Is there an optimal time to initiate adjuvant chemotherapy to predict benefit of survival in non-small cell lung cancer?
Yutao Liu, Xiaoyu Zhai, Junling Li, Zhiwen Li, Di Ma, Ziping Wang
1Department of Medical Oncology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China;2Institute of Reproductive & Child Health, Peking University, Ministry of Health Key Laboratory of Reproductive Health, Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing 100191, China;3Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Thoracic Medical Oncology, Peking University Cancer Hospital &Institute, Beijing 100142, China
*These authors contributed equally to this work.
Is there an optimal time to initiate adjuvant chemotherapy to predict benefit of survival in non-small cell lung cancer?
Yutao Liu1*, Xiaoyu Zhai1*, Junling Li1, Zhiwen Li2, Di Ma1, Ziping Wang3
1Department of Medical Oncology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China;2Institute of Reproductive & Child Health, Peking University, Ministry of Health Key Laboratory of Reproductive Health, Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing 100191, China;3Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Thoracic Medical Oncology, Peking University Cancer Hospital &Institute, Beijing 100142, China
*These authors contributed equally to this work.
Objective:Adjuvant chemotherapy (AC) after curative resection is known to improve the survival of patients with non-small cell lung cancer (NSCLC); however, few studies have reported the correlation between the time to initiation of AC (TTAC) and survival in NSCLC patients.
Methods:The clinical data of 925 NSCLC patients who received curative resection and post-operative AC at the Cancer Hospital of Chinese Academy of Medical Sciences between 2003 and 2013 were retrospectively analyzed. TTAC was measured from the date of surgery to the initiation of AC. Disease-free survival (DFS) was defined as the duration from surgery to the time of tumor recurrence or last follow-up evaluation. The optimal cut-off value of TTAC was determined by maximally selected log-rank statistics. The DFS curve was estimated using the Kaplan-Meier method, and the Cox proportional hazards regression model was used to identify risk factors independently associated with DFS. Propensity score matching (PSM) was performed for survival analysis using the match data.
Results:The optimal discriminating cut-off value of TTAC was set at d 35 after curative resection based on which the patients were assigned into two groups: group A (≤35 d) and group B (>35 d). There was no significant difference in the DFS between the two groups (P=0.246), indicating that the TTAC is not an independent prognostic factor for DFS. A further comparison continued to show no significant difference in the DFS among 258 PSM pairs (P=0.283).
Conclusions:There was no significant correlation between the TTAC and DFS in NSCLC patients. Studies with larger samples are needed to further verify this conclusion.
Non-small cell lung cancer (NSCLC); adjuvant chemotherapy; time to adjuvant chemotherapy (TTAC); disease-free survival
View this article at:https://doi.org/10.21147/j.issn.1000-9604.2017.03.12
Introduction
Lung cancer is a common malignant tumor and the leading cause of cancer-related deaths worldwide (1). Despite optimal surgical resection for localized non-small cell lung cancer (NSCLC), the 5-year survival rate without additional treatment is 73% for stage IA disease and 25% for stage IIIA disease. Platinum-based adjuvantchemotherapy (AC) was reported to improve the survival of NSCLC patients with curative resection in several large randomized controlled trails (RCTs) (2-5); however, few studies have investigated whether the time to initiation of AC (TTAC) impacts the survival of NSCLC patients, although some clinical trials suggest the TTAC as 4—9 weeks after surgery (2-4).
Although multiple studies have shown that the interval from surgery to initiation of AC is significantly associated with survival outcomes in breast and colorectal cancers (6-9), the optimal interval from surgery to initiation of AC in NSCLC patients remains uncertain. The present study was to determine the optimal TTAC and the correlation with survival of NSCLC patients.
Materials and methods
Patients

Figure 1 Flowchart for selection of patients.
In this retrospective study, 925 consecutive patients who underwent curative resection of NSCLC at Cancer Hospital of Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC) between January 2003 and December 2013 (Figure 1) were included. The study protocol was approved by the Institutional Review Board of Cancer Hospital of CAMS & PUMC, in accordance with the Declaration of Helsinki. Ethical approval was obtained from Ethics Committee of Beijing Cancer Hospital. All patients were staged based on the TNM Classification of Malignant Tumors (7th edition) (10) and received post-operative AC. Exclusion criteria were patients with stage IB disease who had received neoadjuvant chemotherapy without high-risk factors, including poorly differentiated tumors, vascular invasion, wedge resection, tumor >4 cm, visceral pleural involvement, and incomplete lymph node sampling [Nx]. Patients were classified on the basis of age at diagnosis, gender, smoking history, histologic subtype, tumor differentiation, lymphatic involvement stage, use of adjuvant radiotherapy, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and comorbidity score. All patients who underwent pulmonary resection were followed up from the day of surgery. The postoperative follow-up evaluations were conducted by means of telephone and outpatient visits, including physical examinations, chest X-ray every 3 months, and chest and abdominal CT every 6 months for the first 2 years, and subsequently, chest X-ray every 6 months, and CT every year.
Comorbidities and outcomes
Based on all non-cancer diagnosis records in the hospital before surgery, comorbid disorders were assessed by Charlson comorbidity index (11-13). TTAC was defined as the time period between the initial operation and the date of the first AC. The outcome of interest was disease-free survival (DFS), which is defined as the duration from the surgery to the recurrence time (local, regional, and/or distant) or death from any cause. Data were censored on the last contact date. Patients who were still alive at the final follow-up evaluation (20 December 2014) were regarded as censored.
Statistical analysis
Approximate 20% of the cases were selected randomly as a training set to determine the cut-off points of the TTAC. Maximally selected log-rank statistics (validated by maxstat package in R, version 3.0.2) were chosen to identify the optimal cut-off points for the continuous variable (measured in days) of the TTAC. Patients were assigned to two groups according to the cut-off value. Comparisons of the baseline differences between the two groups were analyzed by χ2test, and the Fisher’s exact test was used for small samples.
The DFS curve was estimated using the Kaplan-Meier method, and compared using the log-rank test. A Coxproportional hazards regression model was used to identify risk factors independently associated with the DFS. Age, gender, smoking history, histologic subtype, tumor differentiation, lymphatic involvement stage, pathologic stage, use of adjuvant radiotherapy, ECOG PS and the comorbidity score were included as covariates in multivariate analysis. To reduce the influence of potential confounding factors and generate comparable study arms, the propensity score matching (PSM) method was applied (14,15). The variables included gender, age, histologic subtype, smoking history, tumor differentiation, lymphatic involvement stage, pathologic stage, use of adjuvant radiotherapy, ECOG PS, and comorbidity score. For matching, patients with an equivalent propensity score in the two groups were selected using 1:1 matching without replacement, with a caliper width <25% of the standard deviation. After PSM, baseline covariates were compared between the two groups. Subgroup analyses were conducted with a forest plot to determine whether or not any significant difference existed between different ages, genders, and pathologic stages.
GraphPad Prism Software (Version 5.0; GraphPad Software, Inc., La Jolla, USA) was used to generate the survival curve. PSM and forest plots were performed with STATA Software (Version 12; StataCorp LLC, Texas, USA). IBM SPSS Statistics (Version 21.0; IBM Corp., New York, USA) was used for other statistical analyses. A twosided P<0.05 was considered statistically significant.
Results
Patient characteristics

Figure 2 Distribution of time to adjuvant chemotherapy.
The mean age of the patients was 56 years (range, 25—76 years), and the proportions of male and female patients were 52.8% and 47.2%, respectively. The median time from surgery to the initial AC was 5 weeks (range, 1—19 weeks) (Figure 2). The median follow-up time was 48.7 months. Tumor recurrences occurred in 552 patients.
Of the 925 patients, 198 were selected randomly to identify the optimal TTAC. Cut-off point analysis for the DFS determined that 35 d was the optimal TTAC (Figure 3). The remaining 727 patients formed the study population. The patient characteristics are shown in Table 1. The patients included in the analysis were stratified as two groups: group A, in which the patients (n=391) received AC≤35 d after surgery; and group B, in which the patients (n=336) received the same treatment >35 d after surgery.
Impact of TTAC on DFS
The results of Cox proportional hazards regression model analysis are shown in Table 2. Multivariate analysis showed that histologic subtype, pathologic stage and use of AC had a significant impact on patient survival, while the TTAC was not an independent prognostic factor for DFS. There was no significant difference in the DFS between groups A and B (P=0.246) (Figure 4).
After adjustment of PSM and the variables (gender, age, histologic subtype, smoking history, tumor differentiation, lymphatic involvement stage, pathologic stage, use of adjuvant radiotherapy, PS, and comorbidity score), the two groups were well-matched (258 patients each) without significant differences in baseline characteristics (Table 1). Further comparison between the PSM pairs continued to show no significant difference in the DFS (P=0.283) (Figure 4).
Exploratory subgroup analysis

Figure 3 Optimal cut-off values of TTAC by maximally selected log-rank statistics.

Table 1 Patients demographic characteristics and PSM characteristics between two groups

Table 2 Multivariate Cox proportional hazards model of DFS
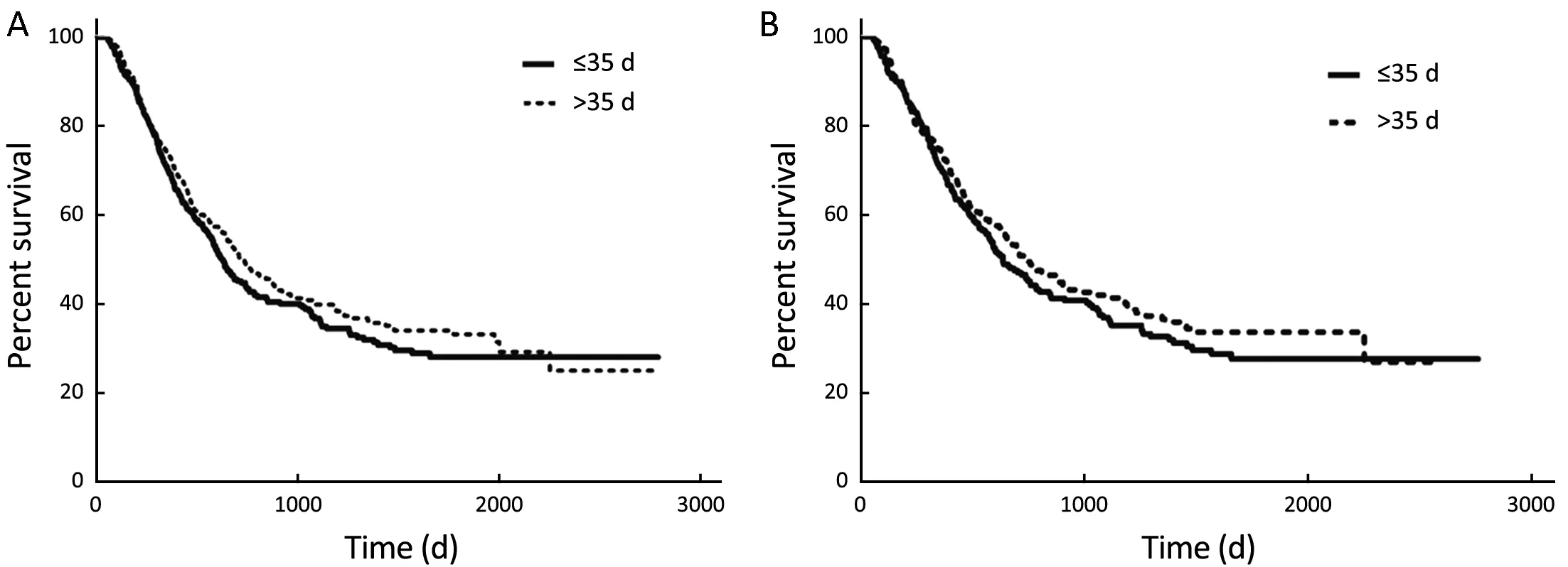
Figure 4 Kaplan-Meier curves of DFS before and after PSM. (A) Before match (P=0.246); (B) after match (P=0.283). DFS, disease-free survival; PSM, propensity score matching.
Exploratory subgroup analysis was performed to determine whether or not the TTAC had any significant impact on survival, independent of age, gender, smoking history, histologic subtype, tumor differentiation, lymphatic involvement stage, pathologic stage, use of adjuvant radiotherapy, PS, or co-morbidity score. The results of the subgroup analysis were similar to the full cohort; specifically, there was no significant difference between any subgroup (Figure 5).
Discussion
Adjuvant chemotherapy is the standard treatment capable of improving the survival outcomes in NSCLC patients after curative resection. Related RCTs arrange the enrolled patients to receive the initial AC 4—9 weeks after surgery (4,5,16). Strictly speaking, the treatment benefits described in most clinical trials are only applicable to patients who receive treatment during the same period as the trials. However, the optimal TTAC remains uncertain, and it is unclear whether or not the same benefit can be achieved when the TTAC is delayed.
Several studies have reported the prognostic effect of early initiation of AC in colorectal or breast cancer patients. In a meta-analysis involving 17,645 colorectal cancer patients, Guetz et al. (17) observed a statistically significant decrease in overall survival (OS) in patients with delayed initiation of AC. In another meta-analysis, Biagi et al. (8) reported a correlation between a 4-week increase in the TTAC and poor survival. In a retrospective analysis of 2,594 early-stage breast cancer patients, Lohrisch et al. (18) found that OS was significantly worse in patients who received AC >12 weeks after surgery compared with patients who received AC within 12 weeks.

Figure 5 Subgroup analysis.
There are various theoretical axioms in favor of initiating AC soon after curative resection. Animal model studies (19-22) have demonstrated that surgery may increase the number of circulating tumor cells and promote invasive metastasis. It is indicated by a classic mathematical model that drug resistance may increase the mutation rate (23). Furthermore, the effect of chemotherapy regimens is inversely proportional to the tumor burden, suggesting that delayed initiation of AC might stimulate tumor growth (24).
Patients selected in clinical trials may not represent the general population since they are usually younger, in better health, and with fewer co-existing diseases. Well-designed retrospective studies can provide important opinions in the“real world”. Furthermore, it is unrealistic to conduct anRCT to compare the effect on survival of patients who receive different TTAC programs for ethical or other reasons (25).
The current study did not observe a significant correlation between TTAC and survival rate in NSCLC, which is consistent with recent reports. A retrospective study from Canada (26) including 1,032 NSCLC patients who received AC at different initiation time points showed that TTAC did not impact the 4-year OS (64% vs. 61%, P=0.758), indicating that there was no significant correlation between delayed TTAC and poor prognosis with respect to OS. Furthermore, Ramsden et al. (27) explored the efficacy of the TTAC using the cut-off points, however, the results also showed that the patients with delayed TTAC did not have a difference in survival. Nevertheless, study designers designed the time interval of these studies. Whether or not there is an optimal TTAC that can be used to predict survival in NSCLC patients remains undefined. Recently, a retrospective study (28) enrolling 12,473 NSCLC patients defined 39—56 d as a reference interval group because of the lowest mortality rate and compared survival outcomes of patients who received the AC earlier (39—56 d), later (57—114 d), or during the reference interval. The results indicated no trend toward poor survival in the patients who initiated chemotherapy during the later period. The study set the OS as the primary endpoint, but it is possible that delays in chemotherapy might not impact OS and could impact progression-free survival (PFS).
In this study, we used maximally selected log-rank statistics and selected 35 d after surgery as the TTAC in an attempt to define the possible cut-off value. The optimal cut-point identified in this analysis was within the time frame in other studies (3,4,26). PSM analysis allowed us to compare survival between patients with similar background characteristics; however, even when potentially confounding variables (age, gender, smoking history, histologic subtype, tumor differentiation, lymphatic involvement stage, stage of disease, use of adjuvant radiotherapy, PS, and comorbidity score) were adjusted, no significant difference in the DFS was elicited between the two groups. Unlike colorectal and breast cancers, we were unable to identify an optimal TTAC that could benefit the survival of NSCLC patients, which may be explained by the host-tumor interaction of lung cancer considerably different from other solid tumors.
The study had some limitations. First, our study was limited by the retrospective nature of the analysis. Although we performed multivariate analysis and used the PSM method to eliminate the selection bias as much as possible, some disparities of known and unknown prognostic factors, such as specific chemotherapy regimens received and total dosage may affect the results. Factors that result in delayed initiation of AC might contribute to inferior outcomes that may not have been considered previously. Second, the exploration of OS in this study was limited. There are different therapies for regional recurrences or distant metastases, depending on different pathologic or mutation types. Several studies (29,30) indicated that in advanced NSCLC patients with epidermal growth factor receptor (EGFR) mutations, first-line therapy with gefitinib resulted in satisfied clinical therapeutic outcomes. In addition, the use of pemetrexed plus cisplatin showed significant benefits to survival in advanced-stage NSCLC patients with adenocarcinoma and large-cell carcinoma (31). Knowing that several factors can affect the OS outcome, we set the DFS as the primary end point for this study. Third, the result of subgroup analysis suggests that the DFS in elderly patients is not shorter than younger patients. The LACE meta-analysis and a retrospective analysis of the JBR.10 trial reported the same findings that chemotherapy significantly improved survival, and treatment-related death did not differ by age (32,33). Thus, AC should not be underestimated in elderly patients. Furthermore, this study was limited by the small number of patients, and research with larger samples is warranted to verify our conclusion.
Conclusions
This study demonstrated that there is no significant correlation between the TTAC and DFS in NSCLC patients, and that AC should not be underestimated in elderly patients. These conclusions warrant verification in future studies with larger samples.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
1.Siegel RL, Miller KD, Jemal A. Cancer statistics,2016. CA Cancer J Clin 2016;66:7-30.
2.Arriagada R, Bergman B, Dunant A, et al. Cisplatinbased adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60.
3.Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51.
4.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected nonsmall-cell lung cancer. N Engl J Med 2005;352:2589-97.
5.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA nonsmall-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006; 7:719-27.
6.Nachiappan S, Askari A, Mamidanna R, et al. The impact of adjuvant chemotherapy timing on overall survival following colorectal cancer resection. Eur J Surg Oncol 2015;41:1636-44.
7.Sun Z, Adam MA, Kim J, et al. Determining the optimal timing for initiation of adjuvant chemotherapy after resection for stage II and III colon cancer. Dis Colon Rectum 2016;59:87-93.
8.Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 2011;305:2335-42.
9.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, et al. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol 2016;2:322-9.
10.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:706-14.
11.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258-67.
12.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9.
13.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83.
14.Haukoos JS, Lewis RJ. The Propensity Score. JAMA 2015;314:1637-8.
15.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424.
16.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008; 26:3552-9.
17.Des Guetz G, Nicolas P, Perret GY, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer 2010;46:1049-55.
18.Lohrisch C, Paltiel C, Gelmon K, et al. Impact on survival of time from definitive surgery to initiation of adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol 2006;24:4888-94.
19.McCulloch P, Choy A. Effect of menstrual phase on surgical treatment of breast cancer. Lancet 1994;344:402-3.
20.Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 1994;79:185-8.
21.Eggermont AM, Steller EP, Sugarbaker PH. Laparotomy enhances intraperitoneal tumor growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery 1987;102:71-8.
22.Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res 1979;39:3861-5.
23.Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep 1979;63:1727-33.
24.Harless W, Qiu Y. Cancer: A medical emergency.Med Hypotheses 2006;67:1054-9.
25.Meyer RM. Generalizing the results of cancer clinical trials. J Clin Oncol 2010;28:187-9.
26.Booth CM, Shepherd FA, Peng Y, et al. Time to adjuvant chemotherapy and survival in non-small cell lung cancer: a population-based study. Cancer 2013;119:1243-50.
27.Ramsden K, Laskin J, Ho C. Adjuvant chemotherapy in resected stage II non-small cell lung cancer: evaluating the impact of dose intensity and time to treatment. Clin Oncol (R Coll Radiol) 2015;27:394-400.
28.Salazar MC, Rosen JE, Wang Z, et al. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol 2017;3:610-9.
29.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 2008;26:2442-9.
30.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57.
31.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51.
32.Pepe C, Hasan B, Winton TL, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol 2007;25:1553-61.
33.Früh M, Rolland E, Pignon JP, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol 2008;26:3573-81.
Cite this article as:Liu Y, Zhai X, Li J, Li Z, Ma D, Wang Z. Is there an optimal time to initiate adjuvant chemotherapy to predict benefit of survival in non-small cell lung cancer? Chin J Cancer Res 2017;29(3):263-271. doi: 10.21147/ j.issn. 1000-9604.2017.03.12
Dr. Ziping Wang. Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Thoracic Medical Oncology, Peking University Cancer Hospital &Institute, Beijing 100142, China.
10.21147/j.issn.1000-9604.2017.03.12
Submitted Dec 27, 2016. Accepted for publication May 09, 2017.
 Chinese Journal of Cancer Research2017年3期
Chinese Journal of Cancer Research2017年3期
- Chinese Journal of Cancer Research的其它文章
- Clinical applications of free medial tibial flap with posterior tibial artery for head and neck reconstruction after tumor resection
- Cancer of head and neck: a multidisciplinary approach
- Glasgow prognostic score after concurrent chemoradiotherapy is a prognostic factor in advanced head and neck cancer
- Neck observation versus elective neck dissection in management of clinical T1/2N0 oral squamous cell carcinoma: a retrospective study of 232 patients
- Recurrence and cancerization of ameloblastoma: multivariate analysis of 87 recurrent craniofacial ameloblastoma to assess risk factors associated with early recurrence and secondary ameloblastic carcinoma
- Enumeration and molecular characterization of circulating tumor cell using an in vivo capture system in squamous cell carcinoma of head and neck
