花生四烯酸对日本沼虾肝胰腺细胞脂质代谢基因表达的影响
丁志丽曹 访罗 娜孔有琴张易祥李景芬叶金云∗
(1.浙江省水生生物资源养护与开发技术研究重点实验室,中国水产科学研究院水生动物繁育与营养重点实验室,湖州师范学院生命科学学院,湖州 313000;2.大连海洋大学水产与生命科学学院,大连 116000)
花生四烯酸对日本沼虾肝胰腺细胞脂质代谢基因表达的影响
丁志丽1曹 访1罗 娜2孔有琴1张易祥1李景芬1叶金云1∗
(1.浙江省水生生物资源养护与开发技术研究重点实验室,中国水产科学研究院水生动物繁育与营养重点实验室,湖州师范学院生命科学学院,湖州 313000;2.大连海洋大学水产与生命科学学院,大连 116000)
本试验旨在评价细胞培养液中花生四烯酸(arachidonic acid,ARA)浓度对日本沼虾肝胰腺细胞活力及脂质代谢相关基因表达的影响。分离日本沼虾肝胰腺细胞,使用M199完全培养液培养5 d后换成含ARA的培养液,ARA浓度分别为0(ARA1)、50(ARA2)、100(ARA3)、200(ARA4)和1 000 μmol/L(ARA5),测定12和24 h时脂质代谢相关基因的表达水平,以及24 h时细胞活力。结果表明:原代肝胰腺细胞使用完全培养液时,生长状况良好,能存活15 d左右;ARA5组24 h时细胞活力显著低于ARA1和ARA2组(P<0.05);高浓度的ARA降低了12和24 h时Δ4脱饱和酶(Δ4FAD)、Δ6脱饱和酶(Δ6FAD)、碳链延长酶6(Elovl6)、B类Ⅰ型清道夫受体(SR-BⅠ)、脂肪酸结合蛋白10(FABP10)、乙酰辅酶A结合蛋白(ACBP)基因表达水平;ARA作用12 h时,ARA2组SR-BⅠ基因表达水平显著高于其余各组(P<0.05),ARA2和ARA3组FABP10基因表达水平显著高于ARA1和ARA5组(P<0.05),ARA3组ACBP基因表达水平显著高于其余各组(P<0.05);ARA作用24 h时,ARA2组SR-BⅠ、FABP10和ACBP基因表达水平显著高于其余各组(P<0.05)。由此可见,细胞培养液中ARA浓度会影响日本沼虾肝胰腺细胞活力及脂质代谢相关基因的表达,过高的ARA浓度(1 000 μmol/L)会降低细胞的活力,适宜的ARA浓度(50~100 μmol/L)可促进脂肪酸脱饱和酶、碳链延长酶及脂肪酸转运相关基因的表达。
日本沼虾;花生四烯酸;细胞培养;基因表达
脂肪酸是一种重要的营养素,能维持细胞膜的流动性,调节机体的生长性能、脂质代谢和免疫功能[1-2]。花生四烯酸(20∶4n-6,ARA)作为一种n-6高不饱和脂肪酸(highly unsaturated fatty acid,HUFA),是类二十烷酸的前体物质[3],能参与机体应激和炎症反应[4-5],调节机体的免疫性能[6]。 此外,ARA及其代谢物能调节过氧化物酶体增殖受体(PPAR)γ[7],从而影响脂质代谢相关基因的转录,调节脂肪酸合成与储存[8-10]。
目前,在水产动物主要集中于通过体内摄食营养来分析ARA对鱼类生长性能和机体脂肪酸组成[5,11-15]、抗 应 激[5,13]、免 疫 性 能[15]以 及 代谢[16-17]的影响,或通过体外鱼头肾细胞培养试验分析ARA对细胞通路基因和脂肪酸代谢相关基因表达[18]、类二十烷酸物质生成[6,18]和免疫功能[19]的影响。此外,在经济虾蟹类等甲壳动物也开展了对ARA的相关研究。Xu等[20]对中国对虾的研究发现,相比亚油酸或亚麻酸,ARA具有更高的营养价值;对斑节对虾的研究表明,当饲料中其他必需脂肪酸满足需求时,添加ARA不能提高其生长性能[21];饲料中添加ARA能改变凡纳滨对虾免疫相关基因的表达[22]。由于传统的摄食营养试验受到体内复杂的细胞代谢通路统一调节,存在饲喂条件及养殖环境应激等诸多影响因素,使得研究某种或某些营养素的生理作用及机制受到一定限制,利用体外细胞培养可以克服这些困难[6,23]。然而,利用甲壳动物的体外细胞培养系统研究脂肪酸的营养代谢未见报道。
脂肪酸被机体吸收后用于胞内甘油三酯的储存或作为燃料用于能量代谢。大量的研究证明,脂肪酸进出细胞是由多个蛋白介导的竞争性的脂肪酸转运体系,这些不同的蛋白能够显著地促进细胞吸收和排出脂肪酸[24-25],如 CD36清道夫受体家族中的B类Ⅰ型清道夫受体(scavenger receptor class B typeⅠ,SR-BⅠ)[26-27]、脂肪酸转位酶(fatty acid translocase,FAT/CD36)等[24]。研究表明,SR-BⅠ能结合各种配体,包括修饰及未经修饰的低密度脂蛋白、极低密度值蛋白及高密度脂蛋白胆固醇酯[26-27]。而在细胞内,脂肪酸主要与脂肪酸结合蛋白(fatty acid-binding protein,FABP)结合,使其溶解性增加,从而促使脂肪酸转运至不同的位点[28]。此外,胞内乙酰辅酶 A结合蛋白(acyl-CoA binding protein,ACBP)主要与长链脂酰辅酶A结合,在细胞内乙酰辅酶A的转运和乙酰辅酶A池的形成方面起着非常重要的作用,ACBP与乙酰辅酶A结合后既可以合成磷脂和甘油三酯,也可以发生β-氧化,产生ATP[29]。
同时,一些生物在体内还可将多不饱和脂肪酸合成HUFA,脂肪酸脱饱和酶和碳链延长酶是HUFA合成的关键酶,前者能将双键引入脂酰链,后者能将碳链进行延长[30]。目前,认为参与HUFA合成的去饱和酶类主要包括Δ6去饱和酶(delta-6 fatty acyl desaturase,Δ6 FAD)、Δ5去饱和酶(delta-5 fatty acyl desaturase,Δ5 FAD)、Δ4去饱和酶(delta-4 fatty acyl desaturase,Δ4 FAD)和 Δ8去饱和酶(delta-8 fatty acyl desaturase,Δ8 FAD)。在哺乳动物,已发现了7种碳链延长酶(elongases of very-long-chain fatty acids-1-7,Elovol1~Elovol7)参与脂肪酸的碳链延长作用,其中Elovl2和Elovl5以C18、C20或C22 PUFA作为延长底物[30]。对水产动物摄食营养试验表明,脂肪的“质”或“量”会影响脂肪酸脱饱和酶和碳链延长酶基因的表达[31-32]。
日本沼虾(Macrobrachium nipponense)又名青虾、河虾,是我国和东南亚一些国家重要的淡水经济养殖种类之一[33]。目前,有关ARA对日本沼虾的生长及营养生理作用还未见报道。肝胰腺是甲壳动物的脂质储存和加工的主要器官[34],也是营养物质代谢的敏感监测器[35-36]。有关日本沼虾肝胰腺细胞的培养可见梁虹[37]摸索的细胞培养条件及王宏伟等[38]研究亚油酸对细胞培养的初步影响,但无后续研究报道。因此,本试验拟培养日本沼虾肝胰腺原代细胞,通过在肝胰腺细胞中添加不同浓度的ARA,分析ARA对肝胰腺细胞活力、脂肪酸脱饱和酶与碳链延长酶基因(Δ4FAD、Δ6FAD和Elovl6)以及脂肪酸转运相关基因(SR-BⅠ、FABP10和ACBP)表达的影响。研究结果可为ARA脂质代谢作用机理研究提供一定的理论基础,同时为其他营养物质的代谢研究提供有益的参考资料。
1 材料与方法
1.1 试验动物
试验用虾购自于湖州日本沼虾养殖基地,暂养1周后,选择健康、体重均匀的日本沼虾用于试验。
1.2 细胞完全培养液的配制
基础培养液为M199培养液(Gibco,美国)添加15%胎牛血清(Gibco,美国)、200 IU/mL的双抗(青霉素和链霉素)、1 g/L葡萄糖、5.2 g/L NaCl、1.43 g/L CaCl2、0.05 g/L MgCl2、0.2 g/L NaHCO3,渗透压为570 mmol/kg,pH为7.0~7.2。
1.3 花生四烯酸-牛血清白蛋白(ARA-BSA)无血清培养液的配制
将10 mg ARA(Sigma,美国)溶解于1 mL无水乙醇,氮气吹干,加入 32.84 mL含 2%BSA(Sigma,美国)的 M199培养液,超声波作用5 min,0.22 μmol/L滤膜过滤除菌,配制成1 000 μmol/L ARA-BSA M199培养液母液,分装后于-20℃保存备用。试验开始前,将母液用含2%BSA的 M199溶液分别稀释成200、100和50 μmol/L的培养液,所有培养液都添加抗氧化剂丁羟基甲苯(0.01%)和双抗(200 IU/mL)。
1.4 日本沼虾肝胰腺细胞的分离与培养
将日本沼虾用75%酒精浸泡3 min,然后用D-hanks平衡盐溶液漂洗3次;无菌条件下取出肝胰腺,用含双抗的D-hanks平衡盐溶液漂洗3次;将组织块剪成1 mm3左右的小块,然后用0.25%的胰酶消化组织块,期间不断吹打使细胞分散;用含胎牛血清的M199培养液终止消化,1 000 r/min离心3 min,弃上清,用完全培养液重新悬浮细胞,调整细胞浓度为1×105个/mL,按每孔200 μL的量接种于96孔细胞培养板,于27℃,5%CO2培养箱中培养,每天观察拍照,4~5 d换液1次。
细胞培养稳定(5 d)后,将细胞完全培养液换成含不同浓度ARA的培养液继续培养,试验分为5组,培养液分别含0(ARA1,对照)、50(ARA2)、100(ARA3)、200(ARA4)和1 000 μmol/L ARABSA(ARA5),并分别在12和24 h收集各组细胞,提取细胞总RNA,用于后续基因表达的测定。
1.5 细胞活力测定
细胞培养5 d时采用H33342/碘化丙啶(PI)染色液检测细胞存活力,即200 μL细胞培养液中加入 H33342 1 μL,PI 2 μL。轻轻振荡混匀后37℃避光孵育15 min,然后在荧光显微镜下统计细胞活力,活细胞为蓝色,死细胞为红色。
将不同浓度的ARA-BSA孵育肝胰腺细胞24 h时,采用噻唑蓝(MTT)细胞增殖-毒性检测试剂盒(南京建成生物工程研究所,南京)测定细胞活力,测定过程按照试剂盒说明书进行。
1.6 总RNA提取和cDNA的合成
使用总RNA提取试剂盒(北京艾德莱生物科技有限公司)提取肝胰腺总RNA,具体操作按照试剂盒说明书进行,电泳检测总RNA的完整性、核酸蛋白测定仪检测其浓度和纯度。用反转录试剂盒(TaKaRa,日本)将总RNA反转录为cDNA,cDNA保存在-20℃用于基因表达分析。
1.7 基因表达的荧光定量PCR(qRT-PCR)分析
采用在线 Primer 3设计 Δ4FAD、Δ6FAD、Elovl6、SR-BⅠ、FABP10和ACBP基因 qRT-PCR所用引物,引物序列见表1。qRT-PCR反应体积为20 μL,包括:2 μL模板,上、下游引物各 0.2 μL(10 μmol/L),10 μL的2×SYBR Green Premix Ex Taq(TaKaRa,日本)以及7.6 μL双蒸水(ddH2O)。反应条件为:95℃预变性30 s;94℃变性 15 s,58℃退火20 s,72℃延伸20 s,共40个循环;PCR后温度以每5 s上升5℃的速度从60℃上升到95℃,绘制熔解曲线,以判断扩增产物的正确性。以日本沼虾β-肌动蛋白(β-actin)为内参,对得到的各样品循环数(Ct)值进行均一化处理,以ARA1组基因为基准,使用 2-ΔΔCt比较 Ct值方法[39]对目的基因相对表达水平进行分析。

表1 qRT-PCR引物序列Table 1 Primer sequence for qRT-PCR
2 结 果
2.1 肝胰腺细胞形态及活力
刚分离的肝胰腺细胞呈现单个圆形状态,2~ 3 d后开始贴壁生长,并缓慢增殖,出现成串状况,生长状况良好,细胞形态见图1。细胞培养5 d时,细胞存活力达到60%左右。原代培养肝胰腺细胞能存活15 d左右。

图1 日本沼虾肝胰腺细胞形态Fig.1 Morphology of cultured hepatopancreas cells(100×)
不同浓度ARA下肝胰腺细胞的活力见图2。由图可见,不同浓度的ARA孵育肝胰腺细胞24 h时,ARA5组细胞活力显著低于ARA1和ARA2组(P<0.05),ARA1、ARA2、ARA3、ARA4组间细胞活力无显著差异(P>0.05)。
2.2 花生四烯酸对肝胰腺细胞脂质代谢基因表达的影响
ARA处理肝胰腺细胞12 h时,各组Δ4FAD、Δ6FAD、Elovl6、SR-BⅠ、FABP10、ACBP和ACC基因表达变化见图3。由图可见,各脂质代谢基因的表达水平随着ARA浓度的增加都呈现先增加后降低趋势。其中,ARA2、ARA3和ARA4组Δ4FAD基因的表达水平显著高于ARA1和ARA5组(P<0.05);ARA1、ARA2和ARA3组Δ6FAD基因的表达水平无显著差异(P>0.05),ARA2组Δ6FAD基因的表达水平显著高于ARA4和ARA5组(P<0.05);ARA4组Elovl6基因表达水平最高,显著高于ARA5组(P<0.05);ARA2组SR-BⅠ基因表达水平最高,显著高于其余各组(P<0.05);ARA2和ARA3组FABP10基因表达水平较高,显著高于ARA1和ARA5组(P<0.05);ARA3组ACBP基因表达水平显著高于其余各组(P<0.05)。
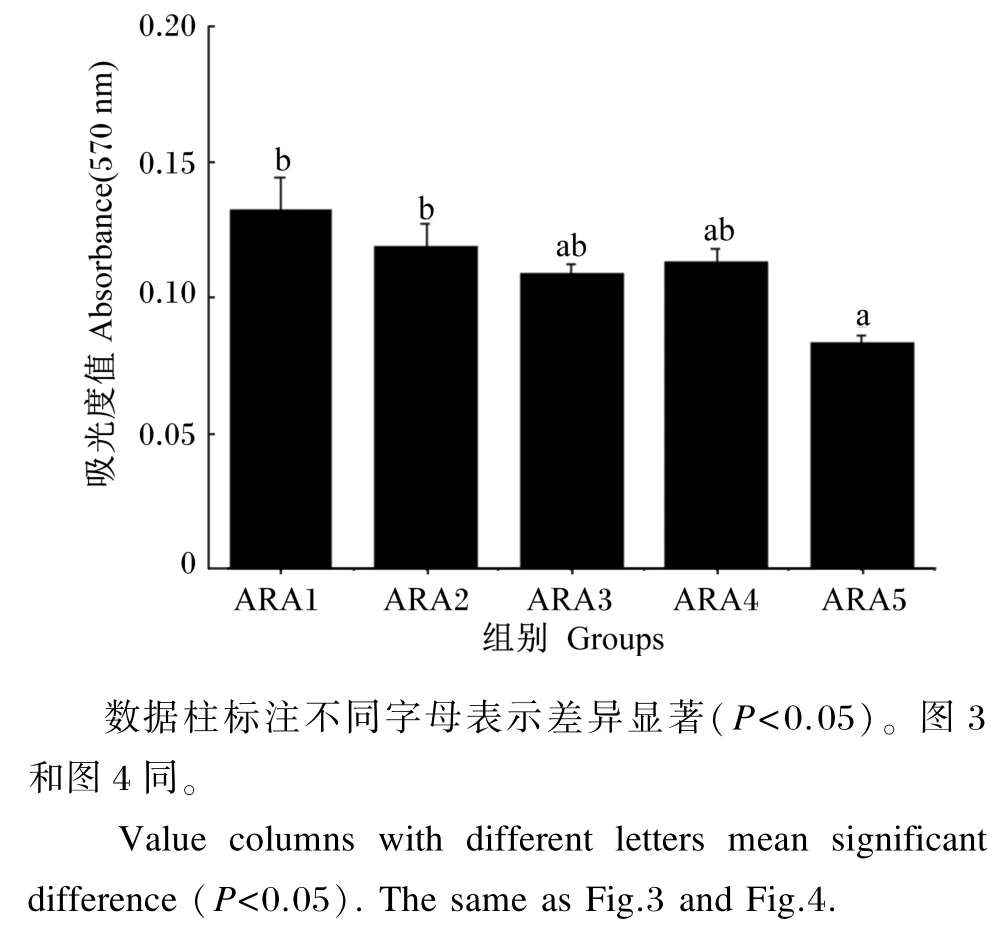
图2 不同浓度ARA下肝胰腺细胞的活力Fig.2 Viability of hepatopancreas cells incubated with different levels of ARA
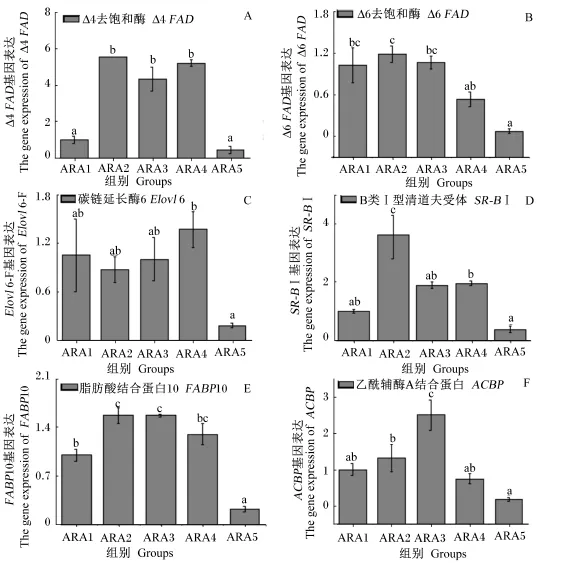
图3 不同浓度ARA下肝胰腺细胞后脂质代谢相关基因表达(12 h)Fig.3 Lipid metabolism-related gene expressions in hepatopancreas cells incubated with different levels of ARA(12 h)
ARA处理肝胰腺细胞24 h时,各组Δ4FAD、Δ6FAD、Elovl6、SR-BⅠ、FABP10、ACBP和ACC基因表达变化见图4。由图可知,各基因的表达变化趋势与ARA处理肝胰腺细胞12 h基本相似。Δ4FAD和Δ6FAD基因表达水平都在ARA2和ARA3组较高;Elovl6基因表达水平在ARA3组达到最大,显著高于其余各组(P<0.05);SR-BⅠ、FABP10和ACBP基因表达水平均在ARA2组达到最大,显著高于其余各组(P<0.05)。
3 讨 论
甲壳动物的细胞培养已经摸索了较长的一段时间,遗憾的是,目前还没有甲壳动物细胞系的培养研究报道,阻碍了对其各种代谢机制和功能的研究。研究人员对虾类各组织的细胞培养研究发现,血细胞培养较为简单,相比而言,肝胰腺细胞的培养难度较大[40-42]。本试验中,肝胰腺细胞培养结果与王宏伟等[38]研究报道类似,肝胰腺原代培养细胞生长状态良好,有利于进行下一步试验。
在研究ARA对日本沼虾肝胰腺细胞影响时,使用不含血清的BSA代替胎牛血清,避免了胎牛血清中含有各种营养物质包括脂肪酸对后续试验可能产生的影响。MTT法测定各试验组细胞活力发现,高浓度的ARA(1 000 μmol/L)降低了肝胰腺细胞的活力,这与 Li等[19]使用不同浓度的ARA对头肾巨噬细胞培养研究结果相似,即高浓度ARA降低了细胞活力。外部脂肪酸可以进入细胞膜,改变细胞脂肪酸和细胞膜的生理特性[43-44],尤其是高水平的脂肪酸可以引起细胞DNA不可逆损伤,胞膜完整性丢失,膜的渗透性混乱,最终导致细胞死亡[45-46]。
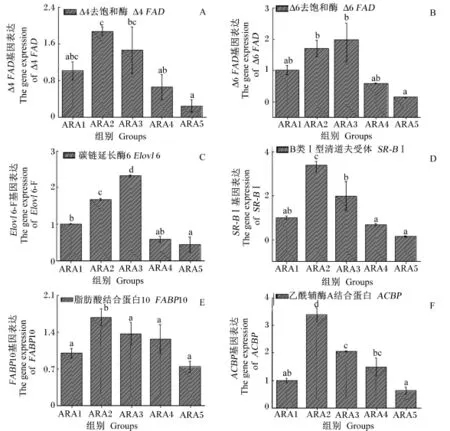
图4 不同浓度ARA下肝胰腺细胞后脂质代谢相关基因表达(24 h)Fig.4 Lipid metabolism-related gene expressions in hepatopancreas cells incubated with different levels of ARA(24 h)
脂肪酸脱饱和酶和碳链延长酶是HUFA合成的关键酶,对高等脊椎动物的研究表明,ARA可以通过碳链延长酶、Δ6 FAD 和 β-氧化生成C22∶5n-6,也可以直接通过Δ4 FAD生成C22∶5n-6;ARA还可以通过Δ17去饱和酶(Δ17 FAD)生成C20∶5n-3(EPA),EPA依次经碳链延长、Δ6去饱和和β-氧化合成C22∶6n-3(DHA),或者EPA转化为22∶5n-3后,在Δ4 FAD作用下直接转化为DHA[47]。本研究发现,不同浓度的ARA处理日本沼虾肝胰腺细胞不同时间后,HUFA合成通路关键酶基因表达都发生改变,高浓度的ARA会降低Δ4FAD、Δ6FAD和Elovl6基因的表达水平,说明ARA是日本沼虾HUFA合成途径中的一个重要调节因子。对乳猪的研究表明,饲料中ARA与DHA比例能调节肝脏脱饱和酶基因的转录水平[48]。在鱼类的摄食营养试验中,研究也发现塞内加尔鳎(Solea Senegalensis)雄鱼在摄食0.7%、2.3%和6.0%ARA时,肝脏Elovl5和Δ4FAD基因表达水平增加[17]。然而,对草鱼(Ctenopharyngodon idellus)的研究表明,脂肪酸脱饱和酶和延长酶基因受到饲料中ARA水平的显著抑制[10]。出现这些差异,可能与不同物种HUFA合成能力不同有关,同时也说明ARA水平能显著影响HUFA合成相关酶基因的表达水平。尽管相关的利用体外培养系统研究脂肪酸浓度对HUFA合成影响的资料有限,但本试验结果表明,体外适宜浓度的脂肪酸能促进日本沼虾HUFA合成途径中关键酶基因表达水平。
有关ARA对脂肪酸转运相关基因表达的影响,报道较少。仅见 Holen等[18]将不同组合的EPA、DHA和ARA添加进大西洋鲑(Salmo salar)头肾细胞,研究发现ARA+EPA能上调脂肪酸转位酶CD36基因的表达。SR-BⅠ属于CD36超家族成员,能维持细胞内胆固醇代谢稳态,在细胞膜脂表达和细胞凋亡等方面具有重要作用[49-50]。本研究发现,肝胰腺细胞中添加不同浓度ARA 12和24 h后,SR-BⅠ基因表达水平均在ARA2组达到最大,说明50 μmol/L的ARA浓度有利于维持细胞的脂质代谢平衡。研究表明,脂肪酸或酰基辅酶A是PPAR的天然配体,能激活PPAR[51],SR-BⅠ的活性可由 PPARα和 PPARγ诱导[52-53]。因此,肝胰腺细胞培养液中一定浓度的脂肪酸可能通过激活PPAR来调节SR-BⅠ基因的表达。对高等动物仓鼠的研究也表明,摄食多不饱和脂肪酸可以增加SR-BⅠ基因和蛋白水平[54]。对奶牛乳腺上皮细胞的体外培养试验表明,脂质代谢相关基因的表达与脂肪酸浓度密切相关[55-56]。FABP10和ACBP都属于胞内脂质结合蛋白,ARA处理肝胰腺细胞 12 h时,ARA2和 ARA3组FABP10以及ARA3组ACBP转录水平达到最高,说明此时50~100 μmol/L的ARA浓度促进了胞内脂肪酸转运。ARA处理肝胰腺细胞24 h时,ARA2组FABP10和ACBP都显著高于其余各组,说明日本沼虾肝胰腺细胞中添加50 μmol/L的ARA可能更有利于促进脂质代谢。大量与脂质合成、分解与代谢的基因都受到 PPAR的调节[57]。体外细胞培养试验结果显示,PPARα与肝型-FABP(L-FABP)具有高度亲和性,说明L-FABP能与PPARα结合调节长链脂肪酸的代谢[58]。同样,PPAR也可激活ACBP基因的表达[59]。因此,适宜浓度的ARA促进了FABP10和ACBP基因的表达机理可能类似于上述SR-BⅠ基因。事实上,FABP10和ACBP属于多功能蛋白,它们在机体的免疫性能方面具有重要的作用[60-61],而研究表明适宜的ARA浓度可以调节调节机体免疫性能[6]。因此,我 们 不 能 排 除 细 胞 培 养 液 中 50~100 μmol/L ARA有利于提高细胞的免疫性能,从而提高了FABP10和ACBP基因的表达。
4 结 论
细胞培养液中ARA浓度会影响日本沼虾肝胰腺细胞活力及脂质代谢相关基因的表达,过高的ARA浓度(1 000 μmol/L)会降低细胞的活力,适宜的ARA浓度(50~100 μmol/L)可促进脂肪酸脱饱和酶、碳链延长酶及脂肪酸转运相关基因的表达。
[1]HIGGS D A,DONG F M.Lipids and fatty acids[M]//STICKNEY R R.Encyclopedia of Aquaculture.New York:John Wiley and Sons,2000:476-496.
[2]TRICHET V V.Nutrition and immunity:an update[J].Aquaculture Research,2010,41(3):356-372.
[3]BELL J G,SARGENT J R.Arachidonic acid in aquaculture feeds:current status and future opportunities[J].Aquaculture,2003,218(1/2/3/4):491-499.
[4]VAN ANHOLT R D,KOVEN W M,LUTZKY S E,et al.Dietary supplementation with arachidonic acid alters the stress response of gilthead seabream (Sparus aurata)larvae[J].Aquaculture,2004,238(1/2/3/4):369-383.
[5]CARRIERⅢJ K,WATANABE W O,HAREL M,et al.Effects of dietary arachidonic acid on larval performance,fatty acid profiles,stress resistance,and expression of Na+/K+ATPase mRNA in black sea bassCentropristis striata[J].Aquaculture,2011,319(1/2):111-121.
[6]FURNE M,HOLEN E,ARAUJO P,et al.Cytokine gene expression and prostaglandin production in head kidney leukocytes isolated from Atlantic cod(Gadus morhua) added different levels of arachidonic acid and eicosapentaenoic acid[J].Fish&Shellfish Immunology,2013,34(3):770-777.
[7]QI C,ZHU Y J,REDDY J K.Peroxisome proliferatoractivated receptors,coactivators,and downstream targets[J].Cell Biochemistry and Biophysics,2000,32(1/2/3):187-204.
[8]BRASH A R.Arachidonic acid as a bioactive molecule[J].The Journal of Clinical Investigation,2001,107(11):1339-1345.
[9]MARTINS D A,ROCHA F, MARTÍNEZRODRÍGUEZ G,et al.Teleost fish larvae adapt to dietary arachidonic acid supply through modulation of the expression of lipid metabolism and stress response genes[J].British Journal of Nutrition,2012,108(5):864-874.
[10]TIAN J J,JI H,OKU H,et al.Effects of dietary arachidonic acid(ARA) on lipid metabolism and health status of juvenile grass carp,Ctenopharyngodon idellus[J].Aquaculture,2014,430:57-65.
[11]CASTELL J D,BELL J G,TOCHER D R,et al.Effects of purified diets containing different combina-tions of arachidonic and docosahexaenoic acid on survival,growth and fatty acid composition of juvenile turbot(Scophthalmus maximus)[J].Aquaculture,1994,128(3/4):315-333.
[12]BELL J G,CASTELL J D,TOCHER D R,et al.Effects of different dietary arachidonic acid:docosahexaenoic acid ratios on phospholipid fatty acid compositions and prostaglandin production in juvenile turbot(Scophthalmus maximus)[J].Fish Physiology and Biochemistry,1995,14(2):139-151.
[13]KOVEN W,BARR Y,LUTZKY S,et al.The effect of dietary arachidonic acid(20∶4n-6)on growth,survival and resistance to handling stress in gilthead seabream (Sparus aurata) larvae[J].Aquaculture,2001,193(1/2):107-122.
[14]FOUNTOULAKI E,ALEXIS M N,NENGAS I,et al.Effects of dietary arachidonic acid(20∶4n-6),on growth,body composition,and tissue fatty acid profile of gilthead bream fingerlings(Sparus aurataL.)[J].Aquaculture,2003,225(1/2/3/4):309-323.
[15]XU H G,AI Q H,MAI K S,et al.Effects of dietary arachidonic acid on growth performance,survival,immune response and tissue fatty acid composition of juvenile Japanese seabass,Lateolabrax japonicus[J].Aquaculture,2010,307(1/2):75-82.
[16]VAN ANHOLT R D,SPANINGS F A T,KOVEN W M,et al.Dietary supplementation with arachidonic acid in tilapia(Oreochromis mossambicus)reveals physiological effects not mediated by prostaglandins[J].General and Comparative Endocrinology,2004,139(3):215-226.
[17]NORAMBUENA F,MORAIS S,ESTÉVEZ A,et al.Dietary modulation of arachidonic acid metabolism in senegalese sole (SoleaSenegalensis) broodstock reared in captivity[J].Aquaculture,2013,372-375:80-88.
[18]HOLEN E,HE J Y,ESPE M,et al.Combining eicosapentaenoic acid,decosahexaenoic acid and arachidonic acid,using a fully crossed design,affect gene expression and eicosanoid secretion in salmon head kidney cellsin vitro[J].Fish&Shellfish Immunology,2015,45(2):695-703.
[19]LI Q F,AI Q H,MAI K S,et al.In vitro effects of arachidonic acid on immune functions of head kidney macrophages isolated from large yellow croaker(Larmichthys crocea)[J].Aquaculture,2012,330-333:47-53.
[20]XU X L,JI W J,CASTELL J D,et al.Essential fatty acid requirement of theChineseprawn,Penaeus chinensis[J].Aquaculture,1994,127(1):29-40.
[21]GLENCROSS B D,SMITH D M.A study of the arachidonic acid requirements of the giant tiger prawn,Penaues monodon[J].Aquaculture Nutrition,2001,7(1):59-69.
[22]赵利斌,王鑫磊,黄旭雄,等.饲料中花生四烯酸水平对凡纳滨对虾免疫相关基因表达及抗菌能力的影响[J].水产学报,2016,40(5):763-775.
[23]HOLEN E,WINTERTHUN S,DU Z Y,et al.Inhibition of p38 MAPK during cellular activation modulate gene expression of head kidney leukocytes isolated from Atlantic salmon(Salmo salar)fed soy bean oil or fish oil based diets[J].Fish&Shellfish Immunology,2011,30(1):397-405.
[24]ABUMRAD N,COBURN C,IBRAHIMI A.Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells:CD36,FATP and FABPm[J].Biochimica et Biophysica Acta(BBA):Molecular and Cell Biology of Lipids,1999,1441(1):4-13.
[25]BONEN A,CHABOWSKI A,LUIKEN J J,et al.Is membrane transport of FFA mediated by lipid,protein,or both?Mechanisms and regulation of proteinmediated cellular fatty acid uptake:molecular,biochemical,and physiological evidence[J].Physiology,2007,22:15-29.
[26]ACTON S L,SCHERER P E,LODISH H F,et al.Expression cloning of SR-BⅠ,a CD36-related class B scavenger receptor[J].The Journal of Biological Chemistry,1994,269(33):21003-21009.
[27]ACTON S,RIGOTTI A,LANDSCHULZ K T,et al.I-dentification of scavenger receptor SR-BⅠ as a high density lipoprotein receptor[J].Science,1996,271(5248):518-520.
[28]ZIMMERMAN A W,VEERKAMP J H.New insights into the structure and function of fatty acid-binding proteins[J].Cellular and Molecular Life Sciences,2002,59(7):1096-1116.
[29]MANDRUP S,HUMMEL R,RAVN S,et al.Acyl-CoA-binding protein/diazepam-binding inhibitor gene and pseudogenes:a typical housekeeping gene family[J].Journal of Molecular Biology,1992,228(3):1011-1022.
[30]JAKOBSSON A,WESTERBERG R,JACOBSSON A.Fatty acid elongases in mammals:their regulation and roles in metabolism[J].Progress in Lipid Re-search,2006,45(3):237-249.
[31]KUAH M K,JAYA-RAM A,SHU-CHIEN A C.The capacity for long-chain polyunsaturated fatty acid synthesis in a carnivorous vertebrate:functional characterisation and nutritional regulation of a Fads2 fatty acyl desaturase with Δ4 activity and an Elovl5 elongase in striped snakehead(Channa striata)[J].Biochimica et Biophysica Acta(BBA):Molecular and Cell Biology of Lipids,2015,1851(3):248-260.
[32]XIE D Z,CHEN F,LIN S Y,et al.Long-chain polyunsaturated fatty acid biosynthesis in the euryhaline herbivorous teleostScatophagusargus:functional characterization,tissue expression and nutritional regulation of two fatty acyl elongases[J].Comparative Biochemistry and Physiology Part B:Biochemistry&Molecular Biology,2016,198:37-45.
[33]YANG Y,XIE S Q,LEI W,et al.Effect of replacement of fish meal by meat and bone meal and poultry by-product meal in diets on the growth and immune response ofMacrobrachium nipponense[J].Fish&Shellfish Immunology,2004,17(2):105-114.
[34]HARRISON K E.The role of nutrition in maturation,reproduction and embryonic development of decapod crustaceans:a review[J].Journal of Shellfish Research,1990,9(1):1-28.
[35]VOGT G,STORCH V,QUINITIO E T,et al.Midgut gland as monitor organ for the nutritional value of diets inPenaeus monodon(Decapoda)[J].Aquaculture,1985,48(1):1-12.
[36]ROSAS C, BOLONGARO-CREVENNA A,SÁNCHEZ A,et al.Role of digestive gland in the energetic metabolism ofPenaeus setiferus[J].The Biological Bulletin,1995,189(2):168-174.
[37]梁虹.日本沼虾细胞培养[D].硕士学位论文.保定:河北大学,2001.
[38]王宏伟,刘瑞兰,郭明申,等.亚油酸对培养日本沼虾肝胰腺细胞的影响[J].河北大学学报:自然科学版,2005,25(1):79-83.
[39]LIVAK K J,SCHMITTGEN T D.Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCtmethod[J].Methods,2001,25(4):402-408.
[40]MULFORD A L,AUSTIN B.Development of primary cell cultures from Nephrops norvegicus[J].Methods in Cell Science,1998,19(4):269-275.
[41]GEORGE S K,DHAR A K.An improved method of cell culture system from eye stalk,hepatopancreas, muscle,ovary,and hemocytes ofPenaeus vannamei[J].In VitroCellular&Developmental Biology:Animal,2010,46(9):801-810.
[42]JAYESH P,JOSE S,PHILIP R,et al.A novel medium for the development ofin vitrocell culture system fromPenaeus monodon[J].Cytotechnology,2013,65(3):307-322.
[43]CALDER P C.The relationship between the fatty acid composition of immune cells and their function[J].Prostaglandins Leukotrienes and Essential Fatty Acids,2008,79(3/4/5):101-108.
[44]TOCHER D R,DICK J R.Polyunsaturated fatty acid metabolism in cultured fish cells:incorporation and metabolism of(n-3)and(n-6)series acids by Atlantic salmon(Salmo salar)cells[J].Fish Physiology and Biochemistry,1990,8(4):311-319.
[45]GORJÃO R,AZEVEDO-MARTINSA K,RODRIGUES H G,et al.Comparative effects of DHA and EPA on cell function[J].Pharmacology&Therapeutics,2009,122(1):56-64.
[46]JI J,ZHANG L,WANG P,et al.Saturated free fatty acid,palmitic acid,induces apoptosis in fetal hepatocytes in culture[J].Experimental and Toxicologic Pathology,2005,56(6):369-376.
[47]TOCHER D R,ZHENG X Z,SCHLECHTRIEM C,et al.Highly unsaturated fatty acid synthesis in marine fish:cloning,functional characterization,and nutritional regulation of fatty acyl Δ6 desaturase of Atlantic cod(Gadus morhuaL.)[J].Lipids,2006,41(11):1003-1016.
[48]WIJENDRAN V,DOWNS L,SRIGLEY C T,et al.Dietary arachidonic acid and docosahexaenoic acid regulate liver fatty acid desaturase(FADS)alternative transcript expression in suckling piglets[J].Prostaglandins,Leukotrienes and EssentialFatty Acids(PLEFA),2013,89(5):345-350.
[49]NECULAI D,SCHWAKE M,RAVICHANDRAN M,et al.Structure of LIMP-2 provides functional insights with implications for SR-BⅠ and CD36[J].Nature,2013,504(7478):172-176.
[50]SHEN W J,HU J,HU Z G,et al.Scavenger receptor class B typeⅠ (SR-BⅠ):a versatile receptor with multiple function and actions[J].Metabolism,2014,63(7):875-886.
[51]SCHOONJANS K,STAELS B,AUWERX J.Role of the peroxisome proliferator-activated receptor(PPAR)in mediating the effects of fibrates and fattyacids on gene expression[J].Journal of Lipid Research,1996,37(5):907-925.
[52]LOPEZ D,MCLEAN M P.Activation of the rat scavenger receptor class B typeⅠ gene by PPARα[J].Molecular and Cellular Endocrinology,2006,251(1/2):67-77.
[53]CHINETTI G,GBAGUIDI F G,GRIGLIO S,et al.CLA-1/SR-BⅠ is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors[J].Circulation,2000,101(20):2411-2417.
[54]SPADY D K,KEARNEY D M,HOBBS H H.Polyunsaturated fatty acids up-regulate hepatic scavenger receptor B1(SR-BⅠ)expression and HDL cholesteryl ester uptake in the hamster[J].Journal of Lipid Research,1999,40(8):1384-1394.
[55]胡菡,王加启,李发弟,等.游离亚麻酸对奶牛乳腺上皮细胞脂肪酸代谢相关基因mRNA转录的影响[J].动物营养学报,2010,22(5):1342-1349.
[56]YONEZAWA T,YONEKURA S,KOBAYASHI Y,et al.Effects of long-chain fatty acids on cytosolic triacylglycerol accumulation and lipid droplet formation in primary cultured bovine mammary epithelial cells[J].Journal of Dairy Science,2004,87(8):2527-2534.
[57]DESVERGNE B,WAHLI W.Peroxisome proliferatoractivated receptors:nuclear control of metabolism[J].Endocrine Reviews,1999,20(5):649-688.
[58]HOSTETLER H A,MCLNTOSH A L,ATSHAVES B P,et al.L-FABP directly interacts with PPARα in cultured primary hepatocytes[J].Journal of Lipid Research,2009,50(8):1663-1675.
[59]SANDBERG M B,BLOKSGAARD M,DURANSANDOVAL D,et al.The gene encoding acyl-CoA-binding protein is subject to metabolic regulation by both sterol regulatory element-binding protein and peroxisome proliferator-activated receptor α in hepatocytes[J].The Journal of Biological Chemistry,2005,280(7):5258-5266.
[60]REN Q,DU Z Q,ZHAO X F,et al.An acyl-CoA-binding protein(FcACBP)and a fatty acid binding protein(FcFABP)respond to microbial infection in Chinese white shrimp,Fenneropenaeus chinensis[J].Fish&Shellfish Immunology,2009,27(6):739-747.
[61]ZHAO Z Y,YIN Z X,WENG S P,et al.Profiling of differentially expressed genes in hepatopancreas of white spot syndrome virus-resistant shrimp(Litopenaeus vannamei)by suppression subtractive hybridisation[J].Fish&Shellfish Immunology,2007,22(5):520-534.
Effects of Arachidonic Acid on Lipid Metabolism-Related Gene Expressions of Hepatopancreas Cells Isolated from Juvenile Oriental River Prawn,Macrobrachium nipponense
DING Zhili1CAO Fang1LUO Na2KONG Youqin1ZHANG Yixiang1LI Jingfen1YE Jinyun1∗
(1.Zhejiang Provincial Key Laboratory of Aquatic Resources Conservation and Development,Key Laboratory of
Aquatic Animal Genetic Breeding and Nutrition,Chinese Academy of Fishery Sciences,College of Life Sciences,Huzhou University,Huzhou313000,China;2.College of Fisheries and Life Science,Dalian Ocean University,Dalian116000,China)
This experiment was conducted to determine the effects of arachidonic acid(ARA)concentration in culture medium on cell viability and lipid metabolism-related gene expressions of hepatopancreas cells isolated from juvenile oriental river prawn,Macrobrachium nipponense.The hepatopancreas cells were dissected from prawns and were cultured with complete culture medium for 5 days.After that,cultured cells were incubated in medium supplemented with graded levels[0(ARA1),50(ARA2),100(ARA3),200(ARA4)and 1 000 μmol/L(ARA5)of ARA.Cell viability at 24 h and gene expressions of lipid metabolism-related genes at 12 and 24 h were examined.The results showed as follows:the hepatopancreas cells showed well growth in complete culture medium,and could survive for 15 days;cell viability was significantly decreased by incubation with higher level(1 000 μmol/L)of ARA(ARA5 group)compared with ARA1 and ARA2 groups(P<0.05)after 24 h;higher level(1 000 μmol/L)of ARA(ARA5 group)caused significant decreases of gene expressions of delta-4 fatty acyl desaturase(Δ4FAD),delta-6 fatty acyl desaturase(Δ6FAD),very-longchain fatty acids-6(Elovl6),scavenger receptor class B typeⅠ (SR-BⅠ),fatty acid-binding protein 10(FABP10)and acyl-CoA binding protein(ACBP)of hepatopancreas cells incubation for both 12 and 24 h;after incubation with ARA for 12 h,the gene expression ofSR-BⅠ of ARA2 group was significantly higher than that of other groups(P<0.05),FABP10 gene expression of ARA2 and ARA3 groups was significantly higher than that of ARA1 and ARA5 groups(P<0.05),and ACBP gene expression of ARA3 group was significantly higher than that of other groups(P<0.05);after incubation with ARA for 24 h,the highest expressions ofSR-BⅠ,FABP10 andACBPwere observed in ARA2 group,which was significantly higher than those of other groups(P<0.05).These findings suggest that ARA can influence cell viability and lipid metabolism-related gene expressions of hepatopancreas cell isolated fromMacrobrachium nipponense.Cell viability can be decreased by incubation with higher level of ARA(1 000 μmol/L).Appropriate levels of ARA(50 to 100 μmol/L)can promote the expressions of genes related to fatty acyl desaturase,elongases of very-longchain fatty acids and fatty acid transport.[Chinese Journal of Animal Nutrition,2017,29(2):536-546]
Macrobrachium nipponense;arachidonic acid;cell culture;gene expression
S968.22
A
1006-267X(2017)02-0536-11
10.3969/j.issn.1006-267x.2017.02.021
(责任编辑 王智航)
2016-08-06
国家自然科学基金(31402308);浙江省自然科学基金(LQ14C190004);浙江省重大科技专项计划项目(2014C02011);浙江省重点研发计划项目(2015C03018)
丁志丽(1979—),女,江苏如皋人,副教授,博士,主要从事水产动物营养与饲料研究。E-mail:dingzhili@zjhu.edu.cn
∗通信作者:叶金云,研究员,博士生导师,E-mail:yjy@zjhu.edu.cn
∗Corresponding author,professor,E-mail:yjy@zjhu.edu.cn

