甘草酸二铵对百草枯诱导肺泡上皮Ⅱ型细胞高迁移率族蛋白1的影响
方 辰,张剑锋,喻 莉,向 沥
甘草酸二铵对百草枯诱导肺泡上皮Ⅱ型细胞高迁移率族蛋白1的影响
方 辰,张剑锋,喻 莉,向 沥
目的 了解甘草酸二铵(DG)对百草枯(PQ)诱导肺泡上皮Ⅱ型细胞(alveolar epithelial cell Ⅱ, AECⅡ)中高迁移率族蛋白1(high mobility group box 1, HMGB1)的影响。方法 AECⅡ用含10%胎牛血清的RPMI 1640培养基,并加入青霉素100 U/ml、链霉素100 μg/L,置于37℃含5% CO2的培养箱中培养。将培养后的细胞分为3组,空白对照组(NS组)不采用任何药物干预培养24 h;模型组(PQ组):以1000 μmol/L PQ培养24 h;DG预治疗组(DG组):先以0.6 mg/ml DG培养2 h,再以0.6 mg/ml DG+1000 μmol/L PQ培养24 h。采用MTT法测定DG对PQ诱导下AECⅡ的增殖作用;采用酶联免疫吸附法检测3组细胞中的HMGB1、Toll样受体4(TLR-4)、髓样分化因子88(MyD88)、细胞核因子-κB(NF-κB)、肿瘤坏死因子(TNF)-α含量;采用实时荧光定量聚合酶链式反应测定3组细胞中HMGB1、TLR-4、MyD88、NF-κB P65 mRNA表达。结果 在1000 μmol/L PQ诱导下,AECⅡ以0.6 mg/ml DG干预24 h时生存率最高,以0 mg/ml DG干预生存率最低。与NS组比较,PQ、DG组HMGB1、TLR-4、MyD88、NF-κB P65、TNF-α水平均明显升高,且DG组升高程度低于PQ组,差异均有统计学意义(P<0.01);与NS组比较,PQ组、DG组HMGB1、TLR-4、MyD88、NF-κB P65 mRNA表达均升高,且DG组升高程度低于PQ组,差异均有统计学意义(P<0.01)。结论 DG可降低HMGB1、TLR-4、MyD88、NF-κB、TNF-α水平,减轻PQ诱导的AECⅡ损伤。
甘草酸二铵;高迁移率族蛋白质类;百草枯
百草枯(PQ)中毒早期即可出现肺水肿、肺出血、肺不张,并可迅速发展为不可逆性肺纤维化[1-3],且在此过程中肺泡上皮Ⅱ型细胞(alveolar epithelial cell Ⅱ, AECⅡ)受到明显损伤[4]。目前PQ中毒并无有效的拮抗剂,如何防治PQ中毒所致的急性肺损伤(acute lung injury, ALI)是临床研究的热点与难点[5]。甘草酸二铵(DG)是从中草药甘草中提取的一种有效成分单体[6],结构与肾上腺皮质激素类似,具有广泛的抗炎、抗氧化、抗病毒、调节免疫、护肝等功效[7]。本课题组前期研究证实,DG可抑制PQ中毒致ALI的SD大鼠肺组织中Toll样受体4(TLR-4)及细胞核因子-κB(NF-κB) P65的异常表达,具有较强的免疫调节和抗炎作用,进而可改善大鼠预后[8];DG还可增强PQ中毒致急性肾损伤SD大鼠肾组织中白细胞介素(IL)-10的表达,并且可抑制TLR-4、髓样分化因子88(MyD88)、NF-κB P65及IL-17的异常表达,从而减轻肾损伤[9-10]。然而,DG调控TLR-4、GR基因及炎性因子的作用机制尚不明确。本研究推测PQ中毒引起ALI的过程中,TLR4-MyD88-NF-κB信号通路中高迁移率族蛋白1(high mobility group box 1, HMGB1)可能发挥重要作用。本研究用DG行预干预,以PQ诱导AECⅡ建立体外模型,经酶联免疫吸附法(ELISA)和实时荧光定量聚合酶链式反应(RT-PCR)测定,了解PQ和DG对HMGB1表达的影响,并探讨DG与HMGB1、TLR4-MyD88-NF-κB信号通路的关系及DG对肺泡上皮细胞保护作用的可能机制。
1 材料与方法
1.1 实验材料 ①实验细胞:大鼠AECⅡ购自上海沪震实业公司;②药品及试剂:胎牛血清、RPMI 1640购自美国Gibco公司,DG购自江苏正大天晴制药有限公司,PQ、米非司酮(以无水乙醇配制)及MTT试剂购自美国Sigma公司,TriPure总RNA抽取试剂盒、反转录试剂盒及FastStart Universal SYBR Green Master购自美国Roche公司,大鼠HMGB1 ELISA试剂盒购自武汉华美生物工程有限公司;③仪器:CO2培养箱购自美国Forma scientific Inc公司,正置显微镜购自日本Olympus公司,酶标仪购自美国BioTek Instruments公司,荧光定量PCR仪购自美国Applied Biosystems StepOne公司。
1.2 实验方法
1.2.1 细胞培养及分组:AECⅡ用含10%胎牛血清的RPMI 1640培养基培养,并加入青霉素100 U/ml、链霉素100 μg/L,置于37℃含5% CO2的培养箱中培养。将实验细胞分为3组:①空白对照组(NS组):未采用任何药物干预,培养24 h;②模型组(PQ组):以1000 μmol/L PQ培养24 h;③DG预治疗组(DG组):先以0.6 mg/ml DG培养2 h,再以0.6 mg/ml DG+1000 μmol/L PQ培养24 h。
1.2.2 MTT法测定细胞存活率:细胞培养及接种同前,分别于96孔板中加入0、0.2、0.4、0.6、0.8、1.0、2.0、4.0 mg/ml DG孵育2 h,再以对应浓度的DG+1000 μmol/L PQ孵育24 h后加入10μl MTT(5 mg/ml),继续培养4 h后加入二甲基亚砜150 μl/孔,酶标仪570 nm处测定吸光度(OD值),计算细胞存活率。存活率(%)=用药组OD值/对照组OD值×100%。
1.2.3 ELISA定量检测质量浓度:取细胞培养物上清,用ELISA试剂盒检测各组大鼠细胞HMGB1、NF-κB P65、TLR4、MyD88、肿瘤坏死因子(TNF)-α质量浓度。操作步骤均按说明书进行。
1.2.4 RT-PCR测定基因表达:取3组大鼠AECⅡ,用TriPure法提取总RNA,以反转录试剂盒反转录成cDNA,采用FastStart Universal SYBR Green Master(ROX)试剂盒以已合成的cDNA为模板进行RT-PCR。HMGB1、TLR-4、MyD88、NF-κB P65的引物序列见表1。聚合酶链式反应条件:95℃预变性10 min,95℃变性15 s,60℃退火/延伸60 s,扩增40个循环。PCR后,用ABI 7500软件分析基因扩增情况,得到相应Ct值,以β-actin作为内参照,每组设3个复孔,基因相对表达量采用2-△△Ct方法计算。

表1 实时荧光定量聚合酶链式反应引物
注:HMGB1为高迁移率族蛋白1,TLR-4为Toll样受体4,MyD88为髓样分化因子88,NF-κB为细胞核因子-κB
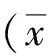
2 结果
2.1 DG对PQ诱导下AECⅡ增殖作用的影响 在1000 μmol/L PQ诱导下,AECⅡ以0.6 mg/ml DG干预24 h生存率最高,以0 mg/ml DG干预生存率最低,见图1。
2.2 ELISA定量检测质量浓度 PQ组、DG组中HMGB1、TLR-4、MyD88、NF-κB P65、TNF-α水平较NS组均有不同程度的升高,差异有统计学意义(P<0.01);但DG组升高程度低于PQ组,差异有统计学意义(P<0.01)。见表2。
2.3 RT-PCR测定基因表达 PQ组、DG组中HMGB1、TLR-4、MyD88、NF-κB P65 mRNA表达均较NS组不同程度升高(P<0.01);DG组升高程度低于PQ组,差异有统计学意义(P<0.01)。见表3。

图1 甘草酸二铵对1000 μmol/L百草枯诱导的大鼠肺泡上皮Ⅱ型细胞增殖作用的影响

表2 3组大鼠肺泡上皮Ⅱ型细胞HMGB1、TLR-4、MyD88、NF-κB P65、TNF-α水平比较
注:NS组未采用任何药物干预培养24 h;PQ组以1000 μmol/L百草枯培养24 h;DG组先以0.6 mg/ml甘草酸二铵培养2 h,再以0.6 mg/ml甘草酸二铵+1000 μmol/L百草枯培养24 h;HMGB1为高迁移率族蛋白1,TLR-4为Toll样受体4,MyD88为髓样分化因子88,NF-κB为细胞核因子-κB,TNF-α为肿瘤坏死因子-α

表3 3组大鼠肺泡上皮Ⅱ型细胞HMGB1、TLR-4、MyD88、NF-κB P65 mRNA表达
注:NS组未采用任何药物干预培养24 h;PQ组以1000 μmol/L百草枯培养24 h;DG组先以0.6 mg/ml甘草酸二铵培养2 h,再以0.6 mg/ml甘草酸二铵+1000 μmol/L百草枯培养24 h;HMGB1为高迁移率族蛋白1,TLR-4为Toll样受体4,MyD88为髓样分化因子88,NF-κB为细胞核因子-κB
3 讨论
PQ的结构与内源性聚胺类似,因肺内存在着一种聚胺转运系统,故PQ进入人体后在肺组织中大量聚集[11],肺组织中浓度要比血液中高10~90倍[12]。AECⅡ主要功能为参与肺泡上皮细胞的再生及分泌表面活性物质。PQ中毒18 h后,AECⅡ开始出现水肿,肺泡表面活性物质减少;24 h后,AECⅡ中线粒体明显水肿、破裂[13];72~96 h后,肺泡上皮细胞出现破裂,成为碎片或完全消失[14]。
目前甘草酸广泛应用于临床抗炎、抗病毒治疗,具有抑制NF-κB活化、炎性细胞因子产生及炎性细胞的迁移等作用。本研究观察DG对PQ诱导AECⅡ的增殖抑制作用,结果显示在一定浓度范围内DG可增加PQ诱导AECⅡ的存活率。
本研究证实,AECⅡ在PQ诱导24 h后,HMGB1 mRNA表达显著增强,而DG预治疗能抑制HMGB1 mRNA的过度表达。HMGB1是一种新发现的炎性介质,其普遍存在于真核细胞中,是一种高度保守的核蛋白,它在核内的主要功能为稳定核小体的结构及调节基因转录[15-16]。细胞在缺氧等病理状态下HMGB1可被释放到包浆与细胞外,参与组织的炎性损伤过程,在各种炎症性疾病中发挥重要作用[17]。目前已有许多研究表明,细胞外HMGB1和许多疾病及缺血/再灌注损伤等病理状态的严重程度呈正相关[18-20];并且在细胞外,HMGB1还可作为致炎因子,在炎性反应中发挥重要作用[21]。本研究显示,PQ组HMGB1水平高于对照组,表明HMGB1可在PQ诱导的肺泡上皮细胞中表达并释放,且可在细胞外发挥细胞因子样功能并激活炎症途径。亦有实验表明,HMGB1经气管内给药即可诱导ALI,引起大量炎性细胞释放和肺水肿[22]。还有实验表明,HMGB1作为晚期炎性介质在脓毒症过程中起重要作用,其血清水平与患者病死率呈正相关[17]。动物实验显示,HMGB1参与了肠黏膜功能障碍和致死性炎性反应等致病过程,在多种炎症性疾病中起重要作用[17,21],且其水平还可反映炎症和组织损伤的严重程度[18]。研究证实HMGB1可作为炎症损伤的治疗靶点,抗HMGB1单克隆抗体为各种炎性反应、缺血灌注损伤等的治疗提供了新的方向[23]。
内毒素、IL-1和TNF均能刺激巨噬细胞、单核细胞、垂体细胞、NK细胞、树突状细胞、内皮细胞和血小板等主动释放HMGB1[24-25]。在受损和坏死的细胞中,HMGB1也可以被动释放,持续性损害周围细胞[26]。研究显示,HMGB1受体主要包括晚期糖基化终末产物受体(RAGE),TLR2,TLR-4,TLR9,巨噬细胞抗原-1,多配体蛋白聚糖-3,CD24,Siglec-10,CXCR4和T细胞免疫球蛋白粘蛋白3等[19,27-28]。有研究认为,TLR-4是HMGB1的主要受体,它可以促进巨噬细胞活化,细胞因子的释放和组织损伤[20,29]。HMGB1结合TLR-4导致了NF-κB表达的上调及细胞因子的释放[20]。HMGB1在嗜中性粒细胞和巨噬细胞中通过TLR4-MyD88-NF-κB信号通路,参与呼吸系统的炎性反应过程[30-32]。也有研究认为,HMGB1通过激活多种信号通路参与机体炎性反应,如激活丝裂原活化蛋白激酶信号通路,使NF-κB易位,促进TNF-α、转化生长因子-β、血管内皮生长因子(VEGF)等炎性因子的表达,引发炎性反应[20,33-34]。这些途径可导致炎性级联反应,促进炎性介质TNF-α、单核细胞趋化蛋白-1、IL-6及IL-8等的释放,从而进一步促进HMGB1的释放,加重组织损伤。且HMGB1还与细胞分化、细胞增殖和凋亡、细胞迁移等密切相关[18]。本实验PQ组HMGB1、TLR-4、MyD88、NF-κB P65、TNF-α水平高于NS组,推测PQ刺激HMGB1释放并经TLR4-MyD88-NF-κB信号通路,参与肺泡上皮损伤过程。
Mollica等[35]认为,甘草酸亦可抑制HMGB1活性和HMGB1易位、分泌,这或许是其抗炎的主要作用机制。甘草酸并不影响HMGB1释放,而是直接抑制其活性[23]。研究表明,甘草酸以适度亲和力(Kd值4.03 mmol/L)结合HMGB1,但不结合血清可溶性晚期糖基化终末产物受体(sRAGE)。甘草酸可抑制HMGB1与sRAGE的结合,半抑制浓度类似HMGB1的甘草甜素Kd值。故与HMGB1结合的甘草甜素可抑制HMGB1与sRAGE的结合,致RAGE信号传导减少。进一步实验显示,甘草甜素与HMGB1分子的A-和B-盒相互作用,经甘草甜素修饰A-和B-框可能强烈干扰与RAGE结合[36-37]。在抗HMGB1单克隆抗体处理的情况下,甘草酸可抑制HMGB1的易位,提示存在着HMGB1释放的循环,诱导其自身易位,并进一步促进HMGB1释放,此循环可被甘草酸素抑制。因甘草甜素抑制HMGB1与sRAGE结合,推测抑制RAGE信号传导可能反过来导致甘草酸对HMGB1易位的抑制。研究证实,甘草酸与HMGB1的直接结合并抑制HMGB1的易位,表明甘草酸可抑制HMGB1分泌[37]。本实验中DG预治疗能部分抑制HMGB1水平表达,考虑DG有与HMGB1相似的抗炎作用。
本研究观察到AECⅡ在PQ诱导24 h后HMGB1 mRNA表达显著增强,而DG预治疗能抑制HMGB1 mRNA过度表达,对肺泡上皮细胞损伤有一定保护作用,揭示HMGB1与PQ中毒致ALI有一定关系,DG可通过调节信号通路抑制炎性反应和预防PQ中毒所致的ALI。
[1] Dinis-Oliveira R J, Duarte J A, Sanchez-Navarro A,etal. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment[J].Crit Rev Toxicol, 2008,38(1):13-71.
[2] 胡凤华,曲东,李杰,等.百草枯中毒1例[J].中国小儿急救医学,2007,14(4):369.
[3] 邱泽武.百草枯中毒的诊断与治疗[J].中国临床医生,2012,40(8):3-5.
[4] Haagsman H P, Schuurmans E A, Batenburg J J,etal. Phospholipid synthesis in isolated alveolar type Ⅱ cells exposed in vitro to paraquat and hyperoxia[J].Biochem J, 1987,245(1):119-126.
[5] 吕方方,魏路清.急性百草枯中毒致肺损伤的发病机制及治疗[J].武警医学院学报,2011,20(7):586-589.
[6] Wu P, Zhang Y, Liu Y,etal. Effects of glycyrrhizin on production of vascular aldosterone and corticosterone[J].Horm Res, 1999,51(4):189-192.
[7] Asl M N, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds[J].Phytother Res, 2008,22(6):709-724.
[8] 李浩,张剑锋,张伟,等.甘草酸二铵对百草枯中毒致急性肺损伤大鼠TLR-4表达的影响[J].蛇志,2014,26(1):1-3,15.
[9] 张伟,张剑锋,李浩,等.甘草酸二铵对百草枯致急性肾损伤大鼠IL-17表达的影响[J].蛇志,2015,27(2):103-105,120.
[10]张伟,张剑锋,李浩,等.甘草酸二铵对百草枯中毒大鼠肾组织TOLL样受体4表达的影响[J].中华急诊医学杂志,2015,24(11):1214-1219.
[11]Dinis-Oliveira R J, De Jesus Valle M J, Bastos M L,etal. Kinetics of paraquat in the isolated rat lung: Influence of sodium depletion[J].Xenobiotica, 2006,36(8):724-737.
[12]Chen C M, Lua A C. Lung toxicity of paraquat in the rat[J].J Toxicol Environ Health A, 2000,60(7):477-487.
[13]Smith P, Heath D. The ultrastructure and time sequence of the early stages of paraquat lung in rats[J].J Pathol, 1974,114(4):177-184.
[14]Dearden L C, Fairshter R D, Morrison J T,etal. Ultrastructural evidence of pulmonary capillary endothelial damage from paraquat[J].Toxicology, 1982,24(3-4):211-222.
[15]American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS)[J].Am J Respir Crit Care Med, 2000,161(2 Pt 1):646-664.
[16]Lin X, Yang H, Sakuragi T,etal. Alpha-chemokine receptor blockade reduces high mobility group box 1 protein-induced lung inflammation and injury and improves survival in sepsis[J].Am J Physiol Lung Cell Mol Physiol, 2005,289(4):L583-L590.
[17]Wang H, Yang H, Czura C J,etal. HMGB1 as a late mediator of lethal systemic inflammation[J].Am J Respir Crit Care Med, 2001,164(10 Pt 1):1768-1773.
[18]Ferhani N, Letuve S, Kozhich A,etal. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease[J].Am J Respir Crit Care Med, 2010,181(9):917-927.
[19]Fiuza C, Bustin M, Talwar S,etal. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells[J].Blood, 2003,101(7):2652-2660.
[20]Zhang J, Xia J, Zhang Y,etal. HMGB1-TLR4 signaling participates in renal ischemia reperfusion injury and could be attenuated by dexamethasone-mediated inhibition of the ERK/NF-κB pathway[J].Am J Transl Res, 2016,8(10):4054-4067.
[21]Yang H, Wang H, Tracey K J. HMG-1 rediscovered as a cytokine[J].Shock, 2001,15(4):247-253.
[22]Kong X, Zhang C, Jin X,etal. The effect of HMGB1 A box on lung injury in mice with acute pancreatitis[J].Biofactors, 2011,37(4):323-327.
[23]Girard J P. A direct inhibitor of HMGB1 cytokine[J].Chem Biol, 2007,14(4):345-347.
[24]Wang H, Vishnubhakat J M, Bloom O,etal. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes[J].Surgery, 1999,126(2):389-392.
[25]Yang H, Wang H, Czura C J,etal. HMGB1 as a cytokine and therapeutic target[J].J Endotoxin Res, 2002,8(6):469-472.
[26]Scaffidi P, Misteli T, Bianchi M E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation[J].Nature, 2002,418(6894):191-195.
[27]Cheng Y, Xiong J, Chen Q,etal. Hypoxia/reoxygenation-induced HMGB1 translocation and release promotes islet proinflammatory cytokine production and early islet graft failure through TLRs signaling[J].Biochim Biophys Acta, 2017,1863(2):354-364.
[28]Thankam F G, Dilisio M F, Dietz N E,etal. TREM-1, HMGB1 and RAGE in the Shoulder Tendon: Dual Mechanisms for Inflammation Based on the Coincidence of Glenohumeral Arthritis[J].PLoS One, 2016,11(10):e0165492.
[29]Wang F C, Pei J X, Zhu J,etal. Overexpression of HMGB1 A-box reduced lipopolysaccharide-induced intestinal inflammation via HMGB1/TLR4 signaling in vitro[J].World J Gastroenterol, 2015,21(25):7764-7776.
[30]Cheng Y, Wang D, Wang B,etal. HMGB1 translocation and release mediate cigarette smoke-induced pulmonary inflammation in mice through a TLR4/MyD88-dependent signaling pathway[J].Mol Biol Cell, 2017,28(1):201-209.
[31]Jiang Z, Zhou Q, Gu C,etal. Depletion of circulating monocytes suppresses IL-17 and HMGB1 expression in mice with LPS-induced acute lung injury[J].Am J Physiol Lung Cell Mol Physiol, 2016:ajplung 00389.2016.
[32]Srikrishna G, Huttunen H J, Johansson L,etal. N -Glycans on the receptor for advanced glycation end products influence amphoterin binding and neurite outgrowth[J].J Neurochem, 2002,80(6):998-1008.
[33]He L, Sun F, Wang Y,etal. HMGB1 exacerbates bronchiolitis obliterans syndrome via RAGE/NF-κB/HPSE signaling to enhance latent TGF-β release from ECM[J].Am J Transl Res, 2016,8(5):1971-1984.
[34]Li J, Huang L, Wang S,etal. Astragaloside Ⅳ attenuates inflammatory reaction via activating immune function of regulatory T-cells inhibited by HMGB1 in mice[J].Pharm Biol, 2016,54(12):3217-3225.
[35]Mollica L, De Marchis F, Spitaleri A,etal. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities[J].Chem Biol, 2007,14(4):431-441.
[36]Scaffidi P, Misteli T, Bianchi M E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation[J].Nature, 2010,418(6894):191-195.
[37]Okuma Y, Liu K, Wake H,etal. Glycyrrhizin inhibits traumatic brain injury by reducing HMGB1-RAGE interaction[J].Neuropharmacology, 2014,85:18-26.
Effects of Diammonium Glycyrrhizinate on the Expression of HMGB1 in Alveolar Epithelial Cell with Paraquat
FANG Chen, ZHANG Jian-feng, YU Li, XIANG Li
( Department of Emergency, Second Affiliated Hospital of Guangxi Medical University, Nanning 530000, China)
Objective To investigate the effects of Diammonium Glycyrrhizinate (DG) on the expression of HMGB1 in rat alveolar epithelial cell Ⅱ(AECⅡ )with paraquat (PQ). Methods The AECⅡ was cultured in RPMI-1640 media containing 10% FBS, 100 U/ml penicillin and 100 μg/L streptomycin at 37°C in 5% CO2. The cells were divided into three groups: The NS group was induced 24 h without any drugs, the PQ group was induced 24 h by 1000 μmol/L PQ ,and the DG group was induced 2 h by 0.6 mg/ml DG, then, was induced 24 h by 0.6 mg/ml DG+1000 μmol/L PQ. MTT assay was used to measure the proliferation effect of DG on AEC Ⅱ with PQ.The ELISA assay was applied to measuring the levels of HMGB1, TLR-4,MyD88,NF-κB, TNF-α in the three groups. The gene expressions of HMGB1,TLR-4, MyD88, NF-кB P65 mRNA in the three groups were detected by R T-PCR.Results The highest survival rate of AECⅡ induced 24 h by 1000 μmol/L PQ was in the group of 0.6 mg/ml DG, the lowest survival rate of AECⅡ induced 24 h by 1000 μmol/L PQ was in the group of 0 mg/ml DG. The levels of HMGB1, TLR4, MyD88, NF-кB P65, TNF-α in PQ and DG group were higher than those in NS group. While, the levels of HMGB1, TLR-4, MyD88, NF-кB P65, TNF-α in DG group were lower than those in PQ group (P<0.01). The expression of HMGB1, TLR-4, MyD88, NF-кB P65, TNF-α mRNA in PQ and DG group were higher than those in NS group.The expression of HMGB1, TLR4,Myd88,NF-кB P65,TNF-αmRNA in DG group were lower than those in PQ group(P<0.01). Conclusion DG can attenuate the injury in rat alveolar epithelial cells caused by paraquat poisoning. And it can decrease the expressions of HMGB1, TLR-4, MyD88, NF-кB and TNF-α.
Diammonium glycyrrhizinate; High mobility group proteins; Paraquat
国家自然科学基金项目(81360290);广西自然科学基金项目(2014GXNSFAA118195)
530000 南宁,广西医科大学第二附属医院急诊科
张剑锋,电话:13977105663
R341.31
A
1002-3429(2017)04-0099-05
10.3969/j.issn.1002-3429.2017.04.035
2016-12-13 修回时间:2017-02-11)

