湿化学法制备钛酸钡基粉体的研究进展
王 艳
(铁电功能材料工程(技术)研究中心 陕西省植物化学重点实验室宝鸡文理学院化学化工学院,陕西 宝鸡 721013)
湿化学法制备钛酸钡基粉体的研究进展
王 艳
(铁电功能材料工程(技术)研究中心 陕西省植物化学重点实验室宝鸡文理学院化学化工学院,陕西 宝鸡 721013)
从反应机理、制备工艺及应用几方面出发,综述了钛酸钡基粉体的湿化学制备方法,包括溶胶-凝胶法、水热法、常压水相法和溶胶-凝胶水热法等,指出了这些方法的技术关键及优缺点,展望了湿化学法制备钛酸钡基粉体的发展前景。
湿化学法;钛酸钡基粉体;制备
钛酸钡(BaTiO3)作为一种典型的铁电材料,是电子陶瓷中使用最广泛的材料之一,被誉为“电子陶瓷工业的支柱”[1],因介电常数高,铁电[2]、压电[3]、绝缘性能[4]优良及环境友好[5]等特点而被广泛应用于多层陶瓷电容器(MLCC)领域。由于电子系统的小型化、轻量化和集成化的发展要求,MLCC正在向大容量、超小超薄的方向快速发展,这就要求陶瓷介质材料的晶粒尺寸达到亚微米甚至纳米级,而陶瓷晶粒的大小正比于初始粉体尺寸[6],故在制备粉体时,须严格控制粒径。
BaTiO3基粉体的制备方法主要包括传统的固相法和湿化学法。固相法所得粉体的平均粒径较大(>1μm),且能耗大、易混入杂质,不能满足当前MLCC的发展需求[7-10]。与固相法相比,湿化学法能够在液相前驱体中将原材料在分子水平上均匀混合,可在较低的温度下制备出纳米级、亚微米级高纯MLCC用BaTiO3基粉体[11-14]。作者在此对BaTiO3基粉体的湿化学制备方法进行了综述,包括溶胶-凝胶法[15]、水热法[16-17]、常压水相法[18]与溶胶-凝胶水热法[19]等,并指出了各方法的技术关键、优缺点以及适用范围。
1 溶胶-凝胶法
溶胶-凝胶法是一个无机聚合的过程(图1):把适当浓度的金属醇盐或无机盐混入有机溶剂中,经过水解和缩聚反应,得到的凝胶经过老化后转化为三维网络结构或线性结构的氧化物凝胶[20]。
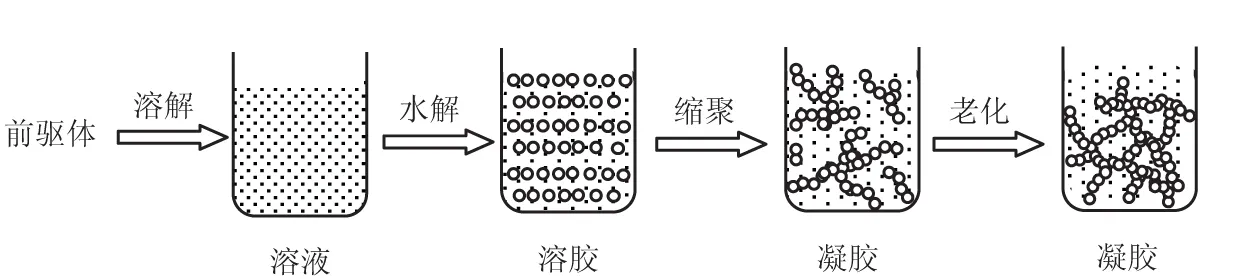
图1 溶胶-凝胶法过程
Fig.1 The process of sol-gel method
溶胶-凝胶法能够很好地控制反应物组分的化学计量比,实现原材料在分子水平上的均匀混合,这对于多组分掺杂的BaTiO3基粉体的制备具有很重要的意义。Xin等[21]采用溶胶-凝胶法制备了Sc掺杂BaTiO3基纳米粉体,粒径为30~40 nm,烧结活性高,烧结温度较低,在1 200 ℃烧结能获得介电温度稳定性较好的细晶陶瓷材料。Zhan等[22]采用溶胶-凝胶法制备了Nb掺杂(Ba0.87Sr0.04Ca0.09)(Ti0.86Zr0.08Sn0.06)O3基粉体,粒径约为50 nm,烧结所得陶瓷晶粒尺寸约为10 μm,室温介电常数超过16 000,符合Y5V标准[当温度在-30~85 ℃之间变化时,容温(ε)变化率为-82%≤(ε-ε25 ℃)/ε25 ℃≤22%]。Wang等[23]采用溶胶-凝胶法制备了粒径约为30 nm的Dy掺杂BaTiO3-Nb-Zn基粉体,当Dy掺杂量为0.4%(摩尔分数)时,陶瓷晶粒尺寸约为3 μm,具有较好的介电性能,室温介电常数达到19 000以上,介电损耗为0.006,符合Y5V标准。
溶胶-凝胶法的优点:易实现多组分的均匀掺杂,无需洗涤,所制备的BaTiO3基粉体组成容易控制、纯度高、粒径小、烧结活性高,被广泛用于制备满足Y5V标准的介电陶瓷。溶胶-凝胶法的缺点:需高温煅烧,粉体易团聚,在制备过程中无法控制粉体的形貌,烧结得到的陶瓷易发生异常长大、开裂或有残留细孔[24-26]。
2 水热法
水热法的基本原理:以水溶液为介质,在密封的反应釜中,将常温下不易合成或不易被氧化的物质通过离子反应的压力平衡、温度(100~400 ℃)及溶剂的联合效应等共同作用得到BaTiO3基粉体。尤其是微波水热法[27-29],与传统的水热法相比更节能、省时且内部加热速度快。Guo等[30]将微波水热法和传统水热法进行了对比,发现2种水热法均可以得到理想的BaTiO3基粉体,但传统水热法反应条件为150 ℃下保温5 h,而微波水热法只需在80 ℃下保温30 min即可得到理想的BaTiO3基粉体。
Velasco-Davalos等[31]利用水热法制备了纳米级BaTiO3基粉体,从而获得性能较好的铁电薄膜材料。与其它湿化学法相比,水热法具有一些独特优点:(1)由于反应是在密封的反应釜中进行,压力相对较高,提供的物理化学环境使前驱体能充分溶解,形成晶元,进而直接结晶得到粉体,这在常压下是无法完成的;(2)可直接在较低温度下得到晶体发育完整的粉体,无需高温煅烧晶化,避免了煅烧过程中可能引起的粉体团聚或者混入杂质;(3)改变水热反应条件,可得到形貌、晶粒尺寸和结构可控的粉体。通过水热法[32]制备的纳米级BaTiO3基粉体具有纯度高、结晶好、颗粒大小分布均匀、无团聚或少团聚、形貌可控(如:多面体形[33]、四方块形[34]、树枝形[35]等)与烧结活性高等优点,能够制备薄层电介质需求的BaTiO3基介质陶瓷材料,此外,还被广泛用于制备铁电薄膜材料。但是粉体组成不易控制,容易发生偏析,且水热法对反应设备和条件要求较高,技术难度大,安全性低,在推广应用时有一定的局限性。
3 常压水相法
常压水相法是在水热法和共沉淀法的基础上发展起来的一种新型的制备BaTiO3基粉体的方法。常压水相法制备BaTiO3基粉体的机理如下[36]:
TiCl4+H2O→TiOCl2+2HCl
(1)
TiOCl2+Ba(OAc)2+4NaOH→BaTiO3+NaOAc+2H2O
(2)
常压水相法的原理与水热法相同,但是常压水相法反应温度较低,一般在100 ℃以下即可获得BaTiO3基粉体。溶剂和原料不同,BaTiO3基粉体的粒径也会不同。通常选择有机溶剂时,粉体粒径能够达到纳米级。Reddy等[37]采用常压水相法在水浴温度为75 ℃的条件下,制备了粒径为30 nm的Ba(ZrxTi1-x)O3基粉体。刘宇等[18]采用常压水相法在水浴温度为80~85 ℃的条件下,制备了形貌可控的单分散Ba(ZrxTi1-x)O3基粉体,粒径为0.47~1.24 μm。崔斌课题组[38-40]采用常压水相法制备了多种亚微米级(平均粒径< 280 nm)单分散球形粉体如BaTiO3、Ba0.97La0.02TiO3[38]和Ba0.991Bi0.006TiO3[39-40]等,并制备了性能优良且体系简单、满足X8R标准的BaTiO3基细晶陶瓷。
与其它方法相比,常压水相法能很好地控制粉体材料的组成、物相、粒度分布及微观形貌,具有物料设备成本低、反应条件易实现、制备工艺简单等特点,可得到粒度分布窄且纯度高、结晶好的纳米级单分散球形BaTiO3基粉体,被广泛用于制备性能优良且满足X8R标准的BaTiO3基细晶陶瓷。但也存在缺点,如:需要多次反复洗涤粉体才能将反应过程中的杂质除去,对技术条件要求较高,如果控制不当,就会引起产物的钛钡比出现较大的波动,进而影响产物的质量。
4 溶胶-凝胶水热法
近年来,随着陶瓷粉体制备技术的不断改进与发展,研究者不再局限于采用单一方法来制备陶瓷粉体,将几种湿化学法相结合制备出成本更低、性能更优异、结构独特的BaTiO3基粉体成为研究热点。如:Yang等[41]将溶胶-凝胶法和水热法相结合制备了中空的纳米级(86.7 nm)BaTiO3基单晶粉体。Sun等[42]采用溶胶-凝胶法与水热法相结合制备了纳米级单分散球形BaTiO3基粉体,在1 350 ℃烧结得到介电温度稳定性较好的BaTiO3基细晶陶瓷。
溶胶-凝胶水热法是当前研究较多的一种制备BaTiO3基粉体新方法[19,43],它结合了溶胶-凝胶法和水热法的优点,以水热处理代替溶胶-凝胶法的高温煅烧过程,使粉体在温和条件下结晶。该方法获得的单分散球形纳米粉体具有组分易控制、结晶性好、纯度高、形貌可控及粒径分布窄等优点。与溶胶-凝胶法相比,所得到的BaTiO3基粉体的分散性好、粒度分布均匀;与水热法相比,更易实现多组分的均匀掺杂,组成更易控制。
5 其它方法
Liu等[39]采用常压水相法与沉淀法相结合制备出粒径约为240 nm的单分散球形BaTiO3基粉体,通过1 180 ℃烧结得到满足X8R标准的细晶陶瓷材料。Yao等[44]采用溶胶-凝胶法与沉淀法相结合得到亚微米级的BaTiO3基粉体,通过烧结得到性能满足X9R标准的细晶陶瓷材料。Shangguan等[45]采用溶胶沉淀法制备了单分散球形亚微米级的Ba0.991Bi0.006TiO3基粉体,通过烧结得到介电温度稳定性较好的细晶陶瓷材料。
6 展望
尽管BaTiO3基粉体的制备方法较多,但从原料、设备、生产工艺及粉体性能等方面综合考虑,常压水相法与溶胶-凝胶水热法极具发展前景。单一湿化学法或多或少存在一些缺点,因此将两种或多种湿化学法相结合,进一步研究和开发更多高效实用的湿化学法尤为迫切。目前的湿化学法研究大都侧重于性能研究,对反应机理认识还不够深入,某些湿化学法仍然处于实验室研究阶段,尚未实现工业化。因此,今后应对湿化学法制备粉体进行更全面深入的研究。
[1] WANG S,HE H,SU H.Effect of synthesized BaTiO3doping on the dielectric properties of ultra temperature-stable ceramics[J].Journal of Materials Science:Materials in Electronics,2012,23(10):1875-1880.
[2] CHEN P Y,CHEN C S,TU C S,et al.Effects of texture on microstructure,Raman vibration,and ferroelectric properties in 92.5%(Bi0.5Na0.5)TiO3-7.5%BaTiO3ceramics[J].Journal of the European Ceramic Society,2016,36(7):1613-1622.
[3] ZHU L F,ZHANG B P,ZHAO L,et al.Large piezoelectric effect of(Ba,Ca)TiO3-xBa(Sn,Ti)O3lead-free ceramics[J].Journal of the European Ceramic Society,2015,36(4):1017-1024.
[4] KIMMEL V A,INIGUEZ J,CAIN G M,et al.Neutral and cha-rged oxygen vacancies induce two-dimensional electron gas near SiO2/BaTiO3interfaces[J].The Journal of Physical Chemistry Letters,2013,4(2):333-337.
[5] DATTA K,THOMAS P A.Structural investigation of a novel pe-rovskite-based lead-free ceramics:xBiScO3-(1-x)BaTiO3[J].Journal of Applied Physics,2010,107(4):043516.
[6] 姚国峰.高温稳定型 MLCC用介质陶瓷材料的制备、结构与性能研究[D].北京:清华大学,2012.
[7] LIN L X,WANG M J,LIU Y R,et al.Decisive role of MgO addition in the ultra-broad temperature stability of multicomponent BaTiO3-based ceramics[J].Ceramics International,2014,40(1):1105-1110.
[8] LIU M Y,HAO H,CHEN W J,et al.Preparation and dielectric properties of X9R core-shell BaTiO3ceramics coated by BiAlO3-BaTiO3[J].Ceramics International,2016,42(1):379-387.
[9] SUN Y,LIU H X,HAO H,et al.The role of Co in the BaTiO3-Na0.5Bi0.5TiO3based X9R ceramics[J].Ceramics International,2015,41(1):931-939.
[10] LU D Y,ZHANG L,SUN X Y.Defect chemistry of a high-k‘Y5V’(Ba0.95Eu0.05)TiO3ceramic[J].Ceramics International,2013,39(6):6369-6377.
[11] LIU B B,WANG X H,ZHAO Q C,et al.Improved energy storage properties of fine-crystalline BaTiO3ceramics by coating po-wders with Al2O3and SiO2[J].Journal of the American Ceramic Society,2015,98(8):2641-2646.
[12] GONG H L,WANG X H,ZHANG S P,et al.Grain size effect on electrical and reliability characteristics of modified fine-grained BaTiO3ceramics for MLCCs[J].Journal of the European Ceramic Society,2014,34(7):1733-1739.
[13] ZHANG Y C,WANG X H,KIM J Y,et al.High performance BaTiO3-based BME-MLCC nanopowder prepared by aqueous chemical coating method[J].Journal of the American Ceramic Society,2012,95(5):1628-1633.
[14] SHEN Z B,WANG X H,GONG H L,et al.Fabrication of high-performance Ba0.95Ca0.05Ti0.85Zr0.15O3ceramics by aqueous chemical coating method[J].Ceramics International,2015,41(S1):S157-S161.
[15] ZHANG Q L,WU F,YANG H.Sol-gel synthesis of A-site-ordered and homogeneous (Nd0.55,Li0.35)TiO3ceramic and its dielectric properties[J].Scripta Materialia,2011,65(9):842-845.
[16] QI H Q,FANG L,XIE W T,et al.Study on the hydrothermal synthesis of barium titanate nano-powders and calcination parameters[J].Journal of Materials Science:Materials in Electronics,2015,26(11):8555-8562.
[17] GAO J B,SHI H Y,DONG H N,et al.Factors influencing formation of highly dispersed BaTiO3nanospheres with uniform sizes in static hydrothermal synthesis[J].Journal of Nanoparticle Research,2015,17(7):286-289.
[18] 刘宇,崔斌,湛海涯,等.单分散BaZrxTi1-xO3亚微米粉体的可控合成[J].粉未冶金技术,2013,31(3):167-173.
[19] WANG W W,CAO L X,LIU W,et al.Low-temperature synthesis of BaTiO3powders by the sol-gel-hydrothermal method[J].Ceramics International,2013,39(6):7127-7134.
[20] KAVIAN R,SAIDI A.Sol-gel derived BaTiO3nanopowders[J].Journal of Alloys and Compounds,2009,468(1/2):528-532.
[21] XIN C R,ZHANG J,LIU Y,et al.Polymorphism and dielectric properties of Sc-doped BaTiO3nanopowders synthesized by solgel method[J].Materials Research Bulletin,2013,48(6):2220-2226.
[22] ZHAN X X,CUI B,XING Y L,et al.A novel process to synthesize high-k‘Y5V’ nanopowder and ceramics[J].Ceramics International,2012,38(1):389-394.
[23] WANG Y,CUI B,ZHANG L L,et al.Phase composition,microstructure,and dielectric properties of dysprosium-doped Ba(Zr0.1Ti0.9)O3-based Y5V ceramics with high permittivity[J].Ceramics International,2014,40(8):11681-11688.
[24] IANCULESCU A C,VASILESCU C A,CRISAN M,et al.Formation mechanism and characteristics of lanthanum-doped BaTiO3powders and ceramics prepared by the sol-gel process[J].Materials Characterization,2015,106:195-207.
[25] DEVI C S,KUMAR G S,PRASAD G.Spectroscopic and electrical studies on Nd3+,Zr4+ions doped nano-sized BaTiO3ferroelectrics prepared by sol-gel method[J].Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy,2015,136(PartB):366-372.
[26] RAMAKANTH S,RAJU K C J.Charge transfer induced magnetism in sol-gel derived nanocrystalline BaTiO3[J].Solid State Communications,2014,187:59-63.
[27] KRZMANC M M,KLEMENT D,JANCAR B,et al.Hydrothermal conditions for the formation of tetragonal BaTiO3particles from potassium titanate and barium salt[J].Ceramics International,2015,41(10):15128-15137.
[28] ZHAO X,LIU W F,CHEN W,et al.Preparation and properties of BaTiO3ceramics from the fine ceramic powder[J].Ceramics International,2015,41(S1):S111-S116.
[29] ZHANG Y,CHEN L J,ZENG J,et al.Aligned porous barium titanate/hydroxyapatite composites with high piezoelectric coefficients for bone tissue engineering[J].Matericals Science and Engineering C,2014,39:143-149.
[30] GUO L T,LUO H J,GAO J Q,et al.Microwave hydrothermal synthesis of barium titanate powders[J].Materials Letters,2006,60(24):3011-3014.
[31] VELASCO-DAVALOS I,AMBRIZ-VARGAS F,GOMEZ-YANEI C,et al.Polarization reversal in BaTiO3nanostructures synthesized by microwave-assisted hydrothermal method[J].Journal of Alloys and Compounds,2016,667:268-274.
[32] MAGNONE E,KIM J R,PAPK J H.The effect of the hydrothermal synthesis variables on barium titanate powders[J].Ceramics International,2016,42(8):10030-10036.
[33] ZHAN H Q,YANG X F,WANG C M,et al.Multiple nucleation and crystal growth of barium titanate[J].Crystal Growth & Design,2012,12(3):1247-1253.
[34] ADIREDDY S,LIN C,CAO B,et al.Solution-based growth of monodisperse cube-like BaTiO3colloidal nanocrystals[J].Chemi-stry of Materials,2010,22(6):1946-1948.
[35] MAXIM F,VIARINHO P,FERREIRA P,et al.Kinetic study of the static hydrothermal synthesis of BaTiO3using titanate nanotubes precursors[J].Crystal Growth & Design,2011,11(8):3358-3365.
[36] OVERAMENKO N A,SHVETS L A,OVCHARENKO F D,et al.Kinetics of hydrothermal synthesis of barium metatianate[J].Izv Akad Nauk SSSR Neorgy Mater,1979,15(11):82-85.
[37] REDDY S B,RAO K P,RAO M S R.Structural and dielectric characterization of Sr substituted Ba(Zr,Ti)O3based functional materials[J].Applied Physics A,2007,89(4):1011-1015.
[38] WANG Y,CUI B,LIU Y,et al.Fabrication of submicron La2O3-coated BaTiO3particles and fine-grained ceramics with temperature-stable dielectric properties[J].Scripta Materialia,2014,90-91:49-52.
[39] LIU Y,CUI B,WANG Y,et al.A novel precipitation-based synthesis for the formation of X8R-type dielectrics composition bas-ed on monodispersed submicron Ba0.991Bi0.006TiO3@Nb2O5particles[J].Journal of the European Ceramic Society,2015,35(9):2461-2469.
[40] LIU Y,CUI B,WANG Y,et al.Core-shell structure and dielectric properties of Ba0.991Bi0.006TiO3@Nb2O5-Co3O4ceramics[J].Journal of the American Ceramic Society,2016,99(5):1664-1670.
[41] YANG X,REN Z H,XU G,et al.Monodisperse hollow perovskite BaTiO3nanostructures prepared by a sol-gel-hydrothermal method[J].Ceramics International,2014,40(7):9663-9670.
[42] SUN Q M,GU Q L,ZHU K J,et al.Stabilized temperature-dependent dielectric properties of Dy-doped BaTiO3ceramics derived from sol-hydrothermally synthesized nanopowders[J].Ceramics International,2016,42(2):3170-3176.
[43] ZANFIR A V,VOICU G,JING A S I,et al.Low-temperature synthesis of BaTiO3nanopowders[J].Ceramics International,2016,42(1):1672-1678.
[44] YAO G F,WANG X H,ZHANG Y C,et al.Nb-modified 0.9BaTiO3-0.1(Bi0.5Na0.5)TiO3ceramics for X9R high-temperature dielectrics application prepared by coating method[J].Journal of the American Ceramic Society,2012,95(11):3525-3531.
[45] SHANGGUAN M Q,CUI B,MA R,et al.Production of Ba0.991Bi0.006TiO3@ZnO-B2O3-SiO2ceramics with a high dielectric constant,a core-shell structure,and a fine-grained microstructure by means of a sol-precipitation method[J].Ceramics International,2016,42(6):7397-7405.
Research Progress on Preparation of BaTiO3-Based Particles by Liquid-State Method
WANG Yan
(EngineeringResearchCenterofAdvancedFerroelectricFunctionalMaterials,ShaanxiKeyLaboratoryofPhytochemistry,CollegeofChemistryandChemicalEngineering,BaojiUniversityofArtsandSciences,Baoji721013,China)
Startingfromreactionmechanism,preparationtechniqueandapplication,theliquid-statemethodsforpreparingBaTiO3-basedparticles,includingsol-gelmethod,hydrothermalmethod,aqueous-phasemethodatatmosphericpressure,sol-gelhydrothermalmethod,aresummarized.Theirkeytechnologiesaswellasmeritsanddemeritsarepointedout.ThedevelopmentprospectofthepreparationofBaTiO3-basedparticlesbyliquid-statemethodisforcasted.
liquid-statemethod;BaTiO3-basedparticle;preparation
陕西省教育厅专项科研计划项目(16JK1040),宝鸡市科技计划项目(16RKX1-4),宝鸡文理学院校级重点项目(ZK16054,ZK16128)
2016-12-06
王艳(1983-),女,陕西蒲城人,博士,讲师,研究方向:功能陶瓷材料的制备与性能,E-mail:wangyan7144279@163.com。
10.3969/j.issn.1672-5425.2017.04.003
TQ028.8 TB34
A
1672-5425(2017)04-0010-04
王艳.湿化学法制备钛酸钡基粉体的研究进展[J].化学与生物工程,2017,34(4):10-13,18.

