锂离子电池负极材料钛酸锂研究进展*
温书剑,张熠霄,陈 阳,宋春阳,崔晓莉
(复旦大学 材料科学系,上海 200433)
锂离子电池负极材料钛酸锂研究进展*
温书剑,张熠霄,陈 阳,宋春阳,崔晓莉
(复旦大学 材料科学系,上海 200433)
锂离子电池被广泛应用于移动电子设备,在电动汽车和各类储能系统有良好的应用前景,是未来最具发展前途的储能电池之一。作为一种锂离子电池负极材料,钛酸锂因具有高的脱嵌锂平台电位,优异的循环性能,突出的热稳定性和安全特性而备受重视。总结了钛酸锂负极材料在结构形貌和电化学性能方面的研究进展,对其微纳米化、表面改性和离子掺杂等方面新的研究成果进行了概述。微纳米化可以赋予材料更大的表面积,有助于锂离子迁移,电极材料与电解液可以更好的接触,产生更大的锂离子迁移电流,有利于提升钛酸锂材料倍率性能;表面改性手段主要以碳包覆、金属-钛酸锂复合材料和形成新表面相为主,通过这些手段可以改善材料导电性和提高电池的循环性能;离子掺杂可使部分Ti4+转变为Ti3+,提高钛酸锂材料的电子导电性。对钛酸锂作为锂离子电池负极材料未来的发展方向进行了展望。
锂离子电池;钛酸锂;负极材料
0 引 言
伴随能源危机和人们对于环境问题的日益关注,寻找可再生绿色能源成为科学研究的重要发展方向。作为清洁能源器件,锂离子电池具有体积小、重量轻、使用寿命长等优点,广泛应用于便携式电子产品,也是理想的电动汽车动力电池之一[1]。
锂离子电池研究的关键是电极材料的选取。碳材料由于其储量丰富,循环稳定性好,性价比高,是商业锂离子电池中最广泛应用的负极材料[2]。但两大缺点制约着碳材料在锂离子电池市场上的进一步发展:一是在充放电过程中,锂离子脱嵌会引起碳电极材料的体积膨胀与收缩,长时间的使用可能使电极材料晶体结构发生坍塌,进而引起电池容量降低[3-4];二是碳电极在脱嵌锂过程中电位接近金属锂,当电池过充时在表面容易形成锂枝晶,引起电池内部短路,因而具有潜在的安全隐患[5-6]。
因此,寻找安全可靠的负极材料势在必行。各类新型负极材料包括钛酸锂(Li4Ti5O12)、 Si- Li4Ti5O12复合材料[7]、硅[8-10]、多种过渡金属氧化物(如TiO2[11-17]、Co3O4[18]、MnO[19]、Fe2O3[20]、Fe3O4[21]及Cr2O3[22])等受到了广泛的关注。其中钛酸锂是最具发展前景的锂离子电池负极材料之一[23]。与碳材料相比,钛酸锂脱嵌锂平台电位较高(1.55 V vs Li/Li+),可避免锂枝晶的产生,保障了电池的安全性;其理论比容量为175 mAh/g,具有平稳的放电平台,容量利用率较高;被称为“零应变”材料,充放电过程中无明显体积变化,能够避免电极材料因反复胀缩而导致的结构破坏,具有稳定的循环性能[24-26]。除此之外,钛酸锂具有高热稳定性[27-28 ]。
然而,钛酸锂材料由于其Ti4+的3d电子层中没有电子[29-30],致使其自身导电性差,影响了其高倍率性能。锂离子嵌入钛酸锂材料包含3个过程:(1)溶剂化锂离子在电解液中的扩散;(2)钛酸锂电极和电解液交界面上发生电荷转移反应;(3)锂离子在钛酸锂电极内的固相扩散[30]。因此,研究者们通常采用2类方法提高钛酸锂材料的倍率性能:一是通过设计纳米结构减小锂离子在钛酸锂电极中的固相扩散距离[6];二是通过表面改性如包覆碳等,通过元素掺杂增强离子扩散和电子传导速率,从而加快电荷转移反应的速度[6]。本文将对以上方法进行讨论,并对钛酸锂材料未来的发展方向进行展望。
1 钛酸锂材料的结构与性能
钛酸锂是由金属锂和过渡金属钛复合的氧化物,为白色晶体,立方密堆积尖晶石结构,具有锂离子的三维扩散通道,固有电子电导率为10-9S/cm。钛酸锂的晶格常数a为0.826 nm,晶体中O2-占据32e位置,Li+中的3/4占据8a位置,余下的Li+与Ti4+共同占据八面体的16d 位置[24]。钛酸锂的结构式为Li[Li1/3Ti5/3]O4,图1为其晶体结构示意图[23],式(1)为锂离子脱嵌过程的相变表达式。
(1)
式(1)中,钛酸锂晶格内Li+的脱嵌表现为两相过程。Li+在外界电流的作用下嵌入钛酸锂晶格,与四面体8a位置的Li+一同占据16c位置,成为淡蓝色岩盐相的Li7Ti5O12。嵌锂过程中Ti4+/Ti3+的变价提高了电子导电性能[31]。在充放电过程中,四面体8a位置和八面体16c位置的Li+可以相互转换,钛酸锂的晶格常数不会受到影响,所以这种变化是高度可逆的。因此,这种两相可逆转变的方式有利于实现钛酸锂材料的充放电循环。
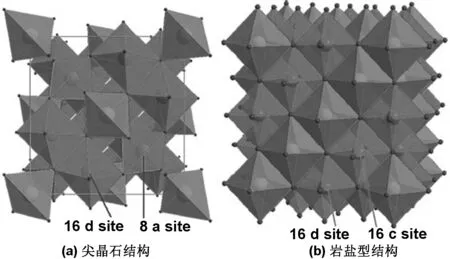
图1 尖晶石结构和岩盐型结构的Li4Ti5O12(黄色四面体代表Li+,绿色八面体代表错位的Li+与Ti4+)[23]
Fig 1 Spinel and rock-salt Li4Ti5O12structures(yellow tetrahedra represent lithium, and green octahedra represent disordered lithium and titanium[23]
2 钛酸锂颗粒的形貌与尺寸调控
相比于传统微尺度级别钛酸锂材料,新型纳米钛酸锂材料因为其出色的倍率性能更加受到人们关注。纳米尺寸的形态赋予材料更大的表面积,缩短锂离子迁移距离,电极材料与电解液可以更好的接触,电解液中可以产生更大的锂离子迁移电流,有利于钛酸锂材料倍率性能的提升[32-34]。更深层的原理可以从结构上分析得出,与微米结构材料相比,纳米结构的钛酸锂材料在充放电过程中增加了单相和固溶态脱嵌锂离子的数量[35]。Borghols等[36]研究发现,固溶体区域中8a与16c的共存促进了锂离子的迁移速率与电导性,对于电池性能的提升做出了主要的贡献。
钛酸锂材料的纳米化研究主要集中在制备纳米颗粒、具有孔洞结构的颗粒和具有微米-纳米混合结构的颗粒。
2.1 纳米颗粒
传统微米级别钛酸锂(800 nm)由TiO2与锂盐通过固相反应合成,这种方法较难对产物的形态与尺寸进行调控,因而限制了钛酸锂材料的倍率性能[37-50]。目前,研究人员尝试利用多种方法制备纳米级别钛酸锂,例如软化学法与熔融盐法。这些方法使得设计与制备纳米尺寸的钛酸锂材料成为可能。
Ren等[37]采用固相合成法制备Li4Ti5O12/C纳米复合物,粒径可控制在50~200 nm,其中碳层厚度为2~4 nm。在0.2与10 C倍率下,产物比容量分别为172和141 mAh/g,且在10 C倍率下200次循环后比容量保持95.7%。Liu等[38]制备的Li4Ti5O12/石墨复合材料平均粒径为200~500 nm。100次循环后,在1和3 C倍率下,产物在1~2 V窗口比容量为144和96.2 mAh/g,高于纯Li4Ti5O12的108和75.4 mAh/g。Han等[39]采用高能球磨法获得粒径在200 nm以下的3组Li4Ti5O12纳米球,在0.1 C倍率下比容量为171,174和167 mAh/g,较普通球磨样品性能有明显提升。图2为Li4Ti5O12纳米球在不同球磨介质尺寸下的扫描电子显微镜图[39]。
2.2 孔洞结构
另一种可以减小锂离子迁移距离并有利于电解液在电极中渗透的方法是制备具有孔洞结构的纳米薄层钛酸锂。纳米颗粒相互连接形成的纳米薄层通过减小扩散距离极大提高了锂离子的运输速率,孔洞结构产生的孔隙可使电解液与电极材料形成有效接触[51]。
Yu等[52]将纳米SiO2与钛酸丁酯作为前驱体, 采用溶胶-凝胶法合成具有核壳结构的SiO2-TiO2。在此基础上,将产物放入LiOH溶液中,除去SiO2后形成了具有孔洞结构的Li4Ti5O12。钛酸锂壳层厚度为50~200 nm,可由反应物钛酸丁酯加入量进行控制。在5和10 C下,产物的比容量为128和115 mAh/g,在20 C的高倍率下,产物依然保持104 mAh/g的可逆比容量。He等[53]以碳作为前驱体制得的Li4Ti5O12同样具有孔洞结构,产物颗粒尺寸为1 μm,平均壁厚60 nm,在10 C倍率下的放电比容量为100 mAh/g,在2 C下连续充放电200次,放电比容量变化不大,保持在150 mAh/g左右。Shao等[54]以单分散聚苯乙烯球作为模板,采用溶胶-凝胶法制备出含孔洞结构的纳米Li4Ti5O12。孔洞壁厚50 nm,孔径为100 nm。在10 C倍率下,产物在0.5~3 V窗口比容量为102 mAh/g。Zhou 等[55]在500~800 ℃范围煅烧水热产物制备直径2 μm的孔洞微球结构Li4Ti5O12(图3)。实验发现,700 ℃煅烧生成产物性能最佳,在1,2, 3, 5, 10, 15和20 C倍率下,比容量分别为157.9, 155.3, 154.1, 150.3, 144.5, 137.8和131.3 mAh/g(图4)。

图2 Li4Ti5O12纳米颗粒经历24 h普通球磨,与尺寸为0.05,0.10, 0.45 mm的球磨介质高能球磨3 h后,在800 ℃下热处理3 h的SEM图[39]
Fig 2 SEM images of the Li4Ti5O12particles heat-treated at 800 ℃ for 3 h after 24 h ball milling, and 3 h high-energy milling using different sizes milling media of 0.05, 0.10, 0.45 mm[39]
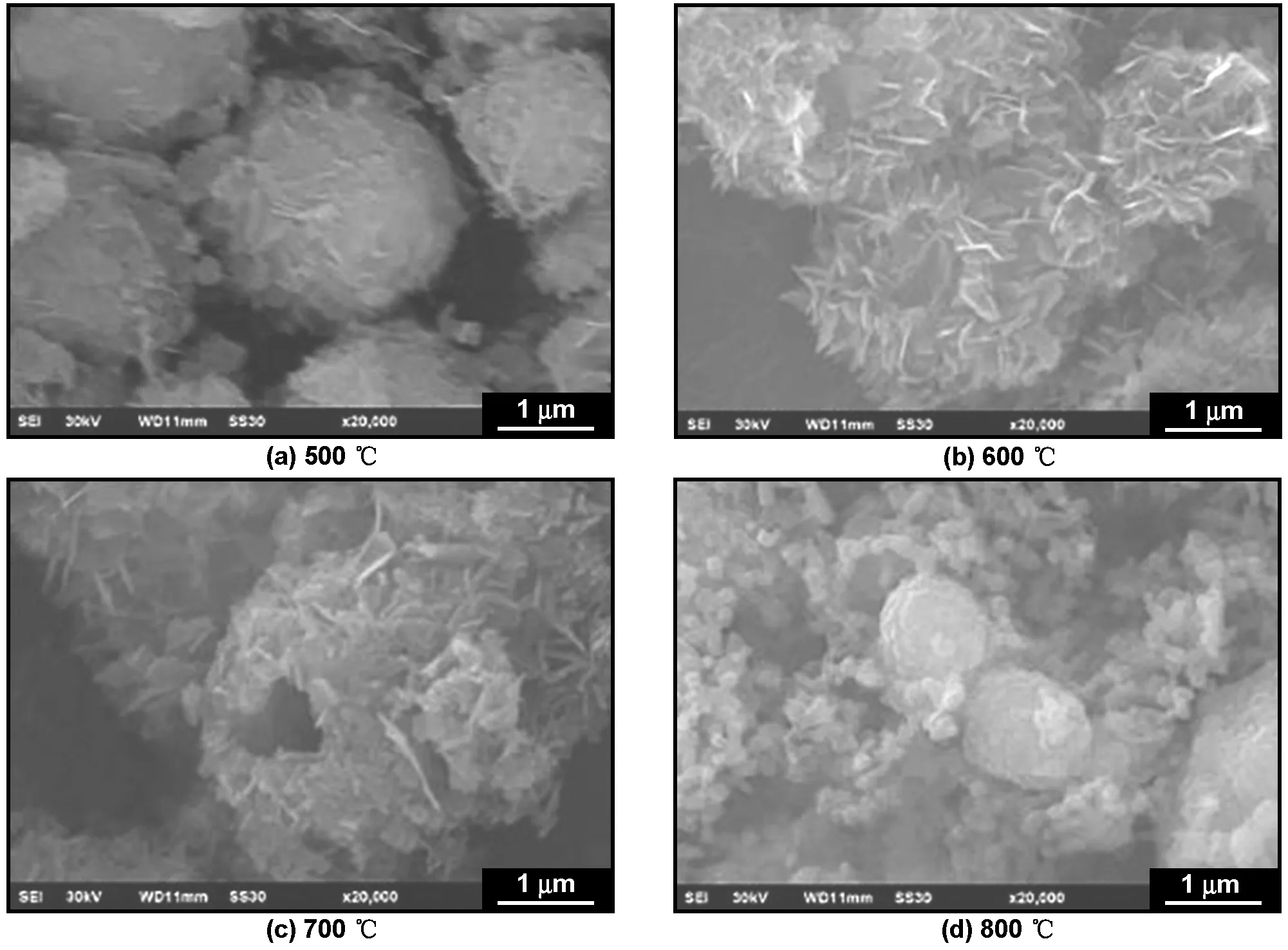
图3 不同温度下煅烧制得的Li4Ti5O12样品SEM图[55]
2.3 微米-纳米混合结构
如前所述,纳米尺寸与孔洞结构有效减小锂离子扩散距离与增加电解液接触面积,提高钛酸锂的倍率性能。然而,随着表面积的增加,振实密度降低且不可逆容量增加,导致电池的体积能量密度降低。为改善这一特点,制备微米-纳米混合结构材料,同时利用两种尺寸材料的优点,是获得高性能钛酸锂锂离子电池的一种新思路。
介孔微球体钛酸锂材料符合这样的设计构思。Tang等[56]制备的单分散性纳米粒子通过堆积生成200~400 nm的Li4Ti5O12颗粒,孔间隙为5~10 nm,可保存纳米粒子的优良特性;纳米粒子堆积的二次微球可保证高振实密度。

图4 1.0~2.5 V电压下700 ℃煅烧制得的Li4Ti5O12样品在不同倍率下的恒流充放电曲线和比容量[55]
Fig 4 Galvanostatic charge-discharge curves and rate performance of the hierarchical Li4Ti5-O12spheres calcinated at 700 ℃ between 1.0 and 2.5 V at different current densities[55]
这种材料在30 C倍率下具有114 mAh/g的高比容量,且以20 C倍率200次循环后仍可保持125 mAh/g的比容量,表现出良好的循环特性,与单一形貌的钛酸锂纳米棒[57]相比具有更好的性能。
图5为钛酸锂微球制备过程中的扫描电子显微镜图[56]。与之类似,通过水热法合成的多孔层状高结晶钛酸锂微球同样具备优良的倍率和循环性能[58]。
尽管目前在纳米钛酸锂材料的制备上取得了巨大进步,其实际应用仍面临一些挑战。首先,低温方法难以使纳米尺寸与最佳结晶度匹配;熔融盐法可使两者有效结合,但形成的钛酸锂纯度不高;其次,纳米粒子的团聚和纳米颗粒间接触的排斥力增强,限制了材料的倍率性能;此外,针对简易低组装密度制作方式的改良尚待进一步研究。
3 钛酸锂材料的表面改性
针对钛酸锂材料的低导电性,通过碳材料、金属材料复合和有机无机复合物材料来对其进行表面改性是一种很好的解决方案。
3.1 碳包覆
碳包覆是改善Li4Ti5O12电化学性能的一种重要方法[59]。在钛酸锂充放电过程中,综合考虑电子电导率和离子扩散速率,对于实现最佳倍率性能是至关重要的。电子电导率是材料传递电子的能力,而离子扩散速率反映了锂离子在材料中迁移速率的大小。通过将含碳物质加热分解,导电碳可以分散或包覆于颗粒表面。

图5 水合氧化钛球体、退火前钛酸锂微球和500 ℃退火1 h后钛酸锂微球 的SEM图[56]
Fig 5 SEM images of hydrous titanium oxide spheres, as-synthesized Li4Ti5O12spheres before annealing and spinel Li4Ti5O12spheres after annealing at 500 ℃ for 1 h[56]
在制备钛酸锂材料过程中,导电碳的添加可以使反应前驱体更加紧密、均匀的混合,使材料导电性和电池的能量密度得到提升。各类碳材料如聚乙炔黑[60-61]、海藻酸[62]、蔗糖[63-64]、聚乙烯醇[65]、柠檬酸[66-67]、沥青[68]、葡萄糖[69]、炭黑[70]、聚苯胺[71]以及石墨烯[72-76]等均运用于Li4Ti5O12的包覆。Guo等[63]以蔗糖作为碳源,分别在TiO2与Li2CO3反应前进行碳包覆,离心煅烧后得到C含量5%的C-Li4Ti5O12复合物。在电化学性能测试中前驱体TiO2预先进行碳包覆的产物性能表现最好,在0.2,5与30 C下比容量分别为171,150和82 mAh/g。Jung等[68]采用沥青作为碳源进行碳包覆,将TiCl4与尿素和硫酸铵混合后在120 ℃ 加热24 h,冷却干燥后在400 ℃下煅烧4 h得到TiO2前驱体,然后与Li2CO3和沥青混合,在900 ℃氩气环境中煅烧20 h制得表面包覆整齐碳层的钛酸锂材料,在1与50 C倍率下充放电,比容量可达163和142 mAh/g。
另一方面,碳包覆层的存在在一定程度上阻碍了锂离子的脱嵌。对此,解决方案之一是包覆具有纳米结构的钛酸锂,诸如纳米杆、孔洞球体和纳米粒子等。尺寸上的减小便于锂离子进行晶格扩散,也有助于电极与电解液的接触。Luo等[69]以葡萄糖作为碳源,采用水热法制备碳包覆钛酸锂纳米棒,碳包覆层厚度为1~3 nm。实验结果表明,产物在10 C倍率下放电比容量为92.7 mAh/g。图6为合成过程中的TiO2纳米管前驱体、Li4Ti5O12和Li4Ti5O12/C的SEM图[69]。
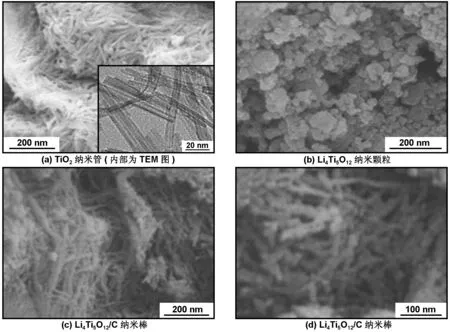
图6 TiO2纳米管的FESEM图(内部为TEM图)、Li4Ti5O12纳米颗粒和Li4Ti5O12/C纳米棒的SEM图[69]
Fig 6 Typical SEM images of TiO2nanotubes (inset shows the TEM image of TiO2nanotubes), Li4Ti5O12nanoparticles and Li4Ti5O12/C nanorods[69]
碳包覆可以提高钛酸锂材料表面的电导率,碳膜的形成可以有效抑制纳米粒子团聚,从而提升了电池的循环稳定性[77-78]。钛酸锂材料中锂离子的扩散主要包括体相扩散、液相扩散和包覆层扩散。材料本身的固有电导率是制约其性能的主要因素,碳包覆对于改善材料本身的导电性具有一定的局限,因此对钛酸锂材料进行金属元素的修饰是提高材料电导率的重要研究方向。
3.2 钛酸锂-金属复合材料
除了碳包覆及与碳材料的复合外,钛酸锂材料也可以通过引入金属元素形成Li4Ti5O12/M (M=Au、Cu、Ag等)新材料。尽管失去了部分比容量,但新复合材料的导电性能得到明显提高,这与离子掺杂情形类似,唯一的区别是金属阳离子无法进入晶格结构,无法与锂离子一同参与电化学反应[79-85]。表1总结了不同金属材料和实验制备方法对钛酸锂-金属复合材料性能的影响。
3.3 通过形成新表面相的改性
除了以上介绍的几种导电材料外,合成新的导电相是提高材料导电性的另一种手段[86-91]。例如Park等[86]研究发现表面氮化可以使钛酸锂表面原子与氮原子形成化学键,在钛酸锂表面附着一层致密导电层。当钛酸锂处于热氨气流中时,会产生氮化钛与碳酸锂位于表面、钛酸锂占据主体的核-壳结构,且合成过程中晶格常数不会变化。混合价态中间相Li4+δTi5O12和导电层TiN的引入极大提高了钛酸锂的电导性能。正因如此,氮改性的钛酸锂在10 C倍率下可以保持165 mAh/g的容量。图7为氮改性前后Li4Ti5O12的SEM与TEM图。
表1 不同金属复合材料种类和制备方法对钛酸锂-金属复合材料性能的影响
Table 1 The effects of different types of metal and preparation process on the performance of Li4Ti5O12-metal composite
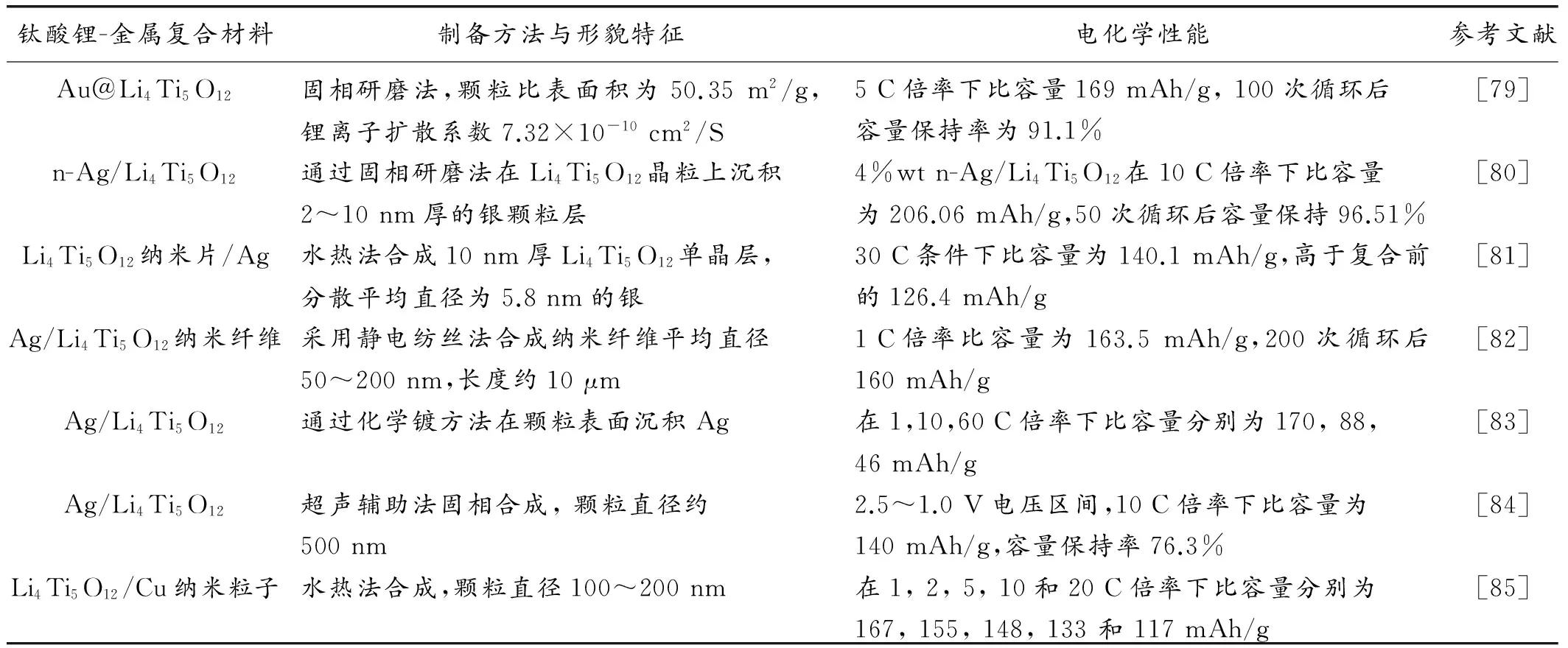
钛酸锂-金属复合材料制备方法与形貌特征电化学性能参考文献Au@Li4Ti5O12固相研磨法,颗粒比表面积为50.35m2/g,锂离子扩散系数7.32×10-10cm2/S5C倍率下比容量169mAh/g,100次循环后容量保持率为91.1%[79]n-Ag/Li4Ti5O12通过固相研磨法在Li4Ti5O12晶粒上沉积2~10nm厚的银颗粒层4%wtn-Ag/Li4Ti5O12在10C倍率下比容量为206.06mAh/g,50次循环后容量保持96.51%[80]Li4Ti5O12纳米片/Ag水热法合成10nm厚Li4Ti5O12单晶层,分散平均直径为5.8nm的银30C条件下比容量为140.1mAh/g,高于复合前的126.4mAh/g[81]Ag/Li4Ti5O12纳米纤维采用静电纺丝法合成纳米纤维平均直径50~200nm,长度约10μm1C倍率比容量为163.5mAh/g,200次循环后160mAh/g[82]Ag/Li4Ti5O12通过化学镀方法在颗粒表面沉积Ag在1,10,60C倍率下比容量分别为170,88,46mAh/g[83]Ag/Li4Ti5O12超声辅助法固相合成,颗粒直径约500nm2.5~1.0V电压区间,10C倍率下比容量为140mAh/g,容量保持率76.3%[84]Li4Ti5O12/Cu纳米粒子水热法合成,颗粒直径100~200nm在1,2,5,10和20C倍率下比容量分别为167,155,148,133和117mAh/g[85]
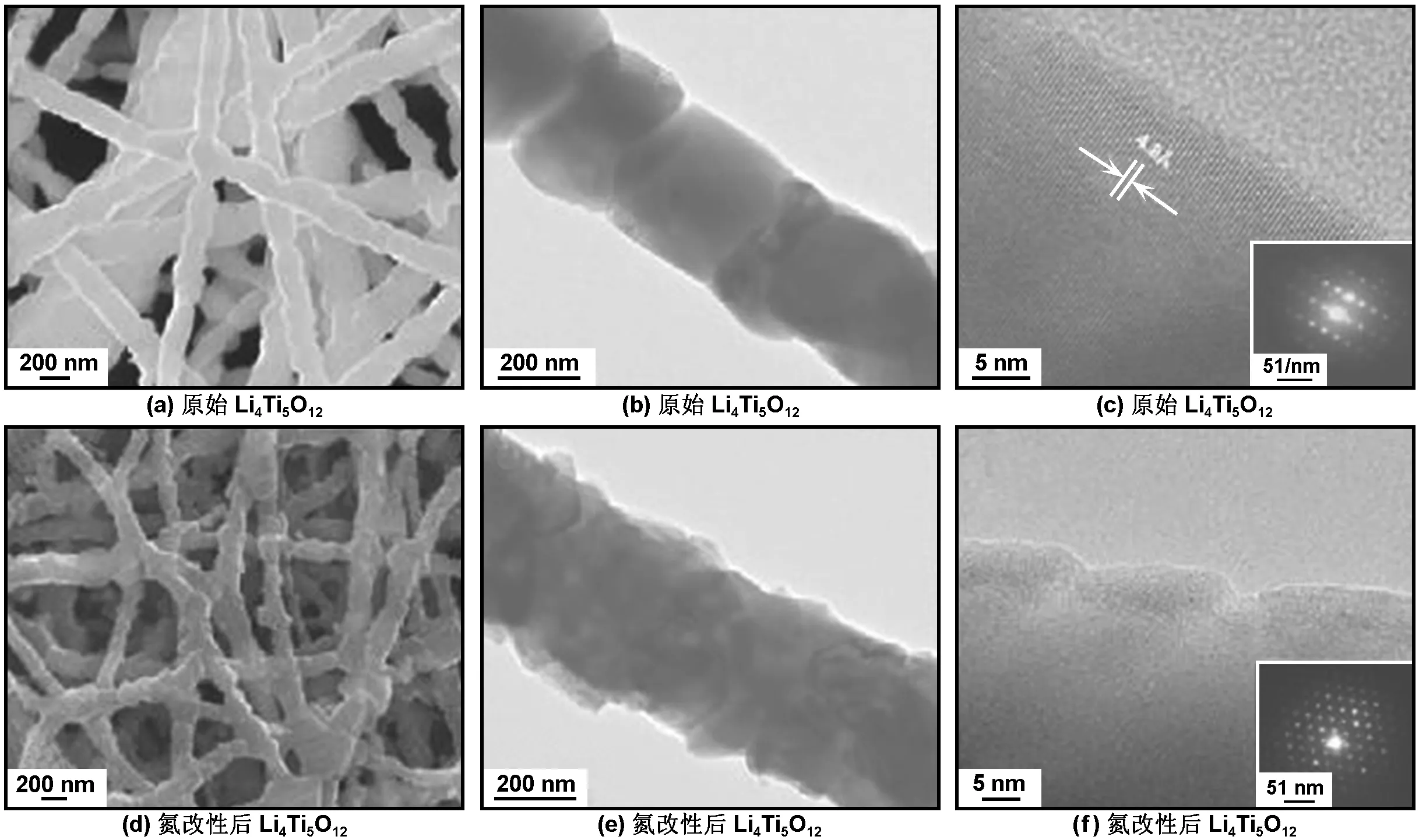
图7 原始Li4Ti5O12与氮改性后Li4Ti5O12的FESEM、TEM与高分辨透射电子显微镜图[86]
Fig 7 SEM, TEM and high resolution-TEM images of pristine Li4Ti5O12nanofibers and nitridated Li4Ti5O12nanofibers, respectively[86]
此外,Gao等[87]还通过氮化的修饰手段得到Li4Ti5O12/TiN复合物。产物在3 C倍率下首次放电比容量为130.8 mAh/g,是Li4Ti5O12的2.6倍;0.1 C下循环100次后,仍有97%的容量保持率。Zhao等[88]将1-乙基3-甲基二氰铵的离子液体作为前驱体,可以产生高温分解的含碳非晶形层与金属性的导电TiNx界面,形成三维导电结构。这比孔状碳包覆不含氮元素的钛酸锂具备更好的倍率性能,体现出钛-氮-碳表面相在提高电导性与钛酸锂表面稳定性上的优势。
通常,合成新导电相可以使导电层与电极材料之间更好接触。值得注意的是,放电容量会因钛酸锂表面的相反应而减小,因此,在一定程度内的表面改性可以使材料在可接受的容量减小范围内获得优良的导电性能。
4 离子掺杂的钛酸锂材料
离子掺杂是提高钛酸锂材料电子导电性的重要方法之一[92],这种方法使部分Ti4+转变为Ti3+,产生Ti3+/Ti4+混合物作为电荷补偿,增加了电子的集中度[93-97]。多种阴阳离子都是可以选择掺杂的对象,诸如Ta5+[98]、Ca2+[99]、Zr4+[100]、V5+[101]、 Mg2+[102]、La3+[103]、Zn2+[104]、Ru4+[105]、Ni2+[106]、Sc3+[107]、Gd3+[108-109]、W6+[110-111]、Nb5+[112]、Na+[113-114]、K+[115]、Ce3+[116-117]、Al3+[118]、Nd3+[119]、Cu2+[120]、Sr2+[121]、F-[122]、Br-[123]等,离子掺杂在Li+, Ti4+和O2-的空位处,相关结构特性、离子价态和导电性能被广泛研究。掺杂后的复合物除了电子导电性提高外,掺杂离子在钛酸锂的晶格结构的引入会产生晶格扭曲,进而影响电池比容量和循环性能。
Guo等[98]对钛酸锂中Ta5+掺杂进行了研究。Ta5+的嵌入增加了钛酸锂的晶格常数,加速锂离子的迁移速率。掺杂后部分Ta5+替换Ti4+进入晶格。作为电荷补偿,Li4Ti5O12材料中部分Ti4+转化为Ti3+,而Ti3+的存在会提高材料整体的电子导电性,从而使材料的电化学性质得到提升。在对电导率的测试中,掺杂后的Li4Ti4.995Ta0.005O12在10 C倍率下比容量为95.1 mAh/g,与未掺杂的钛酸锂50.4 mAh/g的比容量相比提高了88.7%。Zhang等[99]以Li2CO3、TiO2和CaO作为前驱体,采用固相反应制备了Ca2+掺杂的Li4-xCaxTi5O12(x= 0, 0.05, 0.1, 0.15, 0.2),平均粒径为1~2 μm。测试发现当x=0.1时Li3.9Ca0.1Ti5-O12性能最佳,在倍率为1,5和10 C时100次循环后,比容量分别为162.4,148.8和138.7 mAh/g。
掺杂离子会部分进入钛酸锂晶格中,因此推测掺杂可能会影响材料的形貌和颗粒尺寸[124]。Nithya等[100]采用熔融盐法制备Zr4+掺杂的钛酸锂,其粒径可降低至1 μm,减缓团聚作用,有利于倍率性能的提高。其掺杂样品在0.01 C倍率下放电容量为325 mAh/g。
一些离子的掺杂会通过阳极放电至0 V以改变钛酸锂的电化学性能,这类深放电阳极有利于拓宽电化学反应窗口,增加锂离子电池的比容量[125-126]。Yang等[101]发现钒元素掺杂形成的Li4Ti4.95V0.05O12/C。由于V5+的引入降低了电极极化,在0.5~2.5 V的电位窗口与0.1 C倍率下,0,5%,10%与15%的V5+掺杂产物的放电容量分别为158.7,173.2,179.8和181.3 mAh/g。这是因为钒离子与氧离子的结合可以保持局部的对称性,使得材料尖晶石的结构保持稳定。图8为原始Li4Ti5O12,加入呋喃树脂后的Li4Ti5O12和V5+掺杂后Li4Ti5O12的SEM图。

图8 原始Li4Ti5O12,含5%呋喃树脂的Li4Ti5O12/C和10%V掺杂含5%呋喃树脂的Li4Ti5O12/C的SEM图[101]
Fig 8 SEM images of pristine Li4Ti5O12, Li4Ti5O12/C with 5wt% Furan resin and 10%V-doped Li4Ti5O12/C with 5wt% Furan resin[101]
在O位掺杂F-,Br-等负离子,同样可以增加Ti3+的含量,提高Li4Ti5O12的电子导电性,改善材料的电化学性能。比如,Wang等[123]通过在O位上掺杂Br-合成Li4Ti5O11.7Br0.3,在经历100次循环后在10和20 C下比容量为138和104 mAh/g,与未掺杂Li4Ti5O12相比提高了4~7倍。
在离子掺杂中,引入掺杂离子对于钛酸锂材料结构、界面性质、基体和相应离子分布的影响有待深入研究。其中需使用诸如电子顺磁共振(EPR)、微拉曼光谱仪(micro-Raman spectroscopy)和核磁共振(NMR)等新型设备,它们在深刻认识材料结构和电化学性能之间的关系中起到了重要的作用[127-129]。
5 结 语
钛酸锂作为锂离子电池负极材料具有结构稳定、安全性高和循环稳定等优点,在动力储能电池方面具有良好的应用前景。目前,国内外研究人员对钛酸锂进行了深入的研究,针对钛酸锂固有的电子电导率低,大倍率性能差的缺陷,采用减小尺寸、表面改性和离子掺杂等手段改善钛酸锂的电化学特性。这也是未来钛酸锂材料的发展方向,其中的诸多方面值得进一步探讨研究:深入研究Li4Ti5O12不同形貌与尺寸对于性能的影响;选用更多过渡金属离子以及非金属离子对Li4Ti5O12进行掺杂改性,尝试使用两种或多种离子进行双掺杂或共掺杂手段降低Li4Ti5O12高充放电平台,以改善Li4Ti5O12电化学性能;基于Li4Ti5O12自身优异的循环稳定性能,尝试引入其它高容量材料以制备兼具两者优点的复合材料等。
[1] Yi T F, Yang S Y, Xie Y. Recent advances of Li4Ti5O12as a promising next generation anode material for high power lithium-ion batteries[J]. Journal of Materials Chemistry A, 2015, (3): 5750-5777.
[2] Yang S B, Feng X L, Zhi L J, et al. Nanographene-constructed hollow carbon spheres and their favorable electroactivity with respect to lithium storage[J]. Advanced Materials, 2010, 22(7): 838-842.
[3] Chen Z H, Belharouak I, Sun Y K, et al. Titanium-based anode materials for safe lithium-ion batteries[J]. Advanced Functional Materials, 2013, 23(8): 959-969.
[4] Ohzuku T, Ueda A, Yamamoto N. Zero-strain insertion material of Li[Li1/3Ti5/3]O4for rechargeable lithium cells[J]. Journal of the Electrochemical Society, 1995, 142(5): 1431-1435.
[5] Armand M, Tarascon J M. Building better batteries[J]. Nature, 2008, 451(7179): 652-657.
[6] Zhu G N, Wang Y G, Xia Y Y. Ti-based compounds as anode materials for Li-ion batteries[J]. Energy & Environmental Science, 2012,(5): 6652-6667.
[7] Shi J, Liang Y H, Li L L, et al. Evaluation of the electrochemical characteristics of silicon/lithium titanate composite as anode material for lithium ion batteries[J]. Electrochimica Acta, 2015, 155: 125-131.
[8] Hao Shiji, Li Chunli, Zhu Kai, et al. The preparation of high performance porous silicon powders by etching Al-Si alloy in acid solution for lithium ion battery[J]. Journal of Electrochemistry, 2014, (01): 1-4. 郝世吉, 李纯莉, 朱 凱,等. 酸浸蚀Al-Si合金制备锂离子电池高性能多孔硅负极材料[J]. 电化学, 2014, (01): 1-4.
[9] Jiang Z Y, Li C L, Hao S J, et al. An easy way for preparing high performance porous silicon powder by acid etching Al-Si alloy powder for lithium ion battery[J]. Electrochimica Acta, 2014, 115: 393-398.
[10] Li C L, Zhang P, Jiang Z Y. Effect of nano Cu coating on porous Si prepared by acid etching Al-Si alloy powder[J]. Electrochimica Acta, 2015, 161: 408-412.
[11] Tang Y X, Zhang Y Y, Deng J Y, et al. Mechanical force-driven growth of elongated bending TiO2-based nanotubular materials for ultrafast rechargeable lithium ion batteries[J]. Advanced Materials, 2014, 26(35): 6111-6118.
[12] Wang H E, Jin J, Cai Y, et al. Facile and fast synthesis of porous TiO2spheres for use on lithium ion batteries[J]. Journal of Colloid and Interface Science, 2014, 417: 144-151.
[13] Gao L, Li X R, Hu H, et al. TiO2mesoporous microspheres with nanorod structure: facile synthesis and superior electrochemical performance[J]. Electrochimica Acta, 2014, 120: 231-239.
[14] Permana A D C, Nugroho A, Chung K Y, et al. Template-free synthesis of hierarchical porous anatase TiO2microspheres with carbon coating and their electrochemical properties[J]. Chemical Engineering Journal, 2014, 241: 216-227.
[15] Ding S J, Lin T Q, Wang Y M, et al. New facile synthesis of TiO2hollow sphere with an opening hole and its enhanced rate performance in lithium-ion batteries[J]. New Journal of Chemistry, 2013, 37: 784-789.
[16] Zhang G Q, Wu H B, Song T, et al. TiO2hollow spheres composed of highly crystalline nanocrystals exhibit superior lithium storage properties[J]. Angewandte Chemie, 2014, 126(46): 12798-12801.
[17] Chen Y, Ma X Q, Cui X L, et al. In situ synthesis of carbon incorporated TiO2with long-term performance as anode for lithium-ion batteries[J]. Journal of Power Sources, 2016, 302: 233-239.
[18] Lou X W, Deng D, Lee J Y, et al. Self-supported formation of needlelike Co3O4nanotubes and their application as lithium-ion battery electrodes[J]. Advanced Materials, 2008, 20(2): 258-262.
[19] Mai Y J, Zhang D, Qiao Y Q, et al. MnO/reduced graphene oxide sheet hybrid as an anode for Li-ion batteries with enhanced lithium storage performance[J]. Journal of Power Sources, 2012, 216: 201-207.
[20] Wang Y K, Yang L C, Hu R Z, et al. A stable and high-capacity anode for lithium-ion battery: Fe2O3wrapped by few layered graphene[J]. Journal of Power Sources, 2015, 288: 314-319.
[21] Zhang Z H, Wang F, An Q, et al. Synthesis of graphene@Fe3O4@C core-shell nanosheets for high-performance lithium ion batteries[J]. Journal of Materials Chemistry A, 2015, (3): 7036-7043.
[22] Wang F, Li W, Hou M Y, et al. Sandwich-like Cr2O3-graphite intercalation composites as high-stability anode materials for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2015, (3): 1703-1708.
[23] Sun X C, Radovanovic P V, Cui B. Advances in spinel Li4Ti5O12anode materials for lithium-ion batteries[J]. New Journal of Chemistry, 2015, 39: 38-63.
[24] Li N, Chen Z P, Ren W C, et al. Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates[J]. Proceedings of the National Academy of Sciences, 2012, 109(43): 17360-17365.
[25] Ganapathy S, Wagemaker M. Nanosize storage properties in spinel Li4Ti5O12explained by anisotropic surface lithium insertion[J]. ACS Nano, 2012, 6(10): 8702-8712.
[26] Guo J, Zuo W, Cai Y, et al. A novel Li4Ti5O12-based high-performance lithium-ion electrode at elevated temperature[J]. Journal of Materials Chemistry A, 2015, (3): 4938-4944.
[27] Zhang Yonglong, Hu Xuebu, Xu Yunlan, et al. Recent progress of Li4Ti5O12with different morphologies as anode material[J]. Acta Chimica Sinica, 2013, 10: 1341-1353. 张永龙, 胡学步, 徐云兰,等. 不同形貌结构Li4Ti5O12负极材料的最新进展[J]. 化学学报, 2013, 10: 1341-1353.
[28] Liu T, Ni H, Song W L, et al. Enhanced electrochemical performance of Li4Ti5O12as anode material for lithium-ion batteries with different carbons as support[J]. Journal of Alloys and Compounds, 2015, 646: 189-194.
[29] Jhan Y R, Duh J G. Electrochemical performance and low discharge cut-off voltage behavior of ruthenium doped Li4Ti5O12with improved energy density[J]. Electrochimica Acta, 2012, 63: 9-15.
[30] Brousse T, Fragnaud P, Marchand R, et al. All oxide solid-state lithium-ion cells[J]. Journal of Power Sources, 1997, 68: 412-415.
[31] Liu J, Du C Q, Tang Z Y, et al. In situ nickel/carbon coated lithium titanium oxide anode material with improved electrochemical properties[J]. Electrochimica Acta, 2014, 143: 56-62.
[32] Hong Z S, Zheng X Z, Ding X K, et al. Complex spinel titanate nanowires for a high rate lithium-ion battery[J]. Energy & Environmental Science, 2011, 4: 1886-1891.
[33] Wang Y, Li H, He P, et al. Nano active materials for lithium-ion batteries[J]. Nanoscale, 2010, (2): 1294-1305.
[34] Lin C Y, Duh J G. Porous Li4Ti5O12anode material synthesized by one-step solid state method for electrochemical properties enhancement[J]. Journal of Alloys and Compounds, 2011, 509: 3682-3685.
[35] Lin C F, Lai M O, Lu L, et al. Spinel Li4-2xCo3xTi5-xO12(0≤x≤0.5) for lithium-ion batteries: crystal structures, material properties, and battery performances[J]. The Journal of Physical Chemistry C, 2014, 118(26): 14246-14255.
[36] Borghols W J H, Wagemaker M, Lafont U, et al. Size effects in the Li4+xTi5O12spinel[J]. Journal of the American Chemical Society, 2009, 131(49): 17786-17792.
[37] Ren Y R, Lu P, Huang X B, et al. In-situ synthesis of nano-Li4Ti5O12/C composite as an anode material for Li-ion batteries[J]. Solid State Ionics, 2015, 274: 83-87.
[38] Liu H, Wen G, Bi S, et al. Enhanced rate performance of nanosized Li4Ti5O12/graphene composites as anode material by a solid state-assembly method[J]. Electrochimica Acta, 2015, 171: 114-120.
[39] Han S W, Jeong J, Yoon D H. Effects of high-energy milling on the solid-state synthesis of pure nano-sized Li4Ti5O12for high power lithium battery applications[J]. Applied Physics A: Materials Science & Processing, 2014, 114: 925-930.
[40] Kyeremateng N A, Dinh T M, Pech D. Electrophoretic deposition of Li4Ti5O12nanoparticles with a novel additive for Li-ion microbatteries[J]. RSC Advances, 2015, 5: 61502-61507.
[41] Yi T F, Fang Z K, Xie Y, et al. Rapid charge-discharge property of Li4Ti5O12-TiO2nanosheet and nanotube composites as anode material for power lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2014, 6(22): 20205-20213.
[42] Shen Y B, Sondergaard M, Christensen M, et al. Solid state formation mechanism of Li4Ti5O12from an anatase TiO2source[J]. Chemistry of Materials, 2014, 26: 3679-3686.
[43] Hayashi H, Nakamura T, Ebina T. Hydrothermal synthesis of Li4Ti5O12nanoparticles using a supercritical flow reaction system[J]. Journal of the Ceramic Society of Japan, 2014, 122: 78-82.
[44] Wang D, Wu X, Zhang Y, et al. The influence of the TiO2particle size on the properties of Li4Ti5O12anode material for lithium-ion battery[J]. Ceramics International, 2014, 40: 3799-3804.
[45] Kashkooli A G, Lui G, Farhad S, et al. Nano-particle size effect on the performance of Li4Ti5O12spinel[J]. Electrochimica Acta, 2016, 196: 33-40.
[46] Wang D, Wu X Y, Zhang Y Y, et al. The influence of the TiO2particle size on the properties of Li4Ti5O12anode material for lithium-ion battery[J]. Ceramics International, 2014, 40(2): 3799-3804.
[47] Zhu Y R, Wang P, Yi T F, et al. Improved high-rate performance of Li4Ti5O12/carbon nanotube nanocomposite anode for lithium-ion batteries[J]. Solid State Ionics, 2015, 276: 84-89.
[48] Zhang P, Chen M, Shen X, et al. Preparation of Li4Ti5O12nanosheets/carbon nanotubes composites and application of anode materials for lithium-ion batteries[J]. Electrochimica Acta, 2016, 204: 92-99.
[49] Lan C K, Bao Q, Huang Y H, et al. Embedding nano-Li4Ti5O12in hierarchical porous carbon matrixes derived from water soluble polymers for ultra-fast lithium ion batteries anodic materials[J]. Journal of Alloys and Compounds, 2016, 673: 336-348.
[50] Mu D B, Chen Y J, Wu B R, et al. Nano-sized Li4Ti5O12/C anode material with ultrafast charge/discharge capability for lithium ion batteries[J]. Journal of Alloys and Compounds, 2016, 671: 157-163.
[51] Lin Y S, Duh J G. Facile synthesis of mesoporous lithium titanate spheres for high rate lithium-ion batteries[J]. Journal of Power Sources, 2011, 196: 10698-10703.
[52] Yu L, Wu B H, Lou X W. Mesoporous Li4Ti5O12hollow spheres with enhanced lithium storage capability[J]. Advanced Materials, 2013, 25(16): 2296-2300.
[53] He N D, Wang B S, Huang J J. Preparation and electrochemical performance of monodisperse Li4Ti5O12hollow spheres[J]. Journal of Solid State Electrochemistry, 2010, 14(7): 1241-1246.
[54] Shao D, He J R, Luo Y, et al. Synthesis and electrochemical performance of nanoporous Li4Ti5O12anode material for lithium-ion batteries[J]. Journal of Solid State Electrochemistry, 2012, 16(6): 2047-2053.
[55] Zhou Q, Liu L, Guo H P, et al. Synthesis of nanosheets-assembled lithium titanate hollow microspheres and their application to lithium ion battery anodes[J]. Electrochimica Acta, 2015, 151: 502-509.
[56] Tang Y F, Yang L, Qiu Z, et al. Template-free synthesis of mesoporous spinel lithium titanate microspheres and their application in high-rate lithium ion batteries[J]. Journal of Materials Chemistry, 2009, 19: 5980-5984.
[57] Lee S C, Lee S M, Lee J W, et al. Spinel Li4Ti5O12nanotubes for energy storage materials[J]. The Journal of Physical Chemistry C, 2009, 113(42): 18420-18423.
[58] Shen L F, Yuan C Z, Luo H J, et al. Facile synthesis of hierarchically porous Li4Ti5O12microspheres for high rate lithium ion batteries[J]. Journal of Materials Chemistry, 2010, 20: 6998-7004.
[59] Zhang Ning, Liu Yongchang, Tao Zhanliang, et al. Research progress in carbon coating on Li4Ti5O12anode materials[J]. Surface Technology, 2015, (01): 1-7. 张 宁, 刘永畅, 陶占良,等. 钛酸锂表面碳包覆改性研究进展[J]. 表面技术, 2015, (01): 1-7.
[60] Gong L J, Chen Y X, Yu H J, et al. Carbon-coated Li4Ti5O12anode materials synthesized using H2TiO3as Ti source[J]. Journal of Materials Science & Technology, 2014, 30(11): 1092-1095.
[61] Pan M J, Zhang L, Gong L J, et al. Investigation of carbon-coated lithiated Li4+xTi5O12/C for lithium-ion batteries[J]. Materials Research Bulletin, 2015, 71: 48-52.
[62] Tang H Q, Zan L X, Mao W F, et al. Improved rate performance of amorphous carbon coated lithium zinc titanate anode material with alginic acid as carbon precursor and particle size controller[J]. Journal of Electroanalytical Chemistry, 2015, 751: 57-64.
[63] Guo X, Xiang H F, Zhou T P, et al. Morphologies and structures of carbon coated on Li4Ti5O12and their effects on lithium storage performance[J]. Electrochimica Acta, 2014, 130: 470-476.
[64] Zhang H Q, Deng Q J, Mou C X, et al. Surface structure and high-rate performance of spinel Li4Ti5O12coated with N-doped carbon as anode material for lithium-ion batteries[J]. Journal of Power Sources, 2013, 239: 538-545.
[65] Fang W, Cheng X Q, Zuo P J, et al. A facile strategy to prepare nano-crystalline Li4Ti5O12/C anode material via polyvinyl alcohol as carbon source for high-rate rechargeable Li-ion batteries[J]. Electrochimica Acta, 2013, 93: 173-178.
[66] Kuo Y C, Lin J Y. One-pot sol-gel synthesis of Li4Ti5O12/C anode materials for high-performance Li-ion batteries[J]. Electrochimica Acta, 2014, 142: 43-50.
[67] Liu J, Lin Y Q, Lu T X, et al. Characterization and electrochemical properties of Li2MoO4modified Li4Ti5O12/C anode material for lithium-ion batteries[J]. Electrochimica Acta, 2015, 170: 202-209.
[68] Jung H G, Venugopal N L, Scrosati B, et al. A high energy and power density hybrid supercapacitor based on an advanced carbon-coated Li4Ti5O12electrode[J]. Journal of Power Sources, 2013, 221: 266-271.
[69] Luo H J, Shen L F, Rui K, et al. Carbon coated Li4Ti5O12nanorods as superior anode material for high rate lithium ion batteries[J]. Journal of Alloys and Compounds, 2013, 572: 37-42.
[70] Pohjalainen E, Kallioinen J, Kallio T. Comparative study of carbon free and carbon containing Li4Ti5O12electrodes[J]. Journal of Power Sources, 2015, 279: 481-486.
[71] Shi Nannan, Jiang Xue, Zhang Ying, et al. Preparation and performance of N-doped carbon coated Li4Ti5O12as anode material for lithium-ion batteries[J]. Chemical Journal of Chinese Universities, 2015, (05): 981-988. 史楠楠, 姜 雪, 张 莹,等. N掺杂C包覆Li4Ti5O12锂离子电池负极材料的制备与性能[J]. 高等学校化学学报, 2015, (05): 981-988.
[72] Li R Y, Chen T Y, Sun B B, et al. Novel lithium titanate-graphene hybrid containing two graphene conductive frameworks for lithium-ion battery with excellent electrochemical performance[J]. Materials Research Bulletin, 2015, 70: 965-975.
[73] Shi Y W, Zhang D Y, Chang C K, et al. Enhanced electrochemical performance of oxygen-deficient Li4Ti5O12-xanode material induced by graphene oxide[J]. Journal of Alloys and Compounds, 2015, 639: 274-279.
[74] Ni H, Song W L, Fan L Z. A strategy for scalable synthesis of Li4Ti5O12/reduced graphene oxide toward high rate lithium-ion batteries[J]. Electrochemistry Communications, 2014, 40: 1-4.
[75] Li R Y, Chen T Y, Sun B B, et al. Novel lithium titanate-graphene hybrid containing two grapheme conductive frameworks for lithium-ion battery with excellent electrochemical performance[J]. Materials Research Bulletin, 2015, 70: 965-975.
[76] Xue R, Yan J W, Jiang L, et al. Fabrication of lithium titanate/graphene composites with high rate capability as electrode materials for hybrid electrochemical supercapacitors[J]. Materials Chemistry and Physics, 2015, 160: 375-382.
[77] Ming H, Ming J, Li X W, et al. Hierarchical Li4Ti5O12particles co-modified with C&N towards enhanced performance in lithium-ion battery applications[J]. Electrochimica Acta, 2014, 116: 224-229.
[78] Sun X C, Hegde M, Wang J, et al. Structural analysis and electrochemical studies of carbon coated Li4Ti5O12particles used as anode for lithium-ion battery[J]. ECS Transactions, 2014, 58(14): 79-88.
[79] Wang W, Guo Y Y, Liu L X, et al. Gold coating for a high performance Li4Ti5O12nanorod aggregates anode in lithium-ion batteries[J]. Journal of Power Sources, 2014, 245: 624-629.
[80] Krajewski M, Michalska M, Hamankiewicz B, et al. Li4Ti5O12modified with Ag nanoparticles as an advanced anode material in lithium-ion batteries[J]. Journal of Power Sources, 2014, 245: 764-771.
[81] Xu G B, Li W, Yang L W, et al. Highly-crystalline ultrathin Li4Ti5O12nanosheets decorated with silver nanocrystals as a high-performance anode material for lithium ion batteries[J]. Journal of Power Sources, 2015, 276: 247-254.
[82] Kim J G, Shi D Q, Park M S, et al. Controlled Ag-driven superior rate-capability of Li4Ti5O12anodes for lithium rechargeable batteries[J]. Nano Research, 2013, 6(5): 365-372.
[83] Erdas A, Ozcan S, Nalci D, et al. Novel Ag/Li4Ti5O12binary composite anode electrodes for high capacity Li-ion batteries[J]. Surface and Coatings Technology, 2015, 271: 136-140.
[84] Ge H, Chen L, Lin S, et al. Advanced electrochemical performances of Li4Ti5O12/Ag prepared by a facile synthesis route[J]. Ionics, 2014, 20(8): 1189-1192.
[85] Cheng C L, Liu H J, Xue X, et al. Highly dispersed copper nanoparticle modified nano Li4Ti5O12with high rate performance for lithium ion battery[J]. Electrochimica Acta, 2014, 120: 226-230.
[86] Park H J, Song T, Han H, et al. Electrospun Li4Ti5O12nanofibers sheathed with conductive TiN/TiOxNylayer as an anode material for high power Li-ion batteries[J]. Journal of Power Sources, 2013, 244: 726-730.
[87] Gao Hongquan, Wang Xinyu, Zhang Zhian, et al. Modification of Li4Ti5O12anode material with urea as nitrogen source for lithium ion battery[J]. Journal of Inorganic Materials, 2010, 09: 983-988. 高宏权, 王新宇, 张治安,等. 锂离子电池Li4Ti5O12负极材料的尿素掺杂氮化修饰[J]. 无机材料学报, 2010, 09: 983-988.
[88] Zhao L, Hu Y S, Li H, et al. Porous Li4Ti5O12coated with N-doped carbon from ionic liquids for Li-Ion batteries[J]. Advanced Materials, 2011, 23(11): 1385-1388.
[89] Wan Z N, Cai R, Jiang S M, et al. Nitrogen- and TiN-modified Li4Ti5O12: one-step synthesis and electrochemical performance optimization[J]. Journal of Materials Chemistry, 2012, 22: 17773-17781.
[90] Ming H, Ming J, Li X W, et al. Synthesis of N-doped carbon coated metal oxide nanoparticles for enhanced Li-ion storage ability[J]. RSC Advances, 2013,(3): 15613-15617.
[91] Rajagopalan B, Oh E S, Chung J S. The effect of diethylenetriamine on the solvothermal reactions of polyethyleneimine-graphene oxide/lithium titanate nanocomposites for lithium battery anode[J]. Journal of Power Sources, 2015, 275: 702-711.
[92] Wang Dan, Huang Zhao, Wu Xiaoyan, et al. Effect of ion doping on electrochemical properties of Li4Ti5O12as anode material for Li-ion battery[J]. Chinese Journal of Power Sources, 2015,(09): 1826-1827. 王 丹, 黄 昭, 吴晓燕, 等. 离子掺杂对Li4Ti5O12负极材料电化学性能的影响[J]. 电源技术, 2015, (09): 1826-1827.
[93] Kim J G, Shi D Q, Park M S, et al. Zr4+Doping in Li4Ti5O12anode for lithium-ion batteries: open Li+diffusion paths through structural imperfection[J]. Chem Sus Chem, 2014, 7(5): 1451-1457.
[94] Ma Y, Ding B, Ji G, et al. Carbon-encapsulated F-doped Li4Ti5O12as a high rate anode material for Li+batteries[J]. ACS Nano, 2013, 7(12): 10870-10878.
[95] Yu Z J, Zhang X F, Yang G L, et al. High rate capability and long-term cyclability of Li4Ti4.9V0.1O12as anode material in lithium ion battery[J]. Electrochimica Acta, 2011, 56: 8611-8617.
[96] Bai Y J, Gong C, Qi Y X, et al. Excellent long-term cycling stability of La-doped Li4Ti5O12anode material at high current rates[J]. Journal of Materials Chemistry, 2012, 22: 19054-19060.
[97] Wang F, Luo L C, Du J, et al. Nitrogen-doped carbon decorated Li4Ti5O12composites as anode materials for high performance lithium-ion batteries[J]. RSC Advances, 2015, (5): 46359-46365.
[98] Guo M, Wang S Q, Ding L X, et al. Tantalum-doped lithium titanate with enhanced performance for lithium-ion batteries[J]. Journal of Power Sources, 2015, 283: 372-380.
[99] Zhang Q Y, Zhang C L, Li B, et al. Preparation and electrochemical properties of Ca-doped Li4Ti5O12as anode materials in lithium-ion battery[J]. Electrochimica Acta, 2013, 98: 146-152.
[100] Nithya V D, Sharmila S, Vediappan K, et al. Electrical and electrochemical properties of molten-salt-synthesized 0.05 mol Zr- and Si-doped Li4Ti5O12microcrystals[J]. Journal of Applied Electrochemistry, 2014, 44: 647-654.
[101] Yang C C, Hu H C, Lin S J, et al. Electrochemical performance of V-doped spinel Li4Ti5O12/C composite anode in Li-half and Li4Ti5O12/LiFePO4-full cell[J]. Journal of Power Sources, 2014, 258: 424-433.
[102] Lao M, Qian S, Yu H, et al. Enhanced electrochemical properties of Mg2+doped Li2Na2Ti6O14anode material for lithium-ion batteries[J]. Electrochimica Acta, 2016, 196: 642-652.
[103] Wang D, Zhang C M, Zhang Y Y, et al. Synthesis and electrochemical properties of La-doped Li4Ti5O12as anode material for Li-ion battery[J]. Ceramics International, 2013, 39(5): 5145-5149.
[104] Zhang Z W, Cao L Y, Huang J F, et al. Hydrothermal synthesis of Zn-doped Li4Ti5O12with improved high rate properties for lithium ion batteries[J]. Ceramics International, 2013, 39(6): 6139-6143.
[105] Wang W, Wang H L, Wang S B, et al. Ru-doped Li4Ti5O12anode materials for high rate lithium-ion batteries[J]. Journal of Power Sources, 2013, 228: 244-249.
[106] Lin C F, Lai M O, Lu L, et al. Structure and high rate performance of Ni2+doped Li4Ti5O12for lithium ion battery[J]. Journal of Power Sources, 2013, 244: 272-279.
[107] Zhang Y Y, Zhang C M, Lin Y, et al. Influence of Sc3+doping in B-site on electrochemical performance of Li4Ti5O12anode materials for lithium-ion battery[J]. Journal of Power Sources, 2014, 250: 50-57.
[108] Zhang Q Y, Verde M G, Seo J K, et al. Structural and electrochemical properties of Gd-doped Li4Ti5O12as anode material with improved rate capability for lithium-ion batteries[J]. Journal of Power Sources, 2015, 280: 355-362.
[109] Xu G B, Yang L W, Wei X L, et al. Highly-crystalline ultrathin gadolinium doped and carbon-coated Li4Ti5O12nanosheets for enhanced lithium storage[J]. Journal of Power Sources, 2015, 295: 305-313.
[110] Zhang Q Y, Zhang C L, Li B, et al. Preparation and characterization of W-doped Li4Ti5O12anode material for enhancing the high rate performance[J]. Electrochimica Acta, 2013, 107: 139-146.
[111] Zhang Q Y, Lu H S, Zhong H X, et al. W6+, Br--codoped Li4Ti5O12anode with super rate performance for Li-ion batteries[J]. Journal of Materials Chemistry A, 2015, (3): 13706-13716.
[112] Kim S K, Kwon E S, Kim T H, et al. Effects of atmospheric Ti(Ⅲ) reduction on Nb2O5-doped Li4Ti5O12anode materials for lithium ion batteries[J]. Ceramics International, 2014, 40(6): 8869-8874.
[113] Yi T F, Yang S Y, Li X Y, et al. Sub-micrometric Li4-xNaxTi5O12(0≤x≤0.2) spinel as anode material exhibiting high rate capability[J]. Journal of Power Sources, 2014, 246: 505-511.
[114] Wu K Q, Shu J, Lin X T, et al. Phase composition and electrochemical performance of sodium lithium titanates as anode materials for lithium rechargeable batteries[J]. Journal of Power Sources, 2015, 275: 419-428.
[115] Mona S, Jordi C, Marca D. Lepidocrocite-type layered titanate structures: new lithium and sodium ion intercalation anode materials[J]. Chemistry of Materials, 2014, 26: 2502-2512.
[116] Zhang Q Y, Liu Y, Lu H S, et al. Ce3+-doped Li4Ti5O12with CeO2surface modification by a sol-gel method for high-performance lithium-ion batteries[J]. Electrochimica Acta, 2016, 189: 147-157.
[117] Zhou T P, Feng X Y, Guo X, et al. Solid-state synthesis and electrochemical performance of Ce-doped Li4Ti5O12anode materials for lithium-ion batteries[J]. Electrochimica Acta, 2015, 174: 369-375.
[118] Li D, Sun Y, Liu X Z, et al. Doping-induced memory effect in Li-ion batteries: the case of Al-doped Li4Ti5O12[J]. Chemical Science, 2015, 6: 4066-4070.
[119] Xia C Y, Nian C C, Huang Z, et al. One-step synthesis of carbon-coated Li4Ti4.95Nd0.05O12by modified citric acid sol-gel method for lithium-ion battery[J]. Journal of Sol-Gel Science and Technology, 2015, 75(1): 38-44.
[120] Lin C, Ding B, Xin Y, et al. Advanced electrochemical performance of Li4Ti5O12-based materials for lithium-ion battery: Synergistic effect of doping and compositing. Journal of Power Sources, 2014, 248: 1034-1041.
[121] Wu H, Chang S, Liu X, et al. Sr-doped Li4Ti5O12as the anode material for lithium-ion batteries[J]. Solid State Ionics, 2013, 232: 13-18.
[122] Zhao Z, Xu Y L, Ji M D, et al. Synthesis and electrochemical performance of F-doped Li4Ti5O12for lithium-ion batteries[J]. Electrochimica Acta, 2013, 109: 645-650.
[123] Wang J Q, Yang Z Z, Li W H, et al. Nitridation Br-doped Li4Ti5O12anode for high rate lithium ion batteries[J]. Journal of Power Sources, 2014, 266: 323-331.
[124] Yi T F, Liu H P, Zhu Y R, et al. Improving the high rate performance of Li4Ti5O12through divalent zinc substitution[J]. Journal of Power Sources, 2012, 215: 258-265.
[125] Liu J H, Sun X M, Li Y A, et al. Electrochemical performance of LiCoO2/SrLi2Ti6O14batteries for high-power applications[J]. Journal of Power Sources, 2014, 245: 371-376.
[126] Li H S, Shen L F, Ding B, et al. Ultralong SrLi2Ti6O14nanowires composed of single-crystalline nanoparticles: promising candidates for high-power lithium ions batteries[J]. Nano Energy, 2015, 13: 18-27.
[127] Lin X T, Li P, Shao L Y, et al. Lithium barium titanate: A stable lithium storage material for lithium-ion batteries[J]. Journal of Power Sources, 2015, 278: 546-554.
[128] Wu K Q, Wang D J, Lin X T, et al. Comparative study of Na2Li2Ti6O14prepared by different methods as advanced anode material for lithium-ion batteries[J]. Journal of Electroanalytical Chemistry, 2014, 717: 10-16.
[129] Wang P F, Li P, Yi T F, et al. Improved lithium storage performance of lithium sodium titanate anode by titanium site substitution with aluminum[J]. Journal of Power Sources, 2015, 293: 33-41.
The recent development of lithium titanate as anode materials for lithium-ion batteries
WEN Shujian, ZHANG Yixiao, CHEN Yang, SONG Chunyang, CUI Xiaoli
(Department of Materials Science, Fudan University, Shanghai 200433, China)
Lithium-ion batteries are one of the most promising battery systems to be widely used in portable electronics, electric vehicles, and energy storage systems. Lithium titanate (Li4Ti5O12) has been intensively investigated as an important anode material for lithium-ion batteries due to its high potential of around 1.55 V (vs. Li/Li+) during charge and discharge, excellent cycling stability, and high thermal stability and safety. This paper reviews the recent advances in structure and electrochemical performance of lithium titanate involving on new preparation methods of micro/macro particle, surface modification and ion doping. The micro/macro particles can provide greater surface area and shorten the migration distance for Li+. The better contact between the electrode and electrolyte produces benefits transportation of Li+, which improves the cycling performance of Li4Ti5O12. The major methods of surface modification are carbon coating, forming Li4Ti5O12/metal composites and modification by new surface phase. Such methods aim to increase the conductivity and improve the cycling performance of Li4Ti5O12. Doping ions increases the electron concentration and electronic conductivity since the partial Ti4+transform to Ti3+. The future development of lithium titanate as anode materials in lithium-ion batteries is also prospected in this review.
lithium-ion battery; lithium titanate; anode materials
1001-9731(2016)12-12038-12
2016-03-21
2017-07-11 通讯作者:崔晓莉,E-mail: xiaolicui@fudan.edu.cn
温书剑 (1994-),男,北京人,从事锂离子电池负极材料研究。
TB34
A
10.3969/j.issn.1001-9731.2016.12.007

