基于一个四羧酸配体构筑的具有(4,8)-连接的镧系金属-有机框架材料及对小分子的检测试验
刘珍 陈晓 冯云龙(浙江师范大学物理化学研究所,浙江省固体表面反应重点实验室,金华321004)
基于一个四羧酸配体构筑的具有(4,8)-连接的镧系金属-有机框架材料及对小分子的检测试验
刘珍陈晓冯云龙*
(浙江师范大学物理化学研究所,浙江省固体表面反应重点实验室,金华321004)
以3,3′,5,5′-四-(羧基苯基)联苯为配体(H4L),与镧系金属Ln(Ⅲ)盐反应,自组装形成了5个具有三维孔洞结构的镧系金属-有机框架材料:{[Ln3L2(H2O)7]·(OH)·10DMA}n(Ln=Gd(1a);Ln=Ho(2a),{[Ln3L2(H2O)3]·(OH)·mDMA}n(Ln=Er,m=10(1b);Ln=Yb, m=9(2b);Ln=Lu,m=10(3b))。单晶X射线衍射分析表明,这些MOFs属于2种系列的类质同晶化合物,分别属于正交晶系Ccca空间群和单斜晶系C2/c空间群。有机小分子溶剂交换荧光研究发现,2b对小分子二氯甲烷和甲苯荧光有增强效应,表现出良好的荧光探测功能。
3,3′,5,5′-四(羧基苯基)联苯;金属-有机框架材料;晶体结构;荧光性质
0 Introduction
Metal-organic frameworks(MOFs),also known as porous coordination polymers(PCPs),are crystalline solids consisting of metal ions/clusters connected to multidentate organic ligands via coordination bonds. Ever-increasing interest in the design and synthesis of MOFs stems not only from their aesthetic architecturesand topologies[1-3]but also from their possible potential applications such as gas storage/separation,catalysis, ion conductivity,drug delivery[4-13].The lanthanide(Ln) analogues are also highly sought after because Ln ions possesshighcoordinationnumbers,flexible coordinationgeometries,andtheeffectofLn contractionthatcanaffordstructuraldiversity. Moreover,Ln-based compounds can show distinct characteristic luminescent emissions[14-21].In addition, aromaticcarboxylatecoordinationcomplexeshave been extensively investigated in past decades due to theirstrongcoordinationcapability,theirlarge conjugated systems,and the possibility of offering new functional materials.
Amongthediverseorganiclinkers,those containingmulticarboxylategroups,including dicarboxylate,tricarboxylate,tetracarboxylate, hexacarboxylateandoctacarboxylate,havebeen widely employed to synthesize new MOFs because of their preference to stabilize the MOFs through in situ formedsecondarybuildingunits(SBUs)[22].A tetracarboxylic acid,biphenyl-3,3′,5,5′-tetra-(phenyl-4-carboxylic acid)(H4L,Scheme 1),was selected specifically as an organic ligand in this work.It is noteworthy that only three MOFs based on this ligand have been reported to date[23-25].Using H4L,we have successfully synthesized two series of new Ln-MOFs under solvothermal condition,{[Ln3L2(H2O)7]·(OH)· 10DMA}n(Ln=Gd,(1a);Ln=Ho,(2a)),and{[Ln3L2(H2O)3] ·(OH)·mDMA}n(Ln=Er,m=10(1b);Ln=Yb,m=9(2b); Ln=Lu,m=10(3b)).Here we report their structures and luminescent properties.
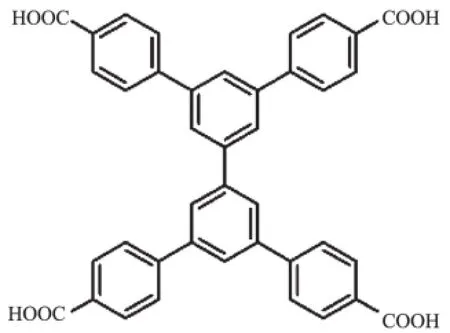
Scheme 1Higand used to construct the Ln-MOFs
1 Experimental
1.1.1Materials and general methods
Biphenyl-3,3′,5,5′-tetra-(phenyl-4-carboxylic acid)(H4L)was prepared according to a literature method[25].All the other reagents were obtained from commercialsourcesandusedwithoutfurther purification.The elemental analyses were performed on a Perkin-Elmer 2 400 element analyzer.The infrared spectra were recorded within the 400~4 000 cm-1region on a Nicolet Impact 410 FTIR spectrometer using KBr pellets.Thermogravimetric analyses were performed on a TGA Q500 V20.10 Build 36 thermogravimetric analyzer in the temperature range of 35~700℃under an air flow with a heating rate of 10℃·min-1.The luminescence spectra were recorded on a JASCO FP-6500spectrofluorimeterinsolidstateatroom temperature.
1.2Preparation of Ln-MOFs
A mixture of Ln(NO3)3·6H2O(0.032 mmol),H4L (0.015 8 mmol),dimethylacetamide(DMA)(2.0 mL), and pyridine(10 μL)was sealed in a 25 mL Teonlined stainless steel container,which was heated at 120℃for 72 h,and then cooled down to room temperature at a rate of 5℃·h-1.Colorless block single crystals were collected and washed with DMA and dried in air to give the products.
{[Gd3(L)2(H2O)7]·(OH)·10DMA}n(1a).Yield:ca. 66%.Anal.Calcd.for C120H149Gd3N10O34(%):C,52.46; H,5.47;N,5.10.Found(%):C,52.78;H,5.82;N, 5.96.FTIR(KBr,cm-1):3 395,2 919,1 603,1 539, 1 23,1 186,1 012,864,787,717,594,473.
{[Ho3(L)2(H2O)7]·(OH)·10DMA}n(2a)Yield:ca. 65%.Anal.Calcd.for C120H149Ho3N10O34(%):C,52.02;H,5.42;N,5.06.Found(%):C,52.36;H,5.78;N, 5.91.FTIR(KBr,cm-1):3 398,2 915,1 609,1 537, 1 427,1 184,860,784,717,586,472.
{[Er3(L)2(H2O)3]·(OH)·10DMA}n(1b).Yield:ca. 69%.Anal.Calcd.for C120H141Er3N10O30(%):C,53.28; H,5.25;N,5.18.Found(%):C,52.58;H,5.62;N, 6.05.FTIR(KBr,cm-1):3 397,2 916,1 605,1 534, 1 425,1 182,858,782,715,583,470.
{[Yb3(L)2(H2O)3]·(OH)·9DMA}n(2b).Yield:ca. 63%.Anal.Calcd.for C116H132Yb3N9O29(%):C,52.86; H,5.05;N,4.78.Found(%):C,53.19;H,5.42;N, 5.96.FTIR(KBr,cm-1):3 396,2 913,5 88,1 533, 1 429,1 247,858,782,711,580,483.
{[Lu3(L)2(H2O)3]·(OH)·10DMA}n(3b).Yield:ca. 63%.Anal.Calcd.for C120H141Lu3N10O30(%):C,52.82; H,5.21;N,5.13.Found(%):C,53.14;H,5.58;N, 6.00.FTIR(KBr,cm-1):3 390,2910,1 558,1 529, 1 420,1 179,549,775,706,575,476.
1.3Crystal structure determination
Data collection of the Ln-MOFs were determined on a Bruker APEXⅡdiffractometer equipped with a graphite-monochromatizedMoKαradiation(λ= 0.071 073 nm)at 296(2)K.Data intensity was corrected by Lorentz-polarization factors and empirical absorption.The structures were solved by the direct methods and expanded with the difference Fourier techniques.The anisotropic displacement parameters were applied to all non-hydrogen atoms in full-matrix least-squares refinements based on F2with SHELXTL-97 program packages[26].The hydrogen atoms were located by geometrically calculations and assigned with isotropic displacement factors and included in thefinalrefinementcycles.X-raystructure refinements indicated that one of benzene rings in 1b, 2b and 3b disordered over two positions and the site occupancy factors are refined to 0.50∶0.50.The solvent molecules are highly disordered,and attempts tolocateandrefinesolventmoleculeswere unsuccessful.Therefore,the SQUEEZE routine of PLATON[27]wasusedtoremovethediffraction contribution from these solvents to produce a set of solvent free diffraction intensities,the structures were then refined again using the data.The free solvent moleculeswereestablishedonthebasisofthe reactants,single-crystalX-raydiffractionstudies, thermogravimetricanalysis.Thedetailed crystallographic and structure refinement data are summarized in Table 1.Selected bond lengths and angles are given in Table 2 and Table 3,respectively.
CCDC:1434268,1a;1434269,2a;1434270,1b; 1434271,2b;1434272,3b.

Table 1Crystal data and structure refinements for the Ln(Ⅱ)-MOFs

Continued Table 1

Table 2Selected bond lengths(nm)and angles(°)for 1a and 2a

Table 3Selected bond lengths(nm)and angles(°)for 1b~3b

Continued Table 3
2 Results and discussion
2.1Crystal structures of 1a and 2a
Single-crystal X-ray diffraction analyses showed that 1a and 2a are isostructural and crystallize in orthorhombic Ccca space group.Therefore,only the structure of 2a as representative is described in detail. Besides the guest solvents,the asymmetric unit of 2a contains one and a half Ho(Ⅱ)ions,two half-L4-ligands,three and a half coordinated water molecules. Ho(1)is seven-coordinated with a distorted onecapped trigonalprismcoordinationgeometrybysix carboxylate oxygen atoms from six L4-ligands(Ho(1)-O 0.220 8(8)~0.231 8(5)nm),one water molecule(Ho (1)-O1W 0.247 5(10)nm).Ho(2)is eight-coordinated with a square antiprism coordination geometry by five carboxylate oxygen atoms from four organic L4-ligands (Ho(2)-O 0.224 8(7)~0.249 5(6)nm),three water molecules(Ho(2)-O(W)0.240 7(9)~0.250 2(9)nm). Three Ho(Ⅱ)ions are connected in the sequence of Ho(2)-Ho(1)-Ho(2)through eight carboxylic groups to form a trinuclear[Ho3(COO)8]SBU.The SBUs are further connected by L4-ligands to construct a 3D framework(Fig.1a).The solvent accessible volume is 64.0%of the volume of the unit cell calculated by PLATON[27]after removal of the guest molecules.

Fig.1(a)View of the coordination environments of Ho(Ⅱ)ions in 2a;(b)The organic L4-viewed as 4-connected node;(c)The trinuclear[Ho3(COO)8]SBU viewed as 8-connected node;(d)View of the(4,8)-connected net
Topologically,each organic L4-ligand links four trinuclear[Ho3(COO)8]SBUsandthuscanbe simplifiedas4-connectednode(Fig.1b).Each trinuclear[Ho3(COO)8]SBU links eight L4-ligands (Fig.1c)and can be considered as 8-connected node. The resulting network can be described as a(4,8)-connected net with Schläfli symbol of(411.614.83)(42.6)2(Fig.1d).
2.2Crystal structures of 1b,2b and 3b
Single-crystal X-ray diffraction analysis revealed that 1b~3b are isostructural and crystallize in the monoclinic C2/c space group.Therefore,only the structure of 1b as representative is described in detail.Besides the guest solvents,the asymmetric unit consists of one and a half Er(Ⅱ)ions,one deprotonated L4-ligand,and one and a half coordinated water molecules.Er(1)is seven-coordinated with a distorted onecapped trigonal prism geometry by six carboxylate oxygen atoms from six L4-ligands(Er(1)-O 0.220 2(4)~0.230 4(3)nm),one watermolecule(Er(1)-O1W 0.237 6(7)nm).Er(2)is six-coordinated with pentagonal pyramid by five carboxylate oxygen atoms from four L4-ligands(Er(2)-O 0.221 6(4)~0.239 2(4)nm),one watermolecule(Er(2)-O2W0.234 9(5)nm).Three Er(Ⅱ)ions are connected by eight carboxylic groups to form a trinuclear[Er3(COO)8]SBU.The SBUs are further connected by L4-ligands to construct a 3D framework(Fig.2a).The solvent accessible volume is 68.7%of the volume of the unit cell calculated by PLATON[27]after removal of the guest molecules.
Topologically,each organic L4-ligand links four trinuclear[Er3(COO)8]SBUs and can be simplified as 4-connected node.Each trinuclear[Er3(COO)8]SBU links eight L4-ligands and can be considered as 8-connectednode.Theresultingnetworkcanbe described as a(4,8)-connected net with Schläfli symbol of(412.612.84)(46)2(Fig.2b).

Fig.2(a)View of the coordination environments of Er(Ⅱ)ions in 1b;(b)View of the(4,8)-connected net
2.3Photoluminescence properties
Considering that the Ln-MOFs are very promising luminescent materials because both the inorganic and organic components constituting MOFs can provide platforms to generate luminescence.The fluorescence properties of 2b were investigated in the solid state andindifferentsolventemulsionsatroom temperature.The free H4L ligand exhibits a broad luminescence peak centered at 391 nm when excited at 357 nm,which is probably assigned to π or n to π*transitions[25].2b shows the characteristic emission bands of2F5/2-2F7/2transition of Yb(Ⅱ)ion at 980 nm when excited at 325 nm.Furthermore,the excitation energy can be transferred from a LMCT state to the 4f levels of the lanthanide ions when the LMCT state lies at high-enough energy[28-29],indicating that the Yb(Ⅱ)ion adopt noncentrosymmetric coordination modes, which is in agreement with the crystal structural analysis.

Fig.3Solid-state photo-luminescent excitation(a)and emission(b)spectra of 2b in different solvents
As mentioned above,it is anticipated that the solvent molecules in the larger porous volume could be replaced by other small organic solvents.In this regard,to examine the potential sensing of small solvent molecules,grinded crystals of 2b(3.0 mg) were dispersed in 5 mL organic reagent such as N,N-dimethylformamide(DMF),dimethylacetamide(DMA), acetone,dichloromethane(CH2Cl2),acetonitrile (MeCN),tetrahydrofuran(THF),acetidin,toluene, methanol(MeOH),sonicated for 30 minutes,and then placed for 24 hours at room temperature in order to form stable suspensions.Interestingly,we found that the 2b exhibited the enhancement of the luminescence intensity when dispersed in CH2Cl2and toluene,which indicates that 2b is a potential luminescent sensing material.
2.4Thermal stability
The TGA diagrams of the MOFs are similar and show two main weight losses in the curves.Therefore, only the TGA curve of 1a as representative is described in detail(Fig.4).The TGA curve of 1a shows the first weight loss of 32.65%occurred in the temperature range of 30~205℃,corresponding to the release of solvent molecules and counter ions(Calcd. 32.33%),Above this temperature(205~650℃),the compound starts to decompose gradually.The final residue may be Gd2O3(Obsd.14.25%,Calcd.13.20%).
3 Conclusions
In summary,five new(4,8)-connected Ln(Ⅱ)-MOFshavebeensuccessfullysynthesizedby solvothermal reactions of Ln(Ⅱ)salts and H4L.The luminescence of 2b can be modulated by small organic molecule exchange.It is expected that the compounds can be used as a promising luminescent sensing material in the future.

Fig.4TGA curves for the Ln(Ⅱ)-MOFs
References:
[1]Moon H R,Lim D W,Suh M P.Chem.Soc.Rev.,2013,42: 1807-1824
[2]Betard A,Fischer R A.Chem.Rev.,2012,112:1055-1083
[3]Zhang J P,Zhang Y B,Lin J B,et al.Chem.Rev.,2012, 112:1001-1033
[4]Han Y H,Tian C B,Lin P,et al.J.Mater.Chem.A,2015,3: 24525-24531
[5]Li J R,Sculley J,Zhou H C,et al.Chem.Rev.,2012,112: 869-932
[6]Agarwal R A,Aijaz A,Ahmad M,et al.Cryst.Growth Des., 2012,12:2999-3005
[7]Li B,Wen H M,Wang H,et al.J.Am.Chem.Soc.,2014, 136:6207-6210
[8]Chen S S,Liu Q,Zhao Y,et al.Cryst.Growth Des.,2014, 14:3727-3741
[9]Wang K,Feng D,Liu T F,et al.J.Am.Chem.Soc., 2014,136:13983-13986
[10]Ward A L,Buckley H L,Lukens W W,et al.J.Am.Chem.Soc.,2013,135:13965-13971
[11]Burtch N C,Jasuja H,Walton K S.Chem.Rev.,2014,114: 10575-10612
[12]Eubank J F,Mouttaki H,Cairns A J,et al.J.Am.Chem. Soc.,2011,133:14204-14207
[13]DVries R F,Snejko N,Iglesias M,et al.Cryst.Growth Des., 2014,14:2516-2521
[14]Yang Q Y,Pan M,Wei S C,et al.Inorg.Chem.,2015,54: 5707-5716.
[15]Han Y H,Tian C B,Li Q H,et al.J.Mater.Chem.C,2014, 2:8065-8070
[16]Zou J P,Peng Q,Wen Z,et al.Cryst.Growth Des.,2010, 10:2613-2619
[17]Jia L N,Hou L,Wei L,et al.Cryst.Growth Des.,2013,13: 1570-1576
[19]Le C Y,Farha O K,Hong B J,et al.J.Am.Chem.Soc., 2011,133:15858-15861
[20]Gong Q,Hu Z,Deibert B J,et al.J.Am.Chem.Soc., 2014,136:16724-16727
[21]Lu Y B,Jian F M,Jin S.Cryst.Growth Des.,2014,14:1684-1694
[22]Liu B,Wu W P,Hou L,et al.Chem.Commun.,2014,50: 8731-8734
[23]Cai J,Yu J,Xu H,et al.Cryst.Growth Des.,2013,13:2094-2097
[24]Sun D,Han L L,Yuan S,et al.Cryst.Growth Des.,2013, 13:377-385
[25]Chen X,He S,Chen F,et al.CrystEngComm,2014,16: 8706-8709
[26]Sheldrick G M.SHELXTL-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany, 1997.
[27]Spek A L.Acta Crystallogr.Sect.D,2009,D65:148-155
[28]Cui Y,Yue Y,Qian G.Chem.Rev.,2012,112:1126-1162
[29]Zhou Y,Li X,Zhang L,et al.Inorg.Chem.,2014,53:3362-3370
Two Series of(4,8)-Connected Lanthanide-Metal Organic Frameworks Based on a Tetra-carboxylate Ligand for Sensing of Small Molecules
LIU ZhenCHEN XiaoFENG Yun-Long*
(Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces,Institute of Physical Chemistry,Zhejiang Normal University, Jinhua,Zhejiang 321004,China)
Biphenyl-3,3′,5,5′-tetra-(phenyl-4-carboxylic acid)(H4L)was used to synthesize five microporous threedimension metal-organic framework with lanthanide metal matrix by intermolecular self-assembly,namely,{[Ln3L2(H2O)7]·(OH)·10DMA}n(Ln=Gd(1a);Ln=Ho(2a)),and{[Ln3L2(H2O)3]·(OH)·mDMA}n(Ln=Er,m=10(1b),Ln=Yb, m=9(2b),Ln=Lu,m=10(3b)).Single-crystal X-ray diffraction revealed the Ln-MOFs are two series of isomorphous metal-organic frameworks,and belong to orthorhombic Ccca space group and monoclinic C2/c space group,respectively.The luminescence intensity of 2b can be modulated by organic small molecule solvents,and 2b exhibited the enhancement of the luminescence intensity when dispersed in CH2Cl2and toluene.CCDC: 1434268,1a;1434269,2a;1434270,1b;1434271,2b;1434272,3b.
biphenyl-3,3′,5,5′-tetra-(phenyl-4-carboxylic acid);metal-organic framework;crystal structure;luminescent property
O614.33+9;O614.343;O614.344;O614.346
A
1001-4861(2016)08-1413-08
10.11862/CJIC.2016.175
2016-03-18。收修改稿日期:2016-06-07。
国家自然科学基金(No.21173197)资助项目。
*通信联系人。E-mail:sky37@zjnu.cn;会员登记号:S06N0984M1401。

