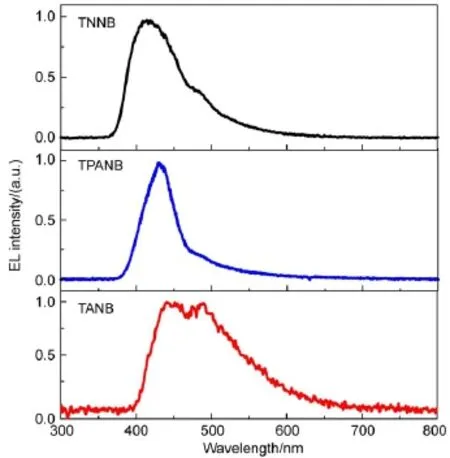
Fig.4 Normalized ELspectra of ITO/PEDOT:PSS/oligomer/ TPBI/LiF/Al devices
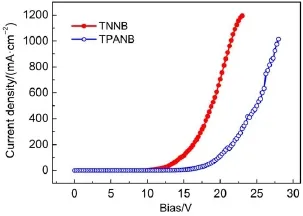
Fig.5 Current density-voltage curves of ITO/PEDOT:PSS/ oligomer/TPBI/LiF/Al devices
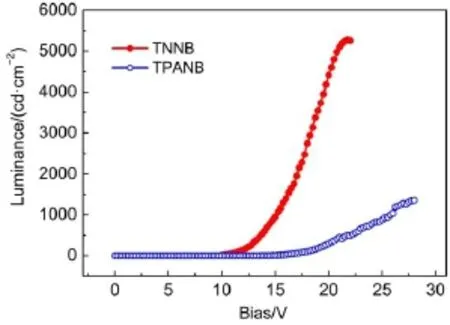
Fig.6 Luminance-voltage curves of ITO/PEDOT:PSS/oligomer/ TPBI/LiF/Al devices
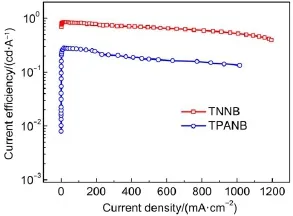
Fig.7 Current efficiency-current density curves of ITO/PEDOT: PSS/oligomer/TPBI/LiF/Al devices
Fig.5 and Fig.6 present the current density-voltage and luminance-voltage characteristics for the devices based on TNNB and TPANB.TPANB shows higher current density and luminance than those of TPANB at the same driving bias;the maximum brightness for TNNB can reach 5273 cd∙m-2,which is far higher than that for TPANB of 1358 cd∙m-2.These differences in their optoelectronic characteristics also clearly reflect on the efficiencies for these two devices:TNNB-based device exhibits greater maximum current efficiency of 0.86 cd∙A-1than that of TPANB for 0.29 cd∙A-1as shown in Fig.7.We speculate that the inferior EL properties for TPANB may be due to the imbalance injection of holes and electrons:although triphenylamine moieties on TPANB can significantly decrease the hole-injection barrier for high-lying HOMO level(-5.11 eV),its relatively high LUMO level for-1.97 eV increases the electron-injection barrier com-pared to that of TNNB(-2.18 eV),which probably results in inefficient hole-electron combination.
4 Conclusions
We have demonstrated a facile approach of the synthesis of a set of propeller-shaped blue-emitting oligomers based on TNB via the combination of acid-promoted cyclotrimerization and Suzuki coupling reactions.Owing to their special propeller-like 3D geometries,their oligomers exhibit blue-emission in solution;TNNB and TPANB also show deep-blue PL emission even in solid state. We fabricated EL devices from the spin-coating films of these oligermers,and the preliminary results demonstrate the device based on TNNB shows a pure blue emission with a brightness and a current efficiency of 5273 cd∙m-2and 0.86 cd∙A-1with CIE coordinates of(0.17,0.11).Future investigation can be focused on device optimization or electrophosphorescent devices using these compounds as host materials.
References
(1)Jou,J.H.;Kumar,S.;Agrawal,A.;Li,T.H.;Sahoo,S.J. Mater.Chem.C 2015,3,2974.doi:10.1039/C4TC02495H
(2)Yu,T.C.;Liu,L.L.;Xie,Z.Q.;Ma,Y.G.Sci.China Chem. 2015,58,907.doi:10.1007/s11426-015-5409-7
(3)Chung,Y.H.;Bian,M.Y.;Zhang,M.X.;Chu,S.S.;Chen,Z. J.;Gong,Q.H.;Xiao,L.X.Acta Phys.-Chim.Sin.2015,31, 1597.[钟耀贤,卞梦颖,张明骁,褚赛赛,陈志坚,龚旗煌,肖立新.物理化学学报,2015,31,1597.]doi:10.3866/PKU. WHXB201505291
(4)Zhu,M.R.;Yang,C.L.Chem.Soc.Rev.2013,42,4963.doi: 10.1039/c3cs35440g
(5)Yook,K.S.;Lee,J.Y.Adv.Mater.2012,24,3169.doi: 10.1002/adma.v24.24
(6)Yang,X.L.;Xu,X.B.;Zhou,G.J.J.Mater.Chem.C 2015,3, 913.doi:10.1039/C4TC02474E
(7)Ouyang,M.;Wu,Q.C.;Yu,Z.W.;Li,H.F.;Zhang,C.Acta Phys.-Chim.Sin.2014,30,1341.[欧阳密,吴启超,余振伟,李洪飞,张诚.物理化学学报,2014,30,1341.]doi:10.3866/ PKU.WHXB201405041
(8)Dai,S.X.;Chen,H.;Lin,Z.H.;Ling,Q.D.Polym.Bull. 2014,3,46.[代水星,陈欢,林正欢,凌启淡.高分子通报, 2014,3,46.]
(9)Chu,Z.Z.;Wang,D.;Zhang,C.;Zou,D.C.Acta Phys.-Chim. Sin.2012,28,2000.[初增泽,王丹,张超,邹德春.物理化学学报,2012,28,2000.]doi:10.3866/PKU. WHXB201206071
(10)Hu,J.Y.;Pu,Y.J.;Satoh,F.;Kawata,S.;Katagiri,H.;Sasabe, H.;Kido,J.Adv.Funct.Mater.2014,24,2064.doi:10.1002/ adfm.v24.14
(11)Zhang,Q.S.;Li,B.;Huang,S.P.;Nomura,H.;Tanaka,H.; Adachi,C.Nat.Photonics 2014,8,326.doi:10.1038/ nphoton.2014.12
(12)Lee,Y.H.;Wu,T.C.;Liaw,C.W.;Wen,T.C.;Feng,S.W.; Lee,J.J.;Wu,Y.T.;Guo,T.F.Org.Electron.2013,14,1064. doi:10.1016/j.orgel.2013.01.021
(13)Jeong,S.J.;Kim,M.K.;Kim,S.H.;Hong,J.I.Org.Electron. 2013,14,2497.doi:10.1016/j.orgel.2013.06.022
(14)Magill,J.H.;Plazek,D.J.Nature 1966,209,70.doi:10.1038/ 209070a0
(15)Whitaker,C.M.;McMahon,R.J.J.Phys.Chem.1996,100, 1081.doi:10.1021/jp9529329
(16)Grilli,S.;Lunazzi,L.;Mazzanti,A.;Pinamonti,M.J.Org. Chem.2002,67,5733.doi:10.1021/jo0258195
(17)Grilli,S.;Lunazzi,L.;Mazzanti,A.;Pinamonti,M. Tetrahedron 2004,60,4451.doi:10.1016/j.tet.2004.01.094
(18)Swallen,S.F.;Kearns,K.L.;Mapes,M.K.;Kim,Y.S.; McMahon,R.J.;Ediger,M.D.;Wu,T.;Yu,L.;Satija,S. Science 2007,315,353.doi:10.1126/science.1135795
(19)Martin,R.E.;Diederich,F.Angew.Chem.Int.Edit.1999,38, 1350.doi:10.1002/(SICI)1521-3773(19990517)38:10<1350:: AID-ANIE1350>3.0.CO;2-6
(20)Shirota,Y.;Kageyama,H.Chem.Rev.2007,107,953.doi: 10.1021/cr050143+
(21)Shirota,Y.J.Mater.Chem.2005,15,75.doi:10.1039/ b413819h
(22)Carreno,M.C.;Ruano,J.L.G.;Sanz,G.;Toledo,M.A.; Urbano,A.Synlett 1997,1241.doi:10.1055/s-1997-1553
(23)Giordano,C.;Villa,M.;Annunziata,R.Synthetic Commun. 1990,20,383.doi:10.1080/00397919008052779
(24)Elmorsy,S.S.;Pelter,A.;Smith,K.;Hursthouse,M.B.;Ando, D.Tetrahedron Lett.1992,33,821.doi:10.1016/S0040-4039 (00)77724-0
(25)Cao,X.Y.;Liu,X.H.;Zhou,X.H.;Zhang,Y.;Jiang,Y.;Cao, Y.;Cui,Y.X.;Pei,J.J.Org.Chem.2004,69,6050.doi: 10.1021/jo049268p
(26)Lyu,Y.Y.;Kwak,J.;Kwon,O.;Lee,S.H.;Kim,D.;Lee,C.; Char,K.Adv.Mater.2008,20,2720.doi:10.1002/adma.v20:14 (27)Chen,G.;Li,W.B.;Zhou,T.R.;Peng,Q.;Zhai,D.;Li,H.X.; Yuan,W.Z.;Zhang,Y.M.;Tang,B.Z.Adv.Mater.2015,27, 4496.doi:10.1002/adma.v27.30
(28)Sobolev,A.N.;Belsky,V.K.;Romm,I.P.;Chernikova,N.Y.; Guryanova,E.N.Acta Crystallogr.Sect.C:Cryst.Struct. Commun.1985,41,967.
(29)Reva,I.;Lapinski,L.;Chattopadhyayc,N.;Faustoa,R.Phys. Chem.Chem.Phys.2003,5,3844.doi:10.1039/b306489a
(30)Yuan,W.Z.;Lu,P.;Chen,S.M.;Lam,J.W.Y.;Wang,Z.M.; Liu,Y.;Kwok,H.S.;Ma,Y.G.;Tang,B.Z.Adv.Mater.2010, 22,2159.doi:10.1002/adma.v22:19
Synthesis of Solution-Processable Propeller-Shaped Oligomers Based on Trinaphthylbenzene and Their Application in Blue Organic Light-Emitting Diodes
CHU Zeng-Ze1WANG Dan2WU Hong-Wei2ZOU De-Chun2,*
(1College of Chemistry and Chemical Engineering,Shenyang Normal University,Shenyang 110034,P.R.China;2Beijing National Laboratory for Molecular Sciences,College of Chemistry and Molecular Engineering, Peking University,Beijing 100871,P.R.China)
Although considerable improvements have been achieved in novel materials and device architectures for organic light-emitting diodes(OLEDs),great challenges remain in efficiency,color purity,and stability for blue emitters because of their intrinsic wide band-gap.In this study,trinaphthylbenzene(TNB),a propeller-shaped conjugated system,is employed as the central moiety for the construction of the organic lightemitting materials.Its nonplanar propeller-shaped structure and easy chemical modification are beneficial for building three-dimensional(3D)π-π conjugated systems to suppress aggregates and excimers.We have demonstrated a facile approach for the synthesis of a set of propeller-shaped blue-emitting oligomers based on the TNB core with peripheral units of naphthalene,anthracene or triphenylamine via the combination of SiCl4-catalyzed cyclotrimerization and Suzuki coupling reactions.The thermal,optical,and electrochemical propertiesof the materials were investigated.The results indicate that the naphthalene and triphenylamine substituted oligomers,1,3,5-tris(3-(1-methoxynaphthalen-2-yl)-4-methoxynaphthalen-1-yl)benzene(TNNB)and 1,3,5-tris (3-(4-(N,N-diphenylamino)phenyl)-4-methoxynaphthalen-1-yl)benzene(TPANB),have the best thermal stability. They exhibit deep blue photoluminescence(PL)emission at 382 and 415 nm in solution,respectively.In comparison with the solution spectra,the emission spectra in films show only a very slight red-shift of 1 nm for TNNB and a blue-shift of 6 nm for TPANB.The electroluminescent device fabricated using TNNB as the emitter has a pure blue emission with a brightness of 5273 cd∙m-2and Commission Internationale de L′Eclairage(CIE)coordinates of(0.17,0.11).
November 12,2015;Revised:February 15,2016;Published on Web:February 19,2016.
Trinaphthylbenzene;Cyclotrimerization reaction;Blue-emitting;Propeller-shaped; Organic light-emitting diode
O644;O649
10.3866/PKU.WHXB201602193
*Corresponding author.Email:dczou@pku.edu.cn;Tel:+86-10-62759799.
The project was supported by the National Natural Science Foundation of China(50833001),National Key Basic Research Program of China(973) (2011CB933300),and Research Starting Foundation for Doctoral Program of Shenyang Normal University,China(054-55440109013).
国家自然科学基金(50833001),国家重点基础研究发展规划项目(973)(2011CB933300)和沈阳师范大学博士科研项目启动基金(054-55440109013)资助

