Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration
Jinwook Kim, Sueun Lee, Sohi Kang, Sung-Jo Kim, Jong-Choon Kim, Miyoung Yang, Changjong Moon,
1 Departments of Veterinary Anatomy and Veterinary Toxicology, College of Veterinary Medicine and BK21 PLUS Project Team, Chonnam National University, Gwangju, South Korea
2 Department of Anatomy, School of Medicine and Institute for Environmental Science, Wonkwang University, Jeonbuk, South Korea
How to cite this article: Kim J, Lee S, Kang S, Kim SH, Kim JC, Yang M, Moon C (2017) Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration. Neural Regen Res 12(10):1733-1741.
Funding: is work was supported by a grant from Wonkwang University in 2017.
Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration
Jinwook Kim1, Sueun Lee1, Sohi Kang1, Sung-Jo Kim1, Jong-Choon Kim1, Miyoung Yang2,*, Changjong Moon1,*
1 Departments of Veterinary Anatomy and Veterinary Toxicology, College of Veterinary Medicine and BK21 PLUS Project Team, Chonnam National University, Gwangju, South Korea
2 Department of Anatomy, School of Medicine and Institute for Environmental Science, Wonkwang University, Jeonbuk, South Korea
How to cite this article: Kim J, Lee S, Kang S, Kim SH, Kim JC, Yang M, Moon C (2017) Brain-derived neurotropic factor and GABAergic transmission in neurodegeneration and neuroregeneration. Neural Regen Res 12(10):1733-1741.
Neurotoxicity induced by stress, radiation, chemicals, or metabolic diseases, is commonly associated with excitotoxicity, oxidative stress, and neuroinflammation.e pathological process of neurotoxicity induces neuronal death, interrupts synaptic plasticity in the brain, and is similar to that of diverse neurodegenerative diseases. Animal models of neurotoxicity have revealed that clinical symptoms and brain lesions can recover over time via neuroregenerative processes. Specifically, brain-derived neurotropic factor (BDNF)and gamma-aminobutyric acid (GABA)-ergic transmission are related to both neurodegeneration and neuroregeneration.is review summarizes the accumulating evidences that suggest a pathogenic role of BDNF and GABAergic transmission, their underlying mechanisms, and the relationship between BDNF and GABA in neurodegeneration and neuroregeneration.is review will provide a comprehensive overview of the underlying mechanisms of neuroregeneration that may help in developing potential strategies for pharmacotherapeutic approaches to treat neurotoxicity and neurodegenerative disease.
brain-derived neurotropic factor; neurotoxicity; gamma-aminobutyric acid-ergic transmission;neurodegenerative diseases; neural regeneration
Introduction
Exposure to neurotoxicity induced by radiation, chemical and neurotoxic agents is common in today’s modern society. Many people suffer from neurodegenerative diseases,including Alzheimer’s disease (AD), Parkinson’s disease(PD), Huntington’s disease (HD), and multiple sclerosis(MS). The causes of neurotoxicity and neurodegenerative diseases vary; however, these conditions commonly share pathological processes, including synaptic dysfunction, neurovascular dysfunction, neuroinflammation, and neuronal death (Mattson and Duan, 1999; Mattson, 2000; Bito and Takemoto-Kimura, 2003; Amor et al., 2010).
To study neurotoxicity and neurodegenerative diseases,several models using neurotoxins have been developed.Kainic acid (KA), an analog of excitotoxic glutamate, can elicit selective neuronal death in rodent brain, resulting in pathological changes that partially mimic neurodegeneration in the central nervous system (CNS) (Wang et al.,2005). KA-induced neurodegeneration in rodents has been used as a model to explore the pathogenesis of excitotoxicity in neurodegenerative diseases (Zheng et al., 2011).Trimethyltin (TMT), a neurotoxic organotin compound,selectively affects neurons in the limbic system, particularly in the hippocampus, and is a useful agent for studying hippocampal neurodegeneration (Chang and Dyer, 1983;Balaban et al., 1988; Ishida et al., 1997; Ishikawa et al.,1997; Lee et al., 2016). Dopaminergic neurotoxins, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), that stimulate the expression of inducible nitric oxide synthase are used to induce pathogenic neurodegeneration that mimics PD (Liberatore et al., 1999; Blum et al., 2001). In addition to neurotoxin treatment, genetically modified animal models are widely used to study human neurodegenerative diseases. For example, transgenic (Tg) mice overexpressing human tau protein have consistently demonstrated neurological deficits and neuronal loss linked to the appearance of neurofibrillary tangles (NFTs). NFTs are a common neuropathological feature found in AD, and have been implicated in mediating neurodegeneration and dementia in AD and other tauopathies (Arriagada et al., 1992; Gomez-Isla et al.,1997; Guillozet et al., 2003). MitoPark mice, which have a disruption in the gene encoding mitochondrial transcription factor A in dopaminergic neurons, showed diverse features of PD, such as progressive motor deficits, neuronal loss, and protein inclusion (Langley et al., 2017). In addition, diverse types of genetic HD models, including R6/2, YAC 128, and BACHD mice, exhibited pathological hallmarks of HD, such as chorea, psychiatric disturbance, gradual dementia, and death. Thus, these models are generally used to study HD(Heng et al., 2008; Liang et al., 2014).
Use of the aforementioned animal models in numerous studies has revealed some of the mechanisms and pathol-ogies of neurotoxicity and neurodegenerative diseases.Brain-derived neurotropic factor (BDNF) and gamma-aminobutyric acid (GABA) are well known to be related to neuronal survival, neuronal protection, and synapse recovery(Levine et al., 1995; Ganguly et al., 2001; Baydyuk and Xu,2014). BDNF and GABAergic transmission are thus believed to contribute to the etiology and regenerative processes of neurodegenerative diseases. However, the regulatory mechanisms and interactions between BDNF and GABA in neurodegenerative diseases are not yet fully understood.
BDNF
BDNF plays pivotal roles in maintaining neuronal function and structure, and supports cellular functions, such as growth, differentiation, and survival in neurons (Maisonpierre et al., 1990). BDNF is synthesized and released in an activity-dependent manner (Lu, 2003), and binds to high-affinity receptors, namely tropomyosin receptor kinase B (TrkB) (Klein et al., 1993). Binding of BDNF to TrkB receptors activates diverse intracellular signaling, including Ras and extracellular signal-regulated kinase (Erk)1 and 2,phospholipase C-γ (PLC-γ), phosphatidylinositol 3-kinase(PI3K), and protein kinase C (PKC) (Nakagawara et al.,1994; Zirrgiebel et al., 1995). Several transcription factors,including c-Jun, c-Fos, and early growth response 1 (Egr-1),are then induced, and cyclic adenosine 3′,5′-monophosphate(cAMP) response element binding protein (CREB) is activated (Nakagawara et al., 1994; Gaiddon et al., 1996; Finkbeiner et al., 1997). In addition to the high-affinity TrkB receptors, BDNF binds to low-affinity receptor p75 neurotrophin receptor (p75NTR) (Berg et al., 1991; Casaccia-Bonnefil et al., 1996), which is known to potentiate Trk-induced survival activity via nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation (Maggirwar et al., 1998;Chittka and Chao, 1999; Hamanoue et al., 1999).
Several studies have reported that changes in BDNF levels occur during neurotoxicity and in neurodegenerative diseases (Ballarin et al., 1991; Lee et al., 2016; Tanila, 2017).Clinical human studies as well as genetic and experimental animal studies have suggested that decreased BDNF levels are associated with synaptic and neuronal loss and cognitive impairment in aging and AD (Tanila, 2017). In patients with AD, the expressions of the precursor form of BDNF(proBDNF) and mature BDNF decreased in the parietal cortex and hippocampus (Phillips et al., 1991; Holsinger et al., 2000; Michalski and Fahnestock, 2003; Peng et al., 2005).Systemic administration of BDNF is not considered to be a suitable approach due to its short plasma half-life and poor blood-brain barrier penetration (Nagahara and Tuszynski,2011). However, neural stem cell injection, which leads to upregulation of hippocampal BDNF, rescues the cognitive phenotype in aged amyloid-β precursor protein (APP)/presenilin (PS)1/tau Tg mice via increased synaptic density and restoration of hippocampal-dependent cognition(Blurton-Jones et al., 2009).erefore, the indirect elevation of BDNF levels in the CNS is considered to be a novel treatment strategy for AD.
In PD, BDNF also has potent effects on the survival and morphology of dopaminergic neurons, and therefore the loss of BDNF is likely to contribute to the death of dopaminergic neurons (Howells et al., 2000). Clinically, reduction in BDNF mRNA and protein expression has been observed in the substantia nigra of patients with PD (Howells et al.,2000). Laboratory animal models using MPTP, which induces hallmark symptoms of PD including loss of dopaminergic neurons in the midbrain (Meredith and Rademacher,2011) showed decreased BDNF protein levels in the lesioned striatum when compared with the same brain regions on the intact side (Kaur and Prakash, 2017). Inversely, over-expression of BDNF in dopaminergic neurons recovers the striatal innervation, dendritic spines and motor behavior in a rat model of PD (Razgado-Hernandez et al., 2015). Degeneration of striatal neurons and reduction in cortical BDNF mRNA and protein levels were observed in a mouse model of HD (Group, 1993; Perez-Navarro et al., 1999; Perez-Navarro et al., 2000; Zuccato et al., 2001; Zuccato and Cattaneo,2007).e levels of transcripts encoding BDNF exons II, IV,and VI were reportedly to be reduced in R6/2 mice (Zuccato et al., 2005). In addition, previous studies regarding multiple sclerosis (MS), a major inflammatory demyelinating disease,also showed decreased plasma BDNF levels, with the exception of a transitory elevation during relapses (Lassmann et al., 1998; Azoulay et al., 2005; Blanco et al., 2005; Vacaras et al., 2017). Together, these reports suggest that reduction in BDNF levels is a common mechanism underlying the development of diverse neurodegenerative diseases (Table 1).
To provide advanced insights into the detailed mechanisms of neurodegeneration, animal models treated with diverse neurotoxins have been widely used (Geloso et al.,2011). Neurotoxins such as KA and TMT induce significant cell death and neuroinflammation, which subsequently result in neurodegeneration. Previous studies have observed the rapid elevation of BDNF levels in neurotoxin-treated neurodegeneration models (Ballarin et al., 1991; Sathanoori et al., 2004; Kim et al., 2014; Lee et al., 2016). Although neurotoxins induce rapid BDNF elevation, neurotoxin-treated models also showed a gradual decrease in BDNF levels over time (Kim et al., 2014), which is similar to the patterns observed in many neurodegenerative models (as summarized inTable 1). The elevation of BDNF in the acute phase of neurotoxin administration is interpreted by two conflicting points of view. First, from a pathogenical view, increased levels of BDNF following neurotoxin administration might increase the severity of excitotoxicity by increasing synaptic activity (Bathina and Das, 2015). Another viewpoint is related to the protective effects of elevated BDNF againstneurotoxins (Casalbore et al., 2010; Corvino et al., 2013).Additionally, Lee et al. (2016) have reported that BDNF treatment significantly reduced neurotoxin-induced cell death via the activation of ERK signaling. Many in vitro and in vivo experiments have shown the protective effects of BDNF in striatal neurons and the therapeutic effects of BDNF or BDNF mimetics (Nakao et al., 1995; Ventimiglia et al., 1995; Bogush et al., 2007). Although further comprehensive studies investigating the precise protective/pathogenic roles of elevated BDNF levels in the acute phase of neurodegenerative process are needed, BDNF seems to play mainly a protective role against neuronal insults. As summarized inTable 1, those studies could provide fundamental data on the role of BDNF in neurodegeneration, and could be useful for the development of novel strategies aimed at increasing BDNF levels in the aforementioned neurodegenerative diseases to ultimately influence the clinical treatment of these conditions.
Table 1 e modulation of BDNF in various neurodegenerative models

Table 1 e modulation of BDNF in various neurodegenerative models
AD: Alzheimer’s disease; APP: amyloid-β precursor protein; BDNF: brain-derived neurotrophic factor; HD: Huntington’s disease; KA: kainic acid;MS: multiple sclerosis; MPTP:1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NSC: neural stem cell; PD: Parkinson’s disease; PI: post-injection;proBDNF: precursor form of BDNF; PS1: presenilin 1; QA: quinolinic acid; rrMS: relapsing-remitting MS; Tg: transgenic; TMT: trimethyltin.
ModelModulation of BDNFReferences ADPatients with AD BDNF mRNA, proBDNF, BDNF ↓Michalski and Fahnestock (2003); Peng et al. (2005)BDNF mRNA ↓ (parietal cortex)Holsinger et al. (2000)BDNF mRNA ↓ (hippocampus)Phillips et al. (1991)Aged Tau/PS1/APP 3xTg mice Hippocampal BDNF (NSC treatment) ↑Blurton-Jones et al. (2009)PDPatients with PD BDNF mRNA, BDNF ↓ (substantia nigra)Howells et al. (2000)MPTP-induced modelBDNF ↓ (lesioned striatum)Kaur and Prakash (2017)Rotenone-induced modelBDNF ↓ (plasma), BDNF ↑ (colon)Johnson et al. (2015)HDQA-induced HD modelBDNF, striatal neurons ↓Perez-Navarro et al. (2000)R6/2 Tg miceBdnfexon II, IV, VI, BDNF ↓Zuccato et al. (2005)MSPatients with MSBDNF ↓ (plasma)Vacaras et al. (2017)Patients with rrMSBDNF ↓ (serum and CSF)Azoulay et al. (2005)OthersKA-induced modelBDNF mRNA ↑(acute phase)Sathanoori et al. (2004)TMT-induced modelBDNF mRNA PI 1–2 d↑, PI 4–8 d ↓Kim et al. (2014)
GABAergic transmission
GABA is the major inhibitory neurotransmitter that activates GABAergic systems (Watanabe et al., 2002; Jin et al.,2003a; Wu et al., 2007b). The GABAergic system plays a pivotal role in maintaining equivalent neurotransmission in the CNS (Barbin et al., 1993; Behar et al., 1996; Taketo and Yoshioka, 2000; Pallotto and Deprez, 2014). Moreover, neuronal networks, including neural migration, differentiation,proliferation, and neurite outgrowth facilitation, are modulated by GABA synthesis, transport, release and reuptake,and GABA receptor composition (Jin et al., 2003b; Wu et al., 2007a).e synthesis of GABA is catalyzed by glutamic acid decarboxylase (GAD), which plays a key role in the regulation of GABAergic transmission (Wu et al., 2007b).GABA transporters, including the GABA transporter (GAT)and the vesicular GABA transporter (VGAT), act to store synthesized GABA and mediate the release and reuptake of GABA from synapses (Nelson and Blaustein, 1982).e action of GABA is terminated by its reuptake from the synaptic clevia membrane-bound GATs, which has been observed in both presynaptic and postsynaptic membranes of differentiated neurons and in the surface of glial cells (Minelli et al., 1995, 1996).
GABAergic transmission is mediated by two distinct receptor classes: ionotropic GABA A receptors (GABAARs)and metabotropic GABA B receptors (GABABRs) (Couve et al., 2000; Sieghart and Sperk, 2002; Bettler and Tiao, 2006).GABAARs are hetero-pentameric chloride channels that mediate fast synaptic inhibition. GABAARs are composed of five subunits selected from at least 19 GABAAR subunits.Interestingly, there is accumulating evidence that individual GABAAR subunits are associated with distinct neuronal structures and subcellular distributions, and that their differential activation is closely correlated with distinct pharmacological and behavioral phenotypes (Rudolph et al., 2001;Kittler et al., 2002; Sieghart and Sperk, 2002). GABAARs are relevant drug targets for anti-convulsant, anxiolytic, and sedative-hypnotic agents (Monteleone et al., 1990; Olsen and Avoli, 1997; Sieghart and Sperk, 2002). GABABR is a G protein-coupled receptor in the CNS that is implicated in neurological and psychiatric disorders (Barnard et al., 1998;Bowery et al., 2002; Calver et al., 2002).erefore, elucidating the modulatory mechanisms of the GABAergic system should be prioritized to understand the inhibitory role of GABA in diverse neurologic conditions. However, the modulation of GABA signal-related molecules in neurodegeneration is not completely understood.
The balance between neuronal excitation and inhibition in neuronal networks is crucial for normal brain function.e dysfunction of neuronal electrical excitability may play an important role in neurodegenerative disease. GABAergic systems have recently become an increasing area of interest in AD research (Rossor and Iversen, 1986; Andrews-Zwilling et al., 2010). In the human APP Tg mouse, inhibitory hippocampal circuits are altered by the sprouting of collat-eral mossy fibers onto GABAergic basket cells (Palop et al.,2007). Tau/PS2/APP 3xTg mice demonstrated significant neurodegeneration of GABAergic septo-hippocampal projection neurons as well as that of their target cells, GABAergic hippocampal neurons (Bowery and Brown, 1974).APP-induced impairment of GABAergic interneurons suggests that dysregulation of the excitatory/inhibitory neurotransmitter balance contributes to neurodegeneration in AD (Wang et al., 2014; Villette and Dutar, 2017). Oyelami et al. (2016) reported the functional and transcriptional deficits in GABAergic pathways in prefrontal cortex in aged APP/PS1 Tg mice.ey observed significant decreases in various GABA-related genes, such as Gabra1, 3, 4, 5, Gabrb2, 3, Gabrg2, Gabarapl1, and Gabarap, and the changes in synaptic function in the prefrontal cortex of 8-month-old APP/PS1 Tg mice (Oyelami et al., 2016). However, VGAT and GAD are not altered in patients with AD or in APP/PS1 Tg mice(Mitew et al., 2013).erefore, understanding the molecular details involved in the alteration of GABAergic transmission may provide insight into the pathogenesis of AD.
Similarly, patients with PD exhibited significantly decreased levels of GABA in the cerebrospinal fluid (CSF) (de Jong et al., 1984). A (3-chlorophenyl)[3,4-dihydro-6,7-dimethoxy-1-[(4-methoxyphenoxy)methyl]-2(1H)-isoquinolinyl]-methanone (CIQ)-induced PD model also showed depressed GABAergic transmission via a cholinergic mechanism in medium spiny projection neurons in the striatum(Feng et al., 2014). In addition, HD caused by expansion of the CAG repeat in exon 1 of the huntingtin gene induced loss of GABAergic medium spiny neurons in the striatum(Kremer et al., 1994; DiFiglia et al., 1997). Furthermore, a pattern of neurodegeneration of GABAergic striatal efferent projection neurons observed in patients with HD, are closely correlated with increasing clinical neuropathological HD grade (Glass et al., 2000). Following quinolinic acid (QA)-induced degeneration of the striatonigral pathway, there was marked loss of GABA immunoreactivity and 59% increase in the density of GABAARs in the substantia nigra pars reticulate (Nicholson et al., 1995). Moreover, QA injection rapidly induced an increase in GABABR subunit 1 or 2 immunoreactivity in the lesioned striatum, despite the neuronal loss(Rekik et al., 2011), indicating that it may be upregulated by reactive astrocytes. GABA insufficiency has also been identified in MS patients.e sensorimotor GABA concentration was abnormally lower in individuals with the secondary progressive form of MS, suggesting that decreased GABA levels are involved in worse motor function (Demakova et al., 2003; Cawley et al., 2015).
Additionally, other neurodegenerative models using neurotoxins, such as KA, induce loss of GABA and GAD when injected into rodent striata (Young et al., 1988).e mRNA levels of GABAAR subunits γ2 and δ also decrease significantly in the hippocampus following TMT-induced seizure (Kim et al., 2015). In 4-aminopyridine-induced excitotoxicity, GABA-mediated transmission may paradoxically boost neuronal hyperexcitation (Pena and Tapia, 2000). As summarized inTable 2, the number of GABAergic neurons is decreased in neurodegenerative stimulation.us, the decrease in the number of GABAergic neurons induces an imbalance of excitatory/inhibitory neurotransmission, which is considered a main causal factor in many neurodegenerative diseases.is suggests that the stable maintenance of GABAergic transmission may be a protective/therapeutic solution in neurodegenerative disease.
Correlation between the BDNF and GABAergic systems
BDNF is synthesized by both neurons and glia and is involved in survival, differentiation, and regeneration of neurons via TrkB binding. BDNF is one of the crucial mediators of long-term potentiation at glutamatergic and GABAergic synapses in the CNS (Korte et al., 1995; Figurov et al., 1996;Lu, 2003). BDNF is attributed to mostly increased presynaptic transmitter release. Specifically, the acute effects of BDNF enhance glutamatergic transmission and reduce GABAergic transmission in the CNS (Zafra et al., 1991; Tanaka et al.,1997). Thus, BDNF could influence GABAergic transmission positively or negatively through various intracellular signaling pathways triggered via TrkB or p75NTR.
BDNF may affect GABAergic transmission, subsequently modulating CNS function, neuronal survival, and plasticity.BDNF binds to TrkB, which couples to the PLC-γ/PKC-δ and ERK/mitogen-activated protein kinase (MAPK) pathway. Activated PLC-γ could induce PI3K activation which increases intracellular Ca2+concentrations (Yamada et al., 1991); consequently, BDNF disrupts GABAAR function through elevated intracellular Ca2+concentration (Tanaka et al., 1997). BDNF-TrkB signaling also affects the presynaptic concentration of GABA through the activation of GAT-1 (Vaz et al., 2011).ese alterations are presumably caused by the upregulation of GAD67 mRNA and neuronal GAT-1. GATs are located in the plasma membrane of neurons and astrocytes and are responsible for the termination of GABAergic transmission.BDNF enhances GAT-1-mediated GABA transport in astrocytes and/or neurons, which requires an active A2Areceptor.In primary hippocampal neurons, BDNF reduces GABAergic miniature inhibitory postsynaptic currents and causes reduction in GABAAR subunit α2, β2, β3, and γ2 immunoreactivity (Brunig et al., 2001). Moreover, BDNF upregulates the expression of GABAAR α4 by Egr-3 stimulation related to PKC pathway activation (Roberts et al., 2005, 2006). BDNF has been shown to selectively regulate GABAAR transcription through activating the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway and BDNF treatment of hippocampal neurons stimulated phosphorylation of STAT3, induced increases in inducible cAMP early repressor (ICER) expression, and decreased transcription of Gabra1 (Lund et al., 2008). BDNF increased GABAAR subunit α4 expression, but decreased GABAAR subunit α1 levels in hippocampal neurons, suggesting that BDNF has a potential role in differentially regulating the expression of extrasynaptic and synaptic GABAARs (Roberts et al., 2006).e influence of BDNF on GABAergic transmission is not only limited todirect regulatory mechanisms, but also includes indirect ionic concentrations.
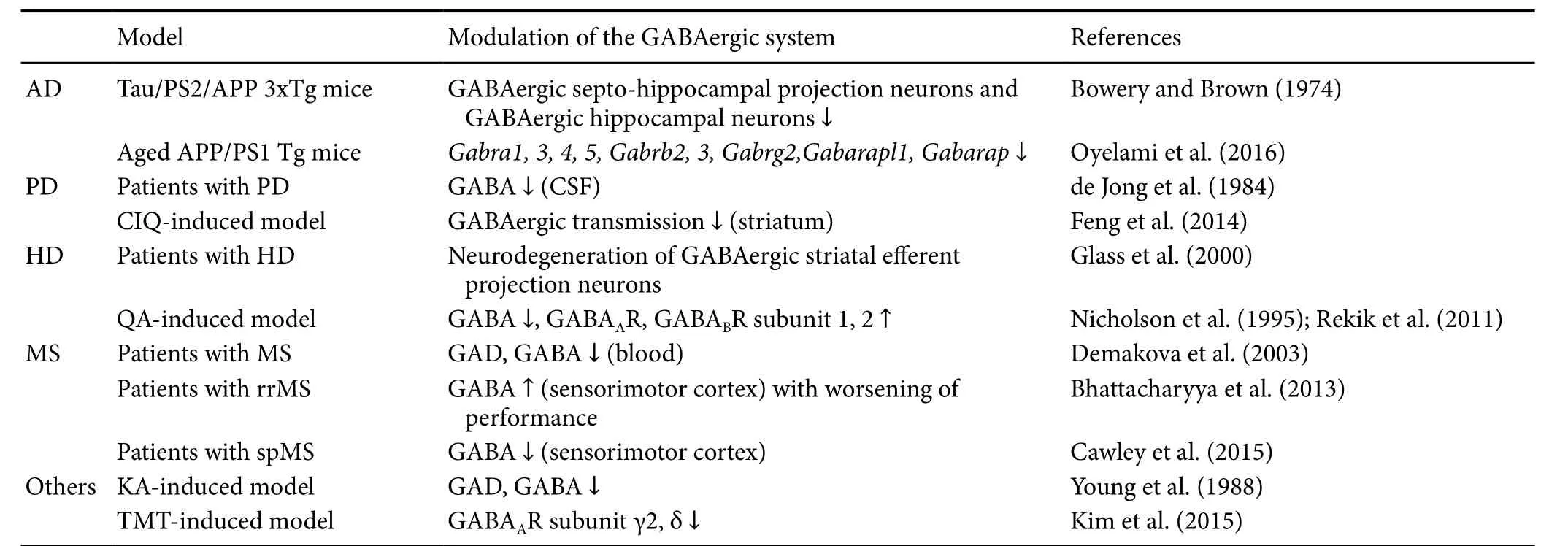
Table 2 Modulation of the GABAergic system in various neurodegenerative diseases
Table 3 e mechanisms underlying BDNF modulation on the GABAergic system

Table 3 e mechanisms underlying BDNF modulation on the GABAergic system
ADORA2A: Adenosine A2A receptor; BDNF: brain-derived neurotrophic factor; Egr: early growth response protein; GABA: γ-aminobutyric acid;GABAAR: GABA A receptor; GAD: glutamic acid decarboxylase; GAT: GABA transporter; JAK: Janus kinase; MAPK: mitogen-activated protein kinase; PKC: protein kinase C; STAT: signal transducer and activator of transcription; TrkB: tyrosine receptor kinase B.
Altered pathway induced by BDNFModulating target References Elevated intracellular Ca2+ concentration by TrkB activationGABAARTanaka et al. (1997)Enhanced GABA transport in astrocytes through ADORA2A signalingGATsVaz et al. (2011)Egr-3 stimulation related to PKC-MAPK pathway activation GABAAR α4Roberts et al. (2005, 2006)JAK/STAT, Gbara1GABAAR α1Lund et al. (2008)Increased GAD activity/elevated GABA uptake activityGABA contentMizuno et al. (1994)Downregulated K+-Cl− cotransporter 2 (KCC2)GABA receptorsWardle and Poo (2003)
BDNF indirectly modulates GABAergic transmission through the postsynaptic regulation of Cl−transport (Wardle and Poo, 2003). Previous studies suggest that the acute postsynaptic downregulation of K+-Cl−cotransporter 2 (KCC2)activity may decrease the efficacy of inhibitory transmission. Moreover, BDNF contributes to the differentiation of striatal GABAergic neurons during development (Mizuno et al., 1994). BDNF injections into the cerebral ventricles of neonatal rats induced an increase in GABA content in the striatum (Mizuno et al., 1994).e elevation of GABA levels mainly resulted from the elevation of GAD activity and GABA uptake activity (Mizuno et al., 1994).erefore,BDNF could alter diverse intracellular mechanisms, which might modulate pre/postsynaptic GABAergic transmission,as summarized inTable 3.e organization of evidence for the correlation between BDNF and GABA during neurodegeneration may be important to understand their underlying mechanisms of neurotoxicity and neurodegenerative diseases.
Based on its role in diverse neurodegenerative processes, BDNF typically enhances the release of presynaptic transmitters, including GABA; in general, its expression is downregulated, except during the acute phase, when it is upregulated. BDNF exhibits an acute excitatory effect via suppression of chloride-dependent fast GABAergic inhibition (Rivera et al., 2002; Canas et al., 2004). In pathological processes including chronic pain and seizure, BDNF/TrkB signaling contributes to downregulation of KCC2 protein expression and its transport function, leading to hyper-excitability (Kong et al., 2014; Tao et al., 2015). Thus, it was suggested that a temporary increase in BDNF during the acute phase of neurodegenerative processes involves hyper-excitability via suppression of chloride transport and decreased efficacy of inhibitory transmission. Additionally,increased BDNF likely disrupts the function of GABAAR by elevating the intracellular Ca2+concentration (Yamada et al., 1991) during the acute phase, resulting in abnormally regulated GABAergic transmission. During the neurodegenerative period, decreased BDNF levels may cause decreases in the intracellular Ca2+concentration, Egr-3 signaling in the post-synapse, as well as GAD65/67 in the pre-synapse(Brunig et al., 2001; Roberts et al., 2005, 2006; Lund et al.,2008; Vaz et al., 2011).us, downregulation of intracellu-lar signaling affecting GABA-related signaling may reduce the inhibitory efficacy of GABAergic transmission, leading to hyper-excitability.erefore, the altered BDNF levels in neurodegenerative processes may perturb the balance in potentiation between glutamatergic and GABAergic synapses in the CNS. An imbalance in excitatory/inhibitory neurotransmission may be one of the key factors for neurodegeneration (as shown inFigure 1). Consequently, targeting the increased levels of BDNF in neurodegenerative diseases could result in a balance in neurotransmission and may further be used as an effective therapeutic strategy in diverse neurodegenerative conditions.
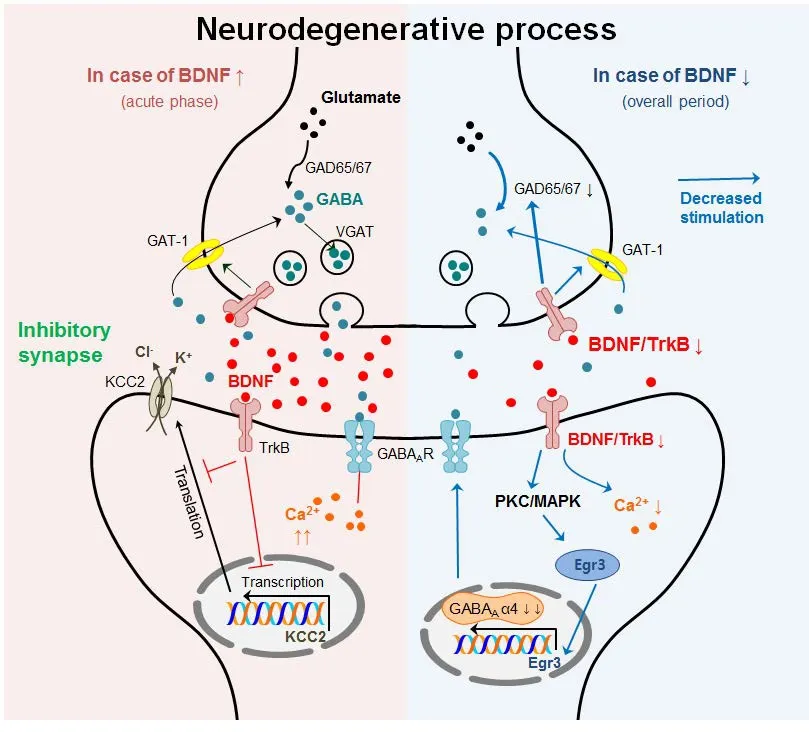
Figure 1 Summarized possible mechanisms between BDNF and GABAergic transmission during neurodegenerative process.
Conclusion
BDNF and GABAergic systems are involved in critical CNS functions, ranging from neuronal development and neuronal survival to learning and memory. In addition, BDNF and GABAergic systems have significant physiological and pathological roles in neurotoxicity and neurodegenerative diseases. In many neurodegenerative diseases, the levels of BDNF are generally decreased, although rapid elevation of BDNF levels occur in the acute phase in response to neuronal insults which may contribute to protective/regenerative processing.e overall reduction of BDNF results in diverse intracellular signalings, leading to an imbalance in excitatory/inhibitory neurotransmission, including dysregulation of GABAergic transmission.erefore, the reduction of BDNF and the subsequent dysregulation of GABAergic transmission may be one of the major mechanisms of neurodegeneration. However, the relationship between BDNF and GABAergic systems remains largely unknown; therefore, further studies investigating the precise interactions between BDNF and GABAergic transmission are needed. Nevertheless,the substantial progress that has been made in the last few decades in elucidating the mechanisms of BDNF and GABAergic systems, may not only further extend our knowledge of normal CNS functions, but may also help to develop potential strategies for pharmacotherapeutic approaches in neurodegeneration.
Acknowledgments:Due to space limitations, we may have omitted some of the relevant literatures in this review.
Author contributions:JK, MY and CM conceived and designed the work, performed the analysis with constructive discussion, and wrote the paper. All authors contributed to literature review and text conception,and approved the final version of this paper.
Conflicts of interest:None declared.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewer:Mitsuhiro Morita, Kobe University, Japan.
Additional file:Open peer review report 1.
Amor S, Puentes F, Baker D, van der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129:154-169.
Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY,Zwilling D, Yan TX, Chen L, Huang Y (2010) Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci 30:13707-13717.
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992)Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42:631-639.
Azoulay D, Vachapova V, Shihman B, Miler A, Karni A (2005) Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. J Neuroimmunol 167:215-218.
Balaban CD, O’Callaghan JP, Billingsley ML (1988) Trimethyltin-induced neuronal damage in the rat brain: comparative studies using silver degeneration stains, immunocytochemistry and immunoassay for neuronotypic and gliotypic proteins. Neuroscience 26:337-361.
Ballarin M, Ernfors P, Lindefors N, Persson H (1991) Hippocampal damage and kainic acid injection induce a rapid increase in mRNA for BDNF and NGF in the rat brain. Exp Neurol 114:35-43.
Barbin G, Pollard H, Gaiarsa JL, Ben-Ari Y (1993) Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci Lett 152:150-154.
Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G,Braestrup C, Bateson AN, Langer SZ (1998) International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50:291-313.
Bathina S, Das UN (2015) Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 11:1164-1178.
Baydyuk M, Xu B (2014) BDNF signaling and survival of striatal neurons. Front Cell Neurosci 8:254.
Behar TN, Li YX, Tran HT, Ma W, Dunlap V, Scott C, Barker JL (1996)GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci 16:1808-1818.
Berg MM, Sternberg DW, Hempstead BL, Chao MV (1991)e low-affinity p75 nerve growth factor (NGF) receptor mediates NGF-induced tyrosine phosphorylation. Proc Natl Acad Sci U S A 88:7106-7110.
Bettler B, Tiao JY (2006) Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacoler 110:533-543.
Bhattacharyya PK, Phillips MD, Stone LA, Bermel RA, Lowe MJ (2013)Sensorimotor cortex gamma-aminobutyric acid concentration correlates with impaired performance in patients with MS. AJNR Am J Neuroradiol 34:1733-1739.
Bito H, Takemoto-Kimura S (2003) Ca(2+)/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium 34:425-430.
Blanco Y, Saiz A, Costa M, Torres-Peraza JF, Carreras E, Alberch J,Jaraquemada D, Graus F (2005) Evolution of brain-derived neurotrophic factor levels aer autologous hematopietic stem cell transplantation in multiple sclerosis. Neurosci Lett 380:122-126.
Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65:135-172.
Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM(2009) Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A 106:13594-13599.
Bogush A, Pedrini S, Pelta-Heller J, Chan T, Yang Q, Mao Z, Sluzas E, Gieringer T, Ehrlich ME (2007) AKT and CDK5/p35 mediate brain-derived neurotrophic factor induction of DARPP-32 in medium size spiny neurons in vitro. J Biol Chem 282:7352-7359.
Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M,Bonner TI, Enna SJ (2002) International Union of Pharmacology.XXXIII. Mammalian gamma-aminobutyric acid(B) receptors: structure and function. Pharmacol Rev 54:247-264.
Bowery NG, Brown DA (1974) Proceedings: On the release of accumulated (3-H)-gamma-aminobutyric acid (GABA) from isolated rat superior cervical ganglia. Br J Pharmacol 52:436P-437P.
Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM (2001)BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci 13:1320-1328.
Calver AR, Davies CH, Pangalos M (2002) GABA(B) receptors: from monogamy to promiscuity. Neurosignals 11:299-314.
Canas N, Pereira IT, Ribeiro JA, Sebastiao AM (2004) Brain-derived neurotrophic factor facilitates glutamate and inhibits GABA release from hippocampal synaptosomes through different mechanisms.Brain Res 1016:72-78.
Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV (1996)Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature 383:716-719.
Casalbore P, Barone I, Felsani A, D’Agnano I, Michetti F, Maira G,Cenciarelli C (2010) Neural stem cells modified to express BDNF antagonize trimethyltin-induced neurotoxicity through PI3K/Akt and MAP kinase pathways. J Cell Physiol 224:710-721.
Cawley N, Solanky BS, Muhlert N, Tur C, Edden RA, Wheeler-Kingshott CA, Miller DH, Thompson AJ, Ciccarelli O (2015) Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain 138:2584-2595.
Chang LW, Dyer RS (1983) Trimethyltin induced pathology in sensory neurons. Neurobehav Toxicol Teratol 5:673-696.
Chittka A, Chao MV (1999) Identification of a zinc finger protein whose subcellular distribution is regulated by serum and nerve growth factor. Proc Natl Acad Sci U S A 96:10705-10710.
Corvino V, Marchese E, Michetti F, Geloso MC (2013) Neuroprotective strategies in hippocampal neurodegeneration induced by the neurotoxicant trimethyltin. Neurochem Res 38:240-253.
Couve A, Moss SJ, Pangalos MN (2000) GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci 16:296-312.
de Jong PJ, Lakke JP, Teelken AW (1984) CSF GABA levels in Parkinson’s disease. Adv Neurol 40:427-430.
Demakova EV, Korobov VP, Lemkina LM (2003) Determination of gamma-aminobutyric acid concentration and activity of glutamate decarboxylase in blood serum of patients with multiple sclerosis.Klin Lab Diagn:15-17.
DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP,Aronin N (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277:1990-1993.
Feng ZJ, Zhang X, Chergui K (2014) Allosteric modulation of NMDA receptors alters neurotransmission in the striatum of a mouse model of Parkinson’s disease. Exp Neurol 255:154-160.
Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B (1996) Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381:706-709.
Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM,Greenberg ME (1997) CREB: a major mediator of neuronal neurotrophin responses. Neuron 19:1031-1047.
Gaiddon C, Loeffler JP, Larmet Y (1996) Brain-derived neurotrophic factor stimulates AP-1 and cyclic AMP-responsive element dependent transcriptional activity in central nervous system neurons. J Neurochem 66:2279-2286.
Ganguly K, Schinder AF, Wong ST, Poo M (2001) GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell 105:521-532.
Geloso MC, Corvino V, Michetti F (2011) Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes. Neurochem Int 58:729-738.
Glass M, Dragunow M, Faull RL (2000)e pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience 97:505-519.
Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT (1997) Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol 41:17-24.
Group THsDCR (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. . Cell 72:971-983.
Guillozet AL, Weintraub S, Mash DC, Mesulam MM (2003) Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 60:729-736.
Hamanoue M, Middleton G, Wyatt S, Jaffray E, Hay RT, Davies AM(1999) p75-mediated NF-kappaB activation enhances the survival response of developing sensory neurons to nerve growth factor. Mol Cell Neurosci 14:28-40.
Heng MY, Detloff PJ, Albin RL (2008) Rodent genetic models of Huntington disease. Neurobiol Dis 32:1-9.
Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M (2000)Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer’s disease. Brain Res Mol Brain Res 76:347-354.
Howells DW, Porritt MJ, Wong JY, Batchelor PE, Kalnins R, Hughes AJ, Donnan GA (2000) Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp Neurol 166:127-135.
Ishida N, Akaike M, Tsutsumi S, Kanai H, Masui A, Sadamatsu M,Kuroda Y, Watanabe Y, McEwen BS, Kato N (1997) Trimethyltin syndrome as a hippocampal degeneration model: temporal changes and neurochemical features of seizure susceptibility and learning impairment. Neuroscience 81:1183-1191.
Ishikawa K, Kubo T, Shibanoki S, Matsumoto A, Hata H, Asai S (1997)Hippocampal degeneration inducing impairment of learning in rats:model of dementia? Behav Brain Res 83:39-44.
Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu CC,Kopke RD, Wu JY (2003a) Demonstration of functional coupling between gamma -aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci U S A 100:4293-4298.
Jin X, Hu H, Mathers PH, Agmon A (2003b) Brain-derived neurotrophic factor mediates activity-dependent dendritic growth in nonpyramidal neocortical interneurons in developing organotypic cultures. J Neurosci 23:5662-5673.
Johnson ME, Lim Y, Senthilkumaran M, Zhou XF, Bobrovskaya L(2015) Investigation of tyrosine hydroxylase and BDNF in a lowdose rotenone model of Parkinson’s disease. J Chem Neuroanat 70:33-41.
Kaur B, Prakash A (2017) Ceriaxone attenuates glutamate-mediated neuro-inflammation and restores BDNF in MPTP model of Parkinson’s disease in rats. Pathophysiology 24:71-79.
Kim J, Son Y, Kim J, Lee S, Kang S, Park K, Kim SH, Kim JC, Kim J,Takayama C, Im HI, Yang M, Shin T, Moon C (2015) Developmental and degenerative modulation of GABAergic transmission in the mouse hippocampus. Int J Dev Neurosci 47:320-332.
Kim J, Yang M, Kim J, Song L, Lee S, Son Y, Kang S, Bae CS, Kim JC, Kim SH, Shin T, Wang H, Moon C (2014) Developmental and degenerative modulation of brain-derived neurotrophic factor transcript variants in the mouse hippocampus. Int J Dev Neurosci 38:68-73.
Kittler JT, McAinsh K, Moss SJ (2002) Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol Neurobiol 26:251-268.
Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL,Barbacid M (1993) Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death.Cell 75:113-122.
Kong S, Cheng Z, Liu J, Wang Y (2014) Downregulated GABA and BDNF-TrkB pathway in chronic cyclothiazide seizure model. Neural Plast 2014:310146.
Korte M, Carroll P, Wolf E, Brem G,oenen H, Bonhoeffer T (1995)Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 92:8856-8860.
Kremer B, Goldberg P, Andrew SE,eilmann J, Telenius H, Zeisler J,Squitieri F, Lin B, Bassett A, Almqvist E, et al. (1994) A worldwide study of the Huntington’s disease mutation. The sensitivity and specificity of measuring CAG repeats. N Engl J Med 330:1401-1406.
Langley M, Ghosh A, Charli A, Sarkar S, Ay M, Luo J, Zielonka J,Brenza T, Bennett B, Jin H, Ghaisas S, Schlichtmann B, Kim D,Anantharam V, Kanthasamy A, Narasimhan B, Kalyanaraman B,Kanthasamy AG (2017) Mito-apocynin prevents mitochondrial dysfunction, microglial activation, oxidative damage, and progressive neurodegeneration in mitopark transgenic mice. Antioxid Redox Signal doi: 10.1089/ars.2016.6905.
Lassmann H, Raine CS, Antel J, Prineas JW (1998) Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J Neuroimmunol 86:213-217.
Lee S, Kang S, Kim J, Yoon S, Kim SH, Moon C (2016) Enhanced expression of immediate-early genes in mouse hippocampus after trimethyltin treatment. Acta Histochem 118:679-684.
Levine ES, Dreyfus CF, Black IB, Plummer MR (1995) Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci U S A 92:8074-8077.
Liang X, Wu J, Egorova P, Bezprozvanny I (2014) An automated and quantitative method to evaluate progression of striatal pathology in Huntington’s disease transgenic mice. J Huntingtons Dis 3:343-350.
Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M,McAuliffe WG, Dawson VL, Dawson TM, Przedborski S (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5:1403-1409.
Lu B (2003) BDNF and activity-dependent synaptic modulation. Learn Mem 10:86-98.
Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR (2008) BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal 1:ra9.
Maggirwar SB, Sarmiere PD, Dewhurst S, Freeman RS (1998) Nerve growth factor-dependent activation of NF-kappaB contributes to survival of sympathetic neurons. J Neurosci 18:10356-10365.
Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ,Furth ME, Lindsay RM, Yancopoulos GD (1990) NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron 5:501-509.
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1:120-129.
Mattson MP, Duan W (1999) “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res 58:152-166.
Meredith GE, Rademacher DJ (2011) MPTP mouse models of Parkinson’s disease: an update. J Parkinsons Dis 1:19-33.
Michalski B, Fahnestock M (2003) Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer’s disease. Brain Res Mol Brain Res 111:148-154.
Minelli A, Brecha NC, Karschin C, DeBiasi S, Conti F (1995) GAT-1, a high-affinity GABA plasma membrane transporter, is localized to neurons and astroglia in the cerebral cortex. J Neurosci 15:7734-7746.
Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F (1996) GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci 16:6255-6264.
Mitew S, Kirkcaldie MT, Dickson TC, Vickers JC (2013) Altered synapses and gliotransmission in Alzheimer’s disease and AD model mice. Neurobiol Aging 34:2341-2351.
Mizuno K, Carnahan J, Nawa H (1994) Brain-derived neurotrophic factor promotes differentiation of striatal GABAergic neurons. Dev Biol 165:243-256.
Monteleone P, Maj M, Iovino M, Steardo L (1990) GABA, depression and the mechanism of action of antidepressant drugs: a neuroendocrine approach. J Affect Disord 20:1-5.
Nagahara AH, Tuszynski MH (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10:209-219.
Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM (1994) Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol 14:759-767.
Nakao N, Brundin P, Funa K, Lindvall O, Odin P (1995) Trophic and protective actions of brain-derived neurotrophic factor on striatal DARPP-32-containing neurons in vitro. Brain Res Dev Brain Res 90:92-101.
Nelson MT, Blaustein MP (1982) GABA efflux from synaptosomes:effects of membrane potential, and external GABA and cations. J Membr Biol 69:213-223.
Nicholson LF, Faull RL, Waldvogel HJ, Dragunow M (1995) GABA and GABAA receptor changes in the substantia nigra of the rat following quinolinic acid lesions in the striatum closely resemble Huntington’s disease. Neuroscience 66:507-521.
Olsen RW, Avoli M (1997) GABA and epileptogenesis. Epilepsia 38:399-407.
Oyelami T, Bondt A, den Wyngaert IV, Hoorde KV, Hoskens L,Shaban H, Kemp JA, Drinkenburg WH (2016) Age-dependent concomitant changes in synaptic dysfunction and GABAergic pathway in the APP/PS1 mouse model. Acta Neurobiol Exp (Wars) 76:282-293.
Pallotto M, Deprez F (2014) Regulation of adult neurogenesis by GABAergic transmission: signaling beyond GABAA-receptors. Front Cell Neurosci 8:166.
Palop JJ, Chin J, Roberson ED, Wang J,win MT, Bien-Ly N, Yoo J,Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L (2007)Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55:697-711.
Pena F, Tapia R (2000) Seizures and neurodegeneration induced by 4-aminopyridine in rat hippocampus in vivo: role of glutamate- and GABA-mediated neurotransmission and of ion channels. Neuroscience 101:547-561.
Peng S, Wuu J, Mufson EJ, Fahnestock M (2005) Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem 93:1412-1421.
Perez-Navarro E, Alberch J, Neveu I, Arenas E (1999) Brain-derived neurotrophic factor, neurotrophin-3 and neurotrophin-4/5 differentially regulate the phenotype and prevent degenerative changes in striatal projection neurons aer excitotoxicity in vivo. Neuroscience 91:1257-1264.
Perez-Navarro E, Canudas AM, Akerund P, Alberch J, Arenas E (2000)Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4/5 prevent the death of striatal projection neurons in a rodent model of Huntington’s disease. J Neurochem 75:2190-2199.
Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA,Winslow JW (1991) BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 7:695-702.
Razgado-Hernandez LF, Espadas-Alvarez AJ, Reyna-Velazquez P, Sierra-Sanchez A, Anaya-Martinez V, Jimenez-Estrada I, Bannon MJ,Martinez-Fong D, Aceves-Ruiz J (2015)e transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PLoS One 10:e0117391.
Rekik L, Daguin-Nerriere V, Petit JY, Brachet P (2011) gamma-Aminobutyric acid type B receptor changes in the rat striatum and substantia nigra following intrastriatal quinolinic acid lesions. J Neurosci Res 89:524-535.
Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M(2002) BDNF-induced TrkB activation down-regulates the K+-Clcotransporter KCC2 and impairs neuronal Cl- extrusion. J Cell Biol 159:747-752.
Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ (2006)Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J Biol Chem 281:29431-29435.
Roberts DS, Raol YH, Bandyopadhyay S, Lund IV, Budreck EC, Passini MA, Wolfe JH, Brooks-Kayal AR, Russek SJ (2005) Egr3 stimulation of GABRA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA(A) receptor alpha4 subunit expression. Proc Natl Acad Sci U S A 102:11894-11899.
Rossor M, Iversen LL (1986) Non-cholinergic neurotransmitter abnormalities in Alzheimer’s disease. Br Med Bull 42:70-74.
Rudolph U, Crestani F, Mohler H (2001) GABA(A) receptor subtypes:dissecting their pharmacological functions. Trends Pharmacol Sci 22:188-194.
Sathanoori M, Dias BG, Nair AR, Banerjee SB, Tole S, Vaidya VA (2004)Differential regulation of multiple brain-derived neurotrophic factor transcripts in the postnatal and adult rat hippocampus during development, and in response to kainate administration. Brain Res Mol Brain Res 130:170-177.
Sieghart W, Sperk G (2002) Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem 2:795-816.
Taketo M, Yoshioka T (2000) Developmental change of GABA(A) receptor-mediated current in rat hippocampus. Neuroscience 96:507-514.
Tanaka T, Saito H, Matsuki N (1997) Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci 17:2959-2966.
Tao W, Chen Q, Wang L, Zhou W, Wang Y, Zhang Z (2015) Brainstem brain-derived neurotrophic factor signaling is required for histone deacetylase inhibitor-induced pain relief. Mol Pharmacol 87:1035-1041.
Vacaras V, Major ZZ, Buzoianu AD (2017) Brain-derived neurotrophic factor levels under chronic natalizumab treatment in multiple sclerosis. A preliminary report. Neurol Neurochir Pol 51:221-226.
Vaz SH, Jorgensen TN, Cristovao-Ferreira S, Duflot S, Ribeiro JA,Gether U, Sebastiao AM (2011) Brain-derived neurotrophic factor(BDNF) enhances GABA transport by modulating the trafficking of GABA transporter-1 (GAT-1) from the plasma membrane of rat cortical astrocytes. J Biol Chem 286:40464-40476.
Ventimiglia R, Mather PE, Jones BE, Lindsay RM (1995)e neurotrophins BDNF, NT-3 and NT-4/5 promote survival and morphological and biochemical differentiation of striatal neurons in vitro. Eur J Neurosci 7:213-222.
Villette V, Dutar P (2017) GABAergic microcircuits in Alzheimer’s disease models. Curr Alzheimer Res 14:30-39.
Wang B, Wang Z, Sun L, Yang L, Li H, Cole AL, Rodriguez-Rivera J,Lu HC, Zheng H (2014) The amyloid precursor protein controls adult hippocampal neurogenesis through GABAergic interneurons.J Neurosci 34:13314-13325.
Wang Q, Yu S, Simonyi A, Sun GY, Sun AY (2005) Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol 31:3-16.
Wardle RA, Poo MM (2003) Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci 23:8722-8732.
Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H (2002)GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol 213:1-47.
Wu CW, Chen YC, Yu L, Chen HI, Jen CJ, Huang AM, Tsai HJ, Chang YT, Kuo YM (2007a) Treadmill exercise counteracts the suppressive effects of peripheral lipopolysaccharide on hippocampal neurogenesis and learning and memory. J Neurochem 103:2471-2481.
Wu H, Jin Y, Buddhala C, Osterhaus G, Cohen E, Jin H, Wei J, Davis K, Obata K, Wu JY (2007b) Role of glutamate decarboxylase (GAD)isoform, GAD65, in GABA synthesis and transport into synaptic vesicles-Evidence from GAD65-knockout mice studies. Brain Res 1154:80-83.
Yamada M, Mizuguchi M, Rhee SG, Kim SU (1991) Developmental changes of three phosphoinositide-specific phospholipase C isozymes in the rat nervous system. Brain Res Dev Brain Res 59:7-16.
Young AB, Greenamyre JT, Hollingsworth Z, Albin R, D’Amato C,Shoulson I, Penney JB (1988) NMDA receptor losses in putamen from patients with Huntington’s disease. Science 241:981-983.
Zafra F, Castren E,oenen H, Lindholm D (1991) Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci U S A 88:10037-10041.
Zheng XY, Zhang HL, Luo Q, Zhu J (2011) Kainic acid-induced neurodegenerative model: potentials and limitations. J Biomed Biotechnol 2011:457079.
Zirrgiebel U, Ohga Y, Carter B, Berninger B, Inagaki N,oenen H,Lindholm D (1995) Characterization of TrkB receptor-mediated signaling pathways in rat cerebellar granule neurons: involvement of protein kinase C in neuronal survival. J Neurochem 65:2241-2250.
Zuccato C, Cattaneo E (2007) Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol 81:294-330.
Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L,MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E (2001) Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 293:493-498.
Zuccato C, Liber D, Ramos C, Tarditi A, Rigamonti D, Tartari M,Valenza M, Cattaneo E (2005) Progressive loss of BDNF in a mouse model of Huntington’s disease and rescue by BDNF delivery. Pharmacol Res 52:133-139.
*Correspondence to:
Changjong Moon, D.V.M., M.S.,Ph.D. or Miyoung Yang, D.V.M.,M.S., Ph.D.,moonc@chonnam.ac.kr or yangm@wku.ac.kr.
orcid:
0000-0003-2451-0374
(Changjong Moon)
0000-0002-4748-6007
(Miyoung Yang)
10.4103/1673-5374.217353
Accepted: 2017-09-19
Edited by Li CH, Song LP, Zhao M
- 中国神经再生研究(英文版)的其它文章
- Matrix bound vesicles and miRNA cargoes are bioactive factors within extracellular matrix bioscaffolds
- Diffusion tensor tractography studies on mechanisms of recovery of injured fornix
- Using 3D bioprinting to produce mini-brain
- Beta secretase activity in peripheral nerve regeneration
- Embracing oligodendrocyte diversity in the context of perinatal injury
- On the road towards the global analysis of human synapses