Microarray gene expression analysis of chemosensitivity for docetaxel, cisplatin and 5-fluorouracil (TPF) combined chemotherapeutic regimen in hypopharyngeal squamous cell carcinoma
Meng Lian, Haizhou Wang, Jugao Fang, Jie Zhai, Ru Wang, Xixi Shen, Yifan Yang, Zhihong Ma, Honggang Liu
1Department of Otorhinolaryngology Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing 100730, China;2Beijing Key Laboratory of Head and Neck Molecular Diagnostic Pathology, Beijing 100730, China
*These authors contributed equally to this work.
Microarray gene expression analysis of chemosensitivity for docetaxel, cisplatin and 5-fluorouracil (TPF) combined chemotherapeutic regimen in hypopharyngeal squamous cell carcinoma
Meng Lian1*, Haizhou Wang1*, Jugao Fang1, Jie Zhai1, Ru Wang1, Xixi Shen1, Yifan Yang1, Zhihong Ma2, Honggang Liu2
1Department of Otorhinolaryngology Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing 100730, China;2Beijing Key Laboratory of Head and Neck Molecular Diagnostic Pathology, Beijing 100730, China
*These authors contributed equally to this work.
Objective:To screen out a set of candidate genes which could help to determine whether patients with hypopharyngeal squamous cell carcinoma (HSCC) could benefit from docetaxel, cisplatin and 5-fluorouracil (TPF) induction chemotherapy.
Methods:Gene-expression profiles in 12 TPF-sensitive patients were compared to 9 resistant controls by microarray analysis. Subsequently, expression levels of potential biomarkers in chemosensitive cell line FaDu after TPF treatment were observed by quantitative real-time polymerase chain reaction (qRT-PCR).
Results:Through microarray analysis, 1,579 differentially expressed genes were identified, of which 815 were up-regulated in TPF chemotherapy-responsive tissues whereas 764 were down-regulated. Gene ontology (GO) analysis suggested these genes participating in physiological processes including transcription and its regulation, cellular signal transduction and metabolic process. Additionally, Kyoto Encyclopedia of Genes and Genomes (KEGG) database revealed that MAPK and Jat/STAT signaling pathways occupied important roles in TPF chemotherapeutic sensitivity. Moreover, in vitro cell culture experiments revealed the expression alternations of IL-6, MAPK14, JUN, CDK5 and CAMK2A exposed to TPF treatment by qRT-PCR, whilst providing an insight into the mechanism underlying TPF chemotherapeutic response in HSCC.
Conclusions:These results provided a battery of genes related to TPF chemotherapeutic sensitivity and might act as molecular targets in HSCC treatment. Moreover, these candidate biomarkers could contribute to HSCC individualized treatment.
Microarray; HSCC; induction chemotherapy; MAPK
View this article at:https://doi.org/10.21147/j.issn.1000-9604.2017.03.06
Introduction
Hypopharyngeal carcinoma (HPC) is an aggressive malignancy that originates from a subsite of the upper aerodigestive tract, and accounts for 0.8%—1.5% of head and neck tumors with poor prognosis (1). Histologically, squamous cell carcinoma is currently the predominant tumor subtype of hypopharynx. Hypopharyngeal squamous cell carcinoma (HSCC) is frequently diagnosed at an advanced stage, hence resulting in a low survival rate andoverall poor prognosis as well as a formidable challenge to treat for these patients.
So far, comprehensive treatment combining surgery and postoperative radiotherapy remains the best choice for standard hypopharyngeal squamous cancer therapy, and chemotherapy and biological therapy also play crucial roles (2-4). For most of the patients who suffer from middlestage or advanced HSCC, receiving surgery may affect laryngeal function, which results in a permanent tracheostomy and has a negative effect on the patients’quality of life. Thus, to preserve the normal physiological function of larynx, induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil (TPF) has been developed and is considered as an alternative remedy to total laryngectomy and this regimen dramatically improved the outcomes in HSCC (5-7).
However, given that the efficacy of these chemotherapeutic agents is somehow hindered by the resistance, we cannot exactly predict whether the treatment will contribute to alleviating symptoms in an individual patient with HSCC. We found that for those patients sensitive to induction chemotherapy, the tumor volume shrank and the odds of functional preservation increased, and even surgery could, in part, ultimately be excluded, whereas in the resistant cases, patients could not benefit from induction chemotherapy and a surgery still remained necessary. While a myriad of genetic factors have been reported to participate in the pathogenesis and tumorigenesis of HSCC, the mechanistic underpinnings for the onset of this resistant phenotype remain elusive.
Compelling evidence suggests that genes are crucially involved in chemosensitivity of carcinoma. Genome-wide analysis using microarrays has emerged as a technology for investigating and addressing issues that were once thought to be non-traceable by simultaneous and large-scale measurement of the expression levels of large gene-sets, which may reflect given treatment response. Unfortunately, the relevant genes involved in HSCC are still little-known, and comprehensive and integrated gene analysis of this disease is hitherto unreported in the literature. In this study, we aim to identify the differentially expressed genes that are probably closely related to the chemosensitivity in HSCC by means of microarrays. The understanding of the genetic differences between those patients who are sensitive to induction chemotherapy and who are not will contribute to the understanding of the molecular basis and the identification of these highly relevant gene biomarkers can help to avoid unnecessary treatment and offer more expectation of desirable individualized treatment.
Materials and methods
Patients, samples and ethics statement
This study was conducted in Department of Otorhinolaryngology Head and Neck Surgery, Beijing Tongren Hospital. A total of 21 patients (aging from 43 to 71 years) who underwent TPF induction chemotherapy for primary HSCC were recruited in the study and sorted accordingly to their chemotherapeutical sensitivities: 12 patients proved to be sensitive to TPF chemotherapy were classified as the experimental group; and the rest 9 insensitive patients were divided into the control group. All patients were diagnosed as squamous cell carcinoma by pathological analysis and classified based on age, the TNM (tumor extension, regional lymph node involvement, and the presence of distant metastasis) stage, the degree of histologic differentiation, and chemotherapy response (Table 1).
The cancer tissues and corresponding adjacent nonneoplastic tissues were collected during surgery. Each specimen was immediately snap-frozen in liquid nitrogen and stored at —80 °C for subsequent study. The pathology analysis was evaluated by pathologists.
The study was approved by the Ethics Committee of Capital Medical University. All patients gave their written informed consent and did not receive any monetary compensation for participating in this study.
Screening of differentially expressed genes
The samples were subjected to microarray hybridization in Beijing Oligo Biotech Co. (Beijing, China). After obtaining the raw data, preliminary processing was conducted. The fold value (the difference in fold) represents the degree of differential expression between drug responsive and resistant patients. The standard used to judge differential expression was as follows: gene expression from the resistant patients (the control group) was used as the valid gene. Compared to the control group, fold change <1.0 was considered as a down-regulated gene, while fold change >1.0 was considered as an up-regulated gene. Genes with a fold change >1.5, or <0.5 compared to the control group were selected for further analysis.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
The differentially expressed genes screened via microarray were verified by qRT-PCR assay. Total RNA was extractedfrom tissue samples using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions and reverse-transcribed into cDNA with Rever-Tra-Ace-α- Transcriptase (Toyobo, Tokyo, Japan) and subsequently amplified by PCR using the SYBR®Premix Ex TaqTMII (Tli RNaseH Plus) (Takara, Tokyo, Japan). β-actin served as an internal control to normalize the RNA abundance. Quantitative PCR was performed on a LightCycler 480 Real-Time PCR system (Roche, Rotkreuz, Switzerland). The comparative Ctmethod (8) was used to calculate the relative-fold changes of mRNA expression in treated cells against control cells, with P<0.05 representing a significant difference, and P<0.01 representing a highly significant difference. All reactions were performed in triplicate, and values are expressed as±s.

Table 1 Clinical data of patients for microarrays
Bioinformatic analysis
The gene ontology (GO) annotations of the differentially expressed genes were analyzed primarily using the online analysis system termed as “Web-Based Gene Set Analysis Toolkit” for the three functional analyses. The distribution ratios of the differentially expressed genes in the three ontologies were used to identify the effect of the particular genes.
In this study, functional analyses using the online system called “Web-Based Gene Set Analysis Toolkit” were performed to identify the differentially expressed genes, and to locate the specific signaling pathways where the genes may participate. P-value was calculated using hypergeometric distribution and the cut-off was set at <0.05.
Cell culture and drug treatment
FaDu cells were cultured in RPMI-1640 (Invitrogen, Carlsbad, CA, USA), which contains 10% (V/V) fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) with 5% CO2at 37 °C.
FaDu cells were seeded in a 6 well plate at a density of105cells per well and incubated overnight, then treated with the indicated reagents for indicated time at the indicated final concentration (paclitaxel 1/10 IC50, fluorouracil 1/5 IC50, cisplatin 1/3 IC50). Cisplatin (Sigma, St. Louis, MO, USA) and fluorouracil (Sigma, St. Louis, MO, USA) were dissolved in 0.9% NaCl. Paclitaxel (Sigma, St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO).
Results
Microarray analysis of mRNA revealed different gene expression profiles between responsive and resistant patients
Expression profile analysis using the mRNA microarray was initially conducted between 12 TPF-responsive HSCC patients (experimental group) and 9 resistant patients (control group). Among the 34,601 genes analyzed, 1,579 genes showed statistically significant differences in the expression levels of tissues from the experimental group compared to the control group (P<0.05). Furthermore, of these 1,579 genes, 815 presented a higher expression in the tissue from experimental group, and 764 were downregulated in the experimental group. Supervised hierarchical clustering analysis revealed that the expression patterns of the selected set of 1,579 differentially expressed genes were able to perfectly distinguish patients who are sensitive to TPF chemotherapy from those resistant ones in a particular set of samples, which suggested the heterogeneity between chemosensitive and resistant cases. The results are also shown in Figure 1.
GO and pathway analysis fulfilled functional annotation of chemoresistance-associated genes in HSCC
The GO database is a controlled vocabulary of terms that provides 3 independent ontologies to describe genes in terms of their biological processes, cellular components and molecular functions. Kyoto encyclopedia of genes and genomes (KEGG) has been widely used as a genome annotation database to display metabolic pathways and relationships between the various pathways in a graphical fashion. To further determine genes that might be used as clinical biomarkers for evaluating TPF chemosensitivity in HSCC, from the identified 1,579 differentially expressed genes, the GO annotation analysis and KEGG pathways were performed.

Figure 1 Hierarchical clustering of differentially expressed mRNAs in experimental group (S1—S12) and control group (N1—N9). For each gene (row), red indicates a higher expression and green a lower one relative to the average level of expression of that gene across the 1,579 samples.
From the GO enrichment analysis, 283 GO terms were significantly related with the identified down-regulated genes in our microarray, whereas 325 were strikingly associated with the up-regulated genes (P<0.05). Besides, the up-regulated genes were involved in the regulation of biological processes including DNA-dependent transcription, signal transduction, and regulation of cell proliferation, etc. (Figure S1). As such, the down-regulated genes participated in processes such as DNA-dependent regulation of transcription, signal transduction, metabolism, protein phosphorylation, as well as responsivity to drugs (Figure S1).
Pathway analysis revealed that several differentially expressed genes were involved in a variety of signaling pathways concerning fat metabolism, cell growth and apoptosis, drug addition, as well as bacteria and virus infection, etc., 35 of which were up-regulated interesting genes and 46 were down-regulated genes (P<0.05, Figure S2).
MAPK signaling pathway plays a key role in HSCC chemosensitivity

Figure 2 Pathway network. Blue cycle nodes represent pathways corresponded to the significantly down-regulated intersecting genes and red cycle nodes represent pathways corresponded to the significantly up-regulated intersecting genes. Yellow cycle nodes indicate pathways that involve genes detected both in up-regulated and down-regulated transcripts. Edges indicate the effect of source pathway on downstream ones.
From the path-net analysis we found that MAPK cascade signaling is a core scaffold pathway in HSCC chemosensitivity, with a branch-point role in several pathways including calcium ion signaling pathway, Jak-STAT signaling pathway and Wnt signaling pathway (Figure 2). It can be found that the degree value of MAPK signaling pathway is the largest in all the signal pathways. The degree value is the value of the number of items between the pathways. The greater the degree is, the more connections with other pathways. The information can be obtained that MAPK pathway plays the most important role in the network. The KEGG data showed that of the 1,579 differentially expressed genes, eleven were involved in a panel of physiological processes, namely, MAPK cascade signaling, calcium ion signaling pathway, Jak-STAT and Wnt signaling pathway. These genes included MAP3K2, MAP2K3, MAPK14, JUN, FOS, CALML6, CAMK2A, CAMK2B, GRIN2A, IL6, and CSF3R (Figure 3), four (MAPK14, CALML6, IL6, CSF3R) of which were overexpressed in experimental group compared to the control, while the other seven (MAP3K2, MAP2K3, JUN, FOS, CAMK2A, CAMK2B, GRIN2A) were just downregulated, as a manifestation of the candidate molecular markers for chemosensitivity to TPF regimen in HSCC. The detailed data are illustrated in Table 2, S1 and S2.
Genes irresponsive to TPF chemotherapy may cause HSCC tolerance
We next investigated whether these genes were instinctively different in responsive and insensitive patients, or they expressed distinctively after TPF chemotherapy. To this end, we measured the expression status of MAPK14, JUN, CAMK2A, IL6, as well as cyclin-dependent kinase 5 (CDK5) in response to TPF treatment, and in a TPF sensitive HPC cell line FaDu. The qRT-PCR results revealed that the expression levels of JUN and CAMK2A were increased significantly after exposure to TPF combination treatment (Figure 4). In particular, CAMK2A remarkably aggrandized (more than 2-fold) compared to untreated cells. Combined with our microarray results, these all together suggested that TPF chemotherapy may induce an increase in JUN and CAMK2A in TPF-sensitive patients, whose levels were otherwise low before TPF therapy. Likewise, the expression of IL6 was significantly suppressed when treated with TPF (Figure 4). However, MAPK14 and CDK5 were up-regulated in FaDu cells after TPF treatment, which may be because MAPK14 was originally overexpressed in TPF-sensitive patients, or the augment of MAPK14 gene expression in sensitive patients was more remarkable in comparison with those insensitive patients. Thus, TPF-tolerance may be because, at least in part, several important genes cannot respond to chemotherapy in TPF-irresponsive HSCC patients.

Figure 3 Signal network. Blue cycle nodes represent genes that showed a lower expression in the experimental group and red cycle nodes represent genes that showed a higher expression in the experimental group. Edges indicate the effect of source gene on target ones.

Table 2 Microarray analysis of 11 genes between experimental and control groups
Discussion
One of the most challenging medical issues today is to bring our genetic knowledge to individualized cancer treatment, which will promise the optimized therapeutic management with more specificity and less toxicity. This study aims to discover several candidate gene hallmarks that can be utilized to estimate whether an individual patient will benefit from TFP chemotherapy regimen.
Gene expression profiling was performed on 34,601 genes in patients with HSCC who benefit from TPF-induction chemotherapy, and compared to those resistant cohorts. Among the 1,579 differentially expressed genes we screened out, eleven genes were implicated in MAPK cascade signaling, calcium ion signaling pathway, Jak-STAT and Wnt signaling pathway, displayed by the KEGG data. Genes of MAPK14, CALML6, IL6, CSF3R were up-regulated in patients who were sensitive to TPF chemotherapy compared to the resistant populations, whereas the expression of the other genes (MAP3K2, MAP2K3, JUN, FOS, CAMK2A, CAMK2B, GRIN2A) was suppressed in sensitive cases. We chose MAPK14, JUN, CAMK2A, CDK5 and IL6 as candidate biomarkers and evaluated the expression of these genes after TPF treatment by using a qRT-PCR. We found that the expression of MAPK14, JUN, CAMK2A, and CDK5 was elevated in response to TPF treatment, whereas IL6 was decreased. Therefore, these genes may become favorable clinical gene hallmarks for early evaluation of TPF-therapeutic outcome.

Figure 4 Quantitative real-time polymerase chain reaction (qRT-PCR) analysis for expression of CAMK2A (A), MAPK14 (B), JUN (C), IL6 (D) and CDK5 (E) in FaDu cells before and after docetaxel, cisplatin and 5-fluorouracil (TPF) treatment. CK means control group of untreated cells. Data are presented as±s. *, P<0.05; **, P<0.01.
In the present study, we found that MAPK signaling plays a central part in HSCC chemosensitivity, with extensive and diverse crosstalk with other tumorigenetic signal transduction pathways including calcium ion signaling pathway, Jak-STAT signaling pathway and Wnt signaling pathway. These results provided insightful perspective in understanding the effect of MAPK signaling, together with the protein kinase components in this pathway, on the chemoresistence. Aberrations in MAPK signaling impinge on evasion of apoptosis insensitivity to anti-growth signals, acquisition of drug resistance, and unlimited replicative potential (9). Recently developed drugs targeting the Ras-MAPK pathway in carcinoma are presenting more selective with the potential for improved therapeutic response and better toxicity as compared to the previous universal MAPK pathway inhibitors (10). Notably, patients with advanced lung cancer are responsive to gefitinib have been reported to carry somatic mutations in EGFR gene (11), a tyrosine kinase receptor that is frequently expressed on the cell membrane and acts as an initiator in MAPK/ERK pathway, and in addition, the EGFR-T790M mutation is associated with acquired resistance to this drug in initially gefitinib-sensitive patients (12,13). Also, dabrafenib inhibits mutated BRAF (V600E and V600K) (14). Another example is that blockage of p38 MAPK pathway could induce a resistant phenotype in response to cisplatin, consistent with results observed in the experimental model of head and neck cancer, hence the conclusion that p38 MAPK pathway is a specific target for the response to cisplatin-based cancer therapy with clinical implications (15). In addition, inhibition of the MAPK pathway increases chemosensitivity to glucocorticoids and possibly other agents (16).
There are several possible reasons responsible for the chemoresistance effect of MAPK pathways. First, aggrandized expression of numerous genes implicated in DNA damage repair has been deemed as contributors to the extreme resistance to conventional DNA-damaging chemotherapeutics. Cisplatin is one such chemotherapeutic that is ineffective against several refractory cancers, and it was reported capable to induce the expression of the DNA repair proteins via MAPK pathway, such as ERCC1 and XPF, which could eliminate cisplatin-induced DNA damage, ultimately resulting in the enhanced resistance to cisplatin (16). The regulation of cell proliferation in multicellular organism is a complex process, which is primarily regulated by external growth factors provided by surrounding cells. On the other hand, the MAPK pathwaysinvolving a series of protein kinase cascades also play a critical role in regulation of cell proliferation (17), and have anti-apoptotic as well as drug resistance effects on cells (18).
As MAPK pathway can interact with CDK and participate in modulating IL6 expression (19,20), we assumed that MAPK signaling plays a critical part in the regulation of HSCC chemosensitivity to TPF therapy. Particularly, TPF chemotherapy was not effective in irresponsive HSCC patients, which may be because these genes barely altered in those people.
Conclusions
These findings suggest that components in MAPK cascade and its interacted tumorigenetic pathways might be candidate biomarkers for evaluating an individual patient’s sensitivity to TPF chemotherapeutic treatment, and MAPK pathway targeting could be an attractive approach in combination with conventional chemotherapies for patients who suffer from chemoresistance in refractory HSCC.
Acknowledgements
This work is supported by grants of the Beijing Municipal Science & Technology Commission (No. Z141107002514003); Beijing Municipal Administration of Hospital Clinical Medicine Development of Special Funding Support (Code. XMLX201311); and the Priming Scientific Research Foundation for the Junior Researcher in Beijing Tongren Hospital, Capital Medical University (2015-YJJ-ZZL-001).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
1.Takes RP, Strojan P, Silver CE, et al. Current trends in initial management of hypopharyngeal cancer: the declining use of open surgery. Head Neck 2012;34:270-81.
2.Lefebvre JL, Andry G, Chevalier D, et al. Laryngeal preservation with induction chemotherapy for hypopharyngeal squamous cell carcinoma: 10-year results of EORTC trial 24891. Ann Oncol 2012;23: 2708-14.
3.Kraus DH, Zelefsky MJ, Brock HA, et al. Combined surgery and radiation therapy for squamous cell carcinoma of the hypopharynx. Otolaryngol Head Neck Surg 1997;116:637-41.
4.Harris BN, Biron VL, Donald P, et al. Primary surgery vs chemoradiation treatment of advancedstage hypopharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg 2015;141:636-40.
5.Pointreau Y, Garaud P, Chapet S, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst 2009;101:498-506.
6.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 2007;357:1695-704.
7.Lian M, Shi Q, Fang J, et al. In vivo gene expression profiling for chemosensitivity to docetaxel-cisplatin-5-FU (TPF) triplet regimen in laryngeal squamous cell carcinoma and the effect of TPF treatment on related gene expression in vitro. Acta Otolaryngol 2017:1-11.
8.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and themethod. Methods 2001;25:402-8.
9.Sharma P, Veeranna, Sharma M, et al. Phosphorylation of MEK1 by cdk5/p35 downregulates the mitogen-activated protein kinase pathway. J Biol Chem 2002;277:528-34.
10.Chow HM, Guo D, Zhou JC, et al. CDK5 activator protein p25 preferentially binds and activates GSK3β. Proc Natl Acad Sci U S A 2014;111:E4887-95.
11.Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol 2005;1:2005.0008.
12.Zarich N, Oliva JL, Martínez N, et al. Grb2 is a negative modulator of the intrinsic Ras-GEF activity of hSos1. Mol Biol Cell 2006;17:3591-7.
13.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39.
14.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancerto gefitinib. N Engl J Med 2005;352:786-92.
15.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73.
16.McCain J. The MAPK (ERK) pathway: investigational combinations for the treatment of BRAF-mutated metastatic melanoma. P T 2013;38:96-108.
17.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002;12:9-18.
18.McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007;1773:1263-84.
19.Veeranna, Shetty KT, Takahashi M, et al. Cdk5 and MAPK are associated with complexes of cytoskeletal proteins in rat brain. Brain Res Mol Brain Res 2000;76:229-36.
20.Sano M, Fukuda K, Sato T, et al. ERK and p38 MAPK, but not NF-κB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res 2001;89:661-9.
Cite this article as:Lian M, Wang H, Fang J, Zhai J, Wang R, Shen X, Yang Y, Ma Z, Liu H. Microarray gene expression analysis of chemosensitivity for docetaxel, cisplatin and 5-fluorouracil (TPF) combined chemotherapeutic regimen in hypopharyngeal squamous cell carcinoma. Chin J Cancer Res 2017;29(3):204-212. doi: 10.21147/j.issn.1000-9604. 2017.03.06
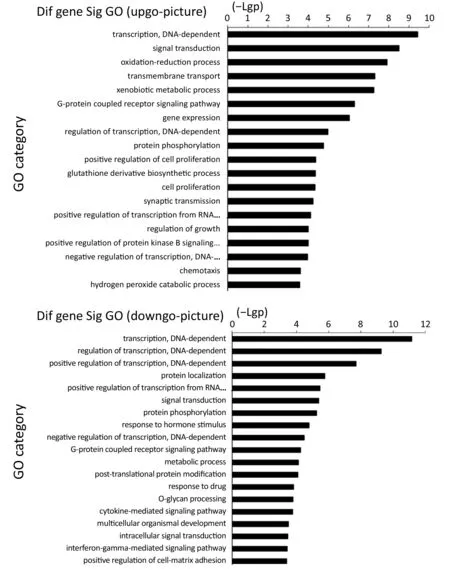
Figure S1 Gene ontology (GO) annotation plot analysis of differentially expressed genes. The upper chart shows the GOs targeted by overexpressed mRNA, and the lower chart shows the GOs targeted by down-regulated mRNA. The vertical axis is the GO category and the horizontal axis is the -Lg P value of the GO category.

Figure S2 Pathway analysis based on mRNA-targeted genes. Significant pathways targeted by down-regulated mRNA are shown. The vertical axis is the pathway category, and the horizontal axis is the enrichment of pathways.

Table S1 Eleven genes in GO database ID

Table S2 Eleven genes in KEGG pathways database ID
Jugao Fang. Department of Otorhinolaryngology Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing 100730, China. Email: fangjugao19651110@163.com.
10.21147/j.issn.1000-9604.2017.03.06
Submitted Feb 26, 2017. Accepted for publication Jun 05, 2017.
 Chinese Journal of Cancer Research2017年3期
Chinese Journal of Cancer Research2017年3期
- Chinese Journal of Cancer Research的其它文章
- Clinical applications of free medial tibial flap with posterior tibial artery for head and neck reconstruction after tumor resection
- Cancer of head and neck: a multidisciplinary approach
- Glasgow prognostic score after concurrent chemoradiotherapy is a prognostic factor in advanced head and neck cancer
- Neck observation versus elective neck dissection in management of clinical T1/2N0 oral squamous cell carcinoma: a retrospective study of 232 patients
- Recurrence and cancerization of ameloblastoma: multivariate analysis of 87 recurrent craniofacial ameloblastoma to assess risk factors associated with early recurrence and secondary ameloblastic carcinoma
- Enumeration and molecular characterization of circulating tumor cell using an in vivo capture system in squamous cell carcinoma of head and neck
