Design of Benzobisthiadiazole Analogues as Promising Anchoring Groups for High Efficient Dye-Sensitized Solar Cells
LI Zhong-Gao LU Tian GAO Heng ZHANG Qing LI Min-Jie,* REN Wei,* LU Wen-Cong,*
Design of Benzobisthiadiazole Analogues as Promising Anchoring Groups for High Efficient Dye-Sensitized Solar Cells
LI Zhong-Gao1LU Tian1GAO Heng2ZHANG Qing1LI Min-Jie1,*REN Wei2,*LU Wen-Cong1,*
(1;2)
This work describes the characteristics of benzobisthiadiazole analogues with different heteroatom substitution patterns as electronwithdrawing anchoring groups in dye-sensitized solar cells (DSSCs). In order to provide a systematic analysis of the effect of the designed anchoring groups, the widely used anchor cyanoacrylic acid was used as the reference. Theoretical calculations show that the newly designed anchors are capable of displaying a decent level of light absorption covering the entire visible range up to the near-IR region of 1000 nm. More importantly, an ultrafast electron injection is observed from the dyes SPN and SPS into the TiO2surface. The quantum dynamics of the interfacial electron transfer (IET) reveal that SPN and SPS anchors provide efficient IET performance. About 90% of the electron injection occurs in the first 15 fs, and is complete after ~100 fs. Furthermore, the pathway of electron injection is direct, leading to very efficient transfer of the wavepacket through the TiO2semiconductor. Therefore, the performances of both the anchors, SPN and SPS, are equivalent and even superior to that of cyanoacrylic acid. These findings are important in the context of providing guidelines for the design of metal-free organic dye sensitizers for high efficient DSSCs.
Benzobisthiadiazole; Anchoring group; Absorption spectra; IET; DSSCs
1 Introduction
Dye-sensitized solar cells (DSSCs) are potential alternative to silicon-based photovoltaics for solar energy conversion, providing low cost and relatively high energy conversion efficiency1–6. In such solar cells, the dyes and the mesoporous TiO2layer represent the key components to achieve good performance7. For many years, the ruthenium-bipyridyl and zinc porphyrin dyes have dominated the highly efficient solar cells8–11. However, these dyes are not ideal due to the lack of abundant nature and the environmental complications, making it necessary to search for the better alternative dyes12–16. The peculiar push-pull metal-free organic dye, composed of an electron-donating group, a linker group, and an electron accepting/anchoring group, has recently attracted much attention thanks to many advantages such as easy fabrication, lower costs, environment friendly, good electron donating/accepting nature and the possibility of extending absorption spectra into the visible range17–26.
As the most important property of DSSCs, incident photon to current conversion efficiency (IPCE) is determined by the electron injection efficiency (inj), the light harvesting efficiency (LHE), and charge collection efficiency (c)27,28. To improve the performance of DSSCs, it is necessary to investigate the electron injection process which is the initial step in charge transport29,30. Naturally, the electron accepting/anchoring groups binding DSSCs sensitizers to metal oxide surfaces are critical to electron injection. Therefore, finding an electron acceptor which is in favour of extending absorption bands and providing rapid electron injection is significant. And much attention has been directed experimentally and theoretically to research different anchoring groups31–33. Among the electron anchoring moieties, cyanoacrylic acid was widely employed for stable grafting of the dye onto the semiconductor surface and a rapid electron injection as observed experimentally by the interfacial electron transfer (IET) for cyanoacrylic acid anchor being completed in the subpicosecond time scale32.
Recently, benzobisthiadiazole analogues with the planar triple-fused-ring have attracted the interest of researchers due to strong electron-accepting feature and excellent intermolecular−interactions. As auxiliary acceptor of dyes they offer some peculiar functionalities such as band gap tuning and photoresponse in the near infrared (NIR) region from our previous work34. They have been also applied in thin film transistors and optoelectronic devices35,36.
In this paper, we present the characteristics of benzobisthiadiazole analoguesdifferent heteroatom substitution as electron anchoring groups in DSSCs for the first time. The electronic structures, HOMO and LUMO levels and absorption spectra of free dyes have been calculated. In addition, the geometrical configuration of dyes on the anatase TiO2(101) surface have been estimated. Meanwhile, as the most important factor of dyes, electron injection has also been evaluated by the quantum dynamics simulations which were successfully applied to investigate the photoinduced IET process in different dye- semiconductor systems37,38. In order to provide a systematic analysis of the designed anchoring groups, we employed the experimentally synthesized dye WS-939using cyanoacrylic acid as the anchor group for the reference. The models of all investigated dyes are shown in Scheme 1.
2 Computational details
The structures of our new free sensitizers were optimized using B3LYP/6-31G() within density functional theory (DFT) as implemented in the Gaussian 09 package40. Local minima were identified by frequency analysis at the same theoretical level as geometry optimization. The UV/Vis absorption spectra were simulated with time-dependent-DFT (TD-DFT), by using the long-range-corrected CAM-B3LYP hybrid density functional41, in conjunction with the 6-31G() basis set. The solvent environment was taken into account by means of conductor-like polarized continuum model (C-PCM) method42(dichloromethane,= 8.93). The reliability of the method for the prediction of absorption spectra has been demonstrated in previous literature43. For assessing the electron injection from dyes into the TiO2semiconductor surface, the (101) surface of anatase TiO2, subjected to periodic boundary conditions, was modelled by TiO2slab comprised of 3 × 3 supercells. A distance of ∼0.77 nm between the top layer and the bottom layer was selected. We also employed the plane-wave DFT method as implemented in the Viennasimulation package (VASP), using the generalized gradient approximation (GGA) exchange-correlation functional and the Perdew-Burke- Ernzerhof (PBE) pseudopotential to optimize the ground state geometry of the TiO2structure as well as the combined dye/TiO2system44. The cutoff energy of TiO2slab relaxation is 500 eV. The first 300 fs electron transfer was simulated by quantum dynamics using IET code developed by Batista and co-workers37,38, based on the optimized ground state structure, and 0.5 ps trajectories were computed with a time step of 1 fs.
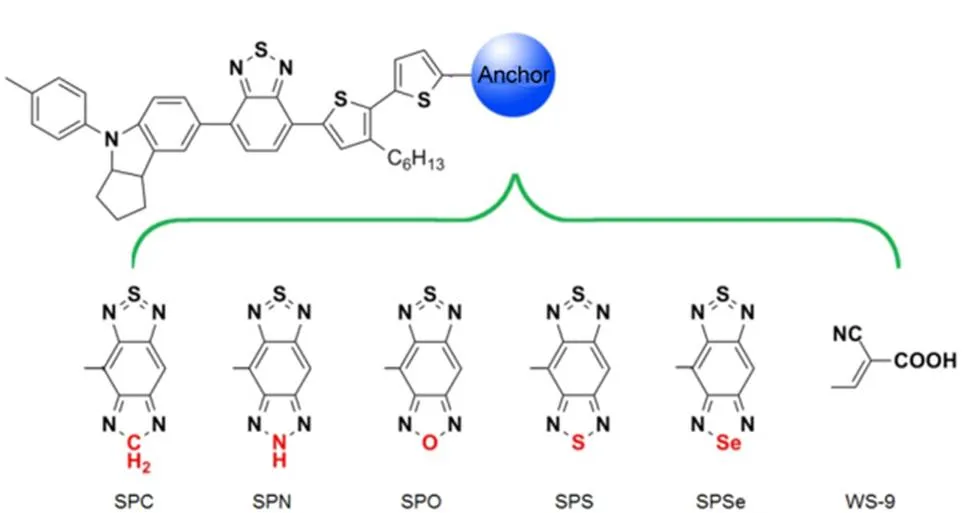
Scheme 1 Chemical structures of five benzobisthiadiazole analogue-based complexes in this study.
3 Results and discussion
3.1 Electronic structures
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies and the band gap between HOMO and LUMO energy for the selected sensitizers are shown in Fig.1. All of the LUMO energy levels of the dye are high enough to enable electron injection into the TiO2conduction band (CB) edge (−4.0 eV), meanwhile their HOMO energy levels are low enough to be regenerated by I−/I3−electrolyte (−4.8 eV). The HOMO and LUMO energy levels of SPS are −5.91 and −2.70 eV, respectively. Replacement of the sulfur atom (S) with the selenium atom (Se) leads to a certain degree ofHOMOelevation andLUMOdecrease due to the selenium atom exhibiting lower electronegativity than sulfur atom, and theHOMOandLUMOof the dye SPSe are −5.88 and −2.79 eV, respectively. The same trend is also observed for SPC and SPN, and the dye SPC displays the narrower band gap. SPO with oxygen (O) atom also possesses a narrow band gap due to the smaller dihedral angle, leading to the effective conjugation length. The dihedral angles of all the dyes were presented in Table S1 of Supporting Information. It is also testified that the narrower band gaps of the investigated dyes are directly correlated with the lower LUMO energy of the benzobisthiadiazole analogue acceptor due to the geometric perturbation and the biradicaloid nature reported by Li34and Thomas45. The heteroatom substitution will enable us to tune energy levels which agree well with Thomas and Bhanuprakash45. The molecules with oxygen atom can behave differently than other dyes, in good agreement with the conclusion in the studies of Johari.36Compared with WS-9, all the designed dyes possess smaller band gaps between HOMO and LUMO energies, which suggests that the new dyes will exhibit significantly red-shifted absorption spectra. From the frontier molecular orbitals (Fig.1), the LUMOs are mainly composed of-chain and acceptor which is beneficial to the efficient electron injection into the conduction band of TiO2spatially.
3.2 Absorption spectra
TD-DFT calculations were applied to investigate the0→Svertical ground-to-excited state transitions and the pertinent photophysical parameters are summarized in Table S2. The excitation from the ground state to the first excited state was caused by HOMO → LUMO transition as observed in WS-9, the designed anchoring groups have substantial influence on the main transitions for intramolecular charge transfer excitation. From Fig.2, the absorption spectra display three main absorption bands except SPN, and the dye with cyanoacrylic acid only exhibiting two absorption bands. It is showed that the designed anchoring groups play a significant role in extending the absorption bands. The longer tail of SPC peak is the most red-shifted absorption band among all the dyes due to the less electronegativity of carbon atom, which agrees well with its narrowest HOMO-LUMO gap. The moderate absorption spectra are even extended to 1045 nm, which covers a broad range in the visible/near-infrared (Vis/NIR) region. The dyes SPN, SPO, SPS and SPSe, with the absorption bands centered at 628, 805, 764 and 842 nm respectively all have obvious red-shift compared with WS-9 (529 nm)34having cyanoacrylic acid as the electron anchoring group.
3.3 Dye adsorption on a (101) TiO2 surface
After the spectroscopic characterization of all the studied dyes described above, in order to simulate the realistic performance of the DSSCs, we focus on the interaction between the sensitizing dye and the semiconductor which is related to the primary charge generation event in DSSCs. A suitable anchoring group should strongly bind to the semiconducting oxide surface and coincide with the dye acceptor moiety. That will promote electronic coupling between the donor levels of the excited dye and the delocalized acceptor levels of the semiconductor conduction band for the charge injection process29,46,47.

Fig.1 Energy levels and frontier orbital distributions of the dyes in CH2Cl2.
There are some possible binding modes for carboxylate group on TiO2, such as unidentate, bidentate chelating, bidentate bridging, etc. Previous investigations have proved that the bidentate bridging mode is one of the stable modes for cyanoacrylic acid anchor on no-hydrated TiO2surfaces, and generally leading to faster electron injection as verified in previous investigations47−49. The reference dye, WS-9, which adopted cyanoacrylic acid as anchor group, absorbed on TiO2surfaces through bidentate bringing model32. In order to make the comparison of different anchors more direct and consistent, the bidentate bridging mode is adopted in this study and the optimized adsorption geometries are illustrated in Fig.3. From Table S3, the interaction between the molecules and the TiO2surface is rather strong, with average calculated distances between the nitrogen and surface titanium ranging from 0.202 to 0.21 nm, which are similar as those seen between the oxygen and titanium in WS-9/TiO2system. The calculated adsorption energies were ranged from −4.11 to −5.43 eV, which indicated that benzobisthiadiazole analogues may bind strongly to the TiO2surface as the cyanoacrylic acid.

Fig.2 UV-Vis absorption spectra of the dyes in CH2Cl2 simulated by TD-DFT calculations.
color online.

Fig.3 Optimized adsorption geometries of the investigated dyes.

Fig.4 Survival probability curves for electron injection of the dye/TiO2 systems.
color online.

Fig.5 Snapshots of electron density calculated at 0, 2, 4, 8, 10 and 100 fs for dye/TiO2.
3.4 IET simulations
Finally, it is important to investigate how the different anchoring groups affect the electron transfer dynamics. The quantum dynamics simulations were carried out and the results are summarized in Fig.4.() is the survival probability of the photoexcited electron that is still in the adsorbate molecule at time t after the photoexcitation of the system. For the SPN/TiO2and SPS/TiO2systems, about 90% of the injection occurs in the first 15 fs, and the electron is completely injected after~ 100 fs. For the SPSe/TiO2system, the overall injection rate is slower and we see about 85% of the injection into the TiO2surface between 0 and 300 fs. For the SPC/TiO2and SPO/TiO2system, the IET time is significantly longer, and the injection efficiency is poor. For WS-9/TiO2system, almost 90% of the injection occurs in the first 60 fs, and the relaxation dynamics finishes after~ 100 fs. From the point of view of electron injection efficiency, the benzobisthiadiazole analogue anchors with nitrogen atom and with sulfur atom are practically equivalent and even superior to the widely used cyanoacrylic acid anchor. The transfer of most of the electronic charge from the adsorbate LUMO into the TiO2conduction band happens within the 100 fs time scale.
To investigate the differences in the relative rates of injection, we further display the plots of electronic isosurfaces of all the dye/TiO2systems taken between 0 and 100 fs in Fig.5. It clearly suggests that, for SPN and SPS systems, the pathway of electron injection is direct at 2 and 10 fs, leading to very efficient transfer of the wavepacket through the TiO2semiconductor, which is similar with the representative snapshots of WS-9 system. This is in agreement with the survival probability trend in Fig.4. However, for SPC, SPO and SPSe system, the injection is initially rapid between 0−10 fs but the electron density is located on the first two layers of the cluster between 10 and 100 fs. The phenomenon is consistent with the injection curve in Fig.4 and the concentration of the electron density wanders between the dye and semiconductor, which may contribute significantly to the lower overall injection efficiency. The snapshots of the electron density distribution show that the heteroatom substitutions with sulfur atom and with nitrogen atom will lead to higher injection efficiency as very promising anchoring groups.
4 Conclusions
In summary, we systematically presented a comparative study between the newly designed benzobisthiadiazole analogue anchors and the widely used cyanoacrylic acid anchor in dye-sensitized solar cells. The designed SPN and SPS anchors are expected to become promising anchoring groups due to the following advantages: UV-Vis spectra of the SPN and SPS dyes display a decent level of light absorption and have an obvious red-shift compared to the dye with cyanoacrylic acid anchoring group, thus leading to a good photocurrent response. In addition, the adsorbability of new anchors on the anatase (101) surface may be strong as the widely-used cyanoacrylic acid, rendering long-term durability to the device. More importantly, inspection of the IET process reveals that SPN and SPS anchors provided efficient IET characteristics, and they guide the electronic flux perpendicularly into the semiconductor surface. About 90% of the injection occurs in the first 15 fs, the electrons can be completely injected after~100 fs. The performance is practically equivalent and even superior to the cyanoacrylic acid. This work is expected to provide some insight for new design of more efficient anchoring groups for DSSCs application.
Supporting Information: available free of chargethe internet at http://www.whxb.pku.edu.cn.
(1) O'Regan, B.; Grätzel, M.1991,, 737.doi: 10.1038/353737a0
(2) Grätzel, M.2001,, 338. doi: 10.1038/35104607
(3) Yuan, W.; Zhao, H.; Hu, H.; Wang, S.; Baker, G. L.2013,, 4155. doi: 10.1021/am4001858
(4) Bella, F.; Sacco, A.; Massaglia, G.; Chiodoni, A.; Pirri, C. F.2015,, 12010. doi: 10.1039/C5NR02286J
(5) Joly, D.; Pelleja, L.; Narbey, S.; Oswald, F.; Meyer, T.; Kervella, Y.; Maldivi, P.; Clifford, J.; Palomares, E.; Demadrille, R.2015,, 2010. doi: 10.1039/C5EE00444F
(6) Cole, J. M.; Low, K. S.; Ozoe, H.; Stathi, P.; Kitamura, C.; Kurata, H.; Rudolf, P.; Kawase, T.2014,, 26684. doi: 10.1039/C4CP02645D
(7) Monti, S.; Pastore, M.; Li, C.; De Angelis, F.; Carravetta, V.2016,, 2787. doi: 10.1021/acs.jpcc.5b11332
(8) Yella, A.; Lee, H. W.; Tsao, H. N.; Yi, C.; Chandiran, A. K.2011,, 629. doi: 10.1126/science.1209688
(9) Santhanamoorthi, N.; Lo, C. M.; Jiang, J. C.2013,, 524. doi: 10.1021/jz302101j
(10) Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B. F.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M. K.; Grätzel, M.2014,, 242.doi: 10.1038/nchem.1861
(11) Urbani, M.; Grätzel, M.; Nazeeruddin, M. K.; Torres, T.2014,, 12330. doi: 10.1021/cr5001964
(12) Hagberg, D. P.; Yum, J.-H.; Lee, H.; De Angelis, F.; Marinado, T.; Karlsson, K. M.; Humphry-Baker, R.; Sun, L.; Hagfeldt, A.; Grätzel, M.2008,, 6259. doi: 10.1021/ja800066y
(13) Ni, J. S.; Yen, Y. C.; Lin, J. T.2015,, 17080. doi: 10.1039/C5CC07105D
(14) Li, H. X.; Zuo, G. F.; Li, Z. F.; Wang, X. F.; Zheng, R. H.2015(5),866. [李会学, 左国防, 李志锋, 王晓峰, 郑仁慧. 物理化学学报, 2015,(5), 886.] doi: 10.3866/PKU.WHXB201503254
(15) Hou, L. M.; Wen, Z.; Li, Y. X.; Hu, H. Y.; Kan, Y. H.; Su, Z. M.2015,, 1504. [侯丽梅, 温 智, 李银祥, 胡华友, 阚玉和, 苏忠民. 物理化学学报,2015,, 1504.]doi: 10.3866/PKU.WHXB201505211
(16) Weng, X. L.; Wang, Y.; Jia, C. Y.; Wan, Z. Q.; Chen, X. M.; Yao, X. J.2016,, 1990. [翁小龙, 王 艳, 贾春阳, 万中全, 陈喜明, 姚小军. 物理化学学报, 2016,, 1990.]doi: 10.3866/PKU.WHXB201605031
(17) Adamo, C.; Le Bahers, T.; Savarese, M.; Wilbraham, L.; García, G.; Fukuda, R.; Ehara, M.; Rega, N.; Ciofini, I.2015,, 166. doi: 10.1016/j.ccr.2015.03.027
(18) Calogero, G.; Bartolotta, A.; Di Marco, G.; Di Carlo, A.; Bonaccorso, F.2015,, 3244.doi: 10.1039/C4CS00309H
(19) Wang, C. L.; Zhang, M.; Hsiao, Y. H.; Tseng, C. K.; Liu, C. L.; Xu, M.; Wang, P.; Lin, C. Y.2016,, 200.doi: 10.1039/C5EE02505B
(20) Wu, J.; Li, G.; Zhang, L.; Zhou, G.; Wang, Z. S.2016,, 3342. doi: 10.1039/C5TA09763K
(21) Biswas, A. K.; Das, A.; Ganguly, B.2015,, 31093. doi: 10.1039/C5CP05144D
(22) Muenmart, D.; Prachumrak, N.; Tarsang, R.; Namungruk, S.; Jungsuttiwong, S.; Sudyoadsuk, T.; Pattanasattayavong, P.; Promarak, V.2016,, 38481. doi: 10.1039/C6RA06220B
(23) Panneerselvam, M.; Kathiravan, A.; Solomon, R. V.; Jaccob, M.2017,, 6153.doi: 10.1039/C6CP07768D
(24) Sivanadanam, J.; Ganesan, P.; Gao, P.; Nazeeruddin, M. K.; Emeline, A.; Bahnemann, D.; Rajalingam, R.2016,, 37347. doi: 10.1039/C6RA01185C
(25) Kathiravan, A.; Srinivasan, V.; Khamrang, T.; Velusamy, M.; Jaccob, M.; Pavithra, N.; Anandan, S.; Velappan, K.2017,, 3125. doi: 10.1039/C6CP08180K
(26) Hao, X. L.; Zhao, J. G.; Gao, J. R.; Han, L.2015,, 1977.[郝学良, 赵金鸽, 高建荣, 韩 亮. 物理化学学报, 2015,, 1977.]doi: 10.3866/PKU.WHXB201509075
(27) Liang, M.; Chen, J.2013,, 3453. doi: 10.1039/C3CS35372A
(28) Rahman, M. M.; Ko, M. J.; Lee, J. J.2015,, 3526. doi: 10.1039/C4NR06645F
(29) Pastore, M.; De Angelis, F.2013,, 956. doi: 10.1021/jz302147v
(30) Yang, L.; Li, Y.; Chen, S.; Zhang, J.; Zhang, M.; Wang, P.2016,, 329. [杨 林, 李 阳, 陈 淑, 张 静, 张 敏, 王 鹏. 物理化学学报, 2016,, 329.]doi: 10.3866/PKU.WHXB201511031
(31) Mai, C. L.; Moehl, T.; Hsieh, C. H.; Décoppet, J. D.; Zakeeruddin, S. M.; Grätzel, M.; Yeh, C. Y.2015,, 14975. doi: 10.1021/acsami.5b03783
(32) Li W.; Rego,L. G. C.; Bai,F. Q.; Wang, J.; Jia, R.; Xie, L. M.; Zhang, H. X.2014,, 3992.doi: 10.1021/jz501973d
(33) Ooyama, Y.; Furue, K.; Enoki, T.; Kanda, M.; Adachi, Y.; Ohshita, J.2016,, 30662.doi: 10.1039/C6CP06513A
(34) Li, M. J.; Kou, L.; Diao, L.; Zhang, Q.; Li, Z. G.; Wu, Q.; Lu, W. C.; Pan, D. Y.; Wei, Z.2015,, 9782.doi: 10.1021/acs.jpcc.5b03667
(35) Huang, S.; Kannadorai, R. K.; Chen, Y.; Liu, Q.; Wang, M.2015,, 4223.doi: 10.1039/C4CC09399B
(36) Johari, P.; Singh, S. P.2015,, 14890.doi: 10.1021/acs.jpcc.5b02404
(37) Abuabara, S. G.; Rego, L. G. C.; Batista, V. S.2005,, 18234.doi: 10.1021/ja055185u
(38) Li, C.; Koenigsmann, C.; Ding, W.; Rudshteyn, B.; Yang, K. R.; Regan, K. P.; Konezny, S. J.; Batista, V. S.; Brudvig, G. W.; Schmuttenmaer, C. A.2015,, 1520.doi: 10.1021/ja5111078
(39) Wu, Y.; Marszalek, M.; Zakeeruddin, S. M.; Zhang, Q.; Tian, H.; Grätzel, M.; Zhu, W.2012,, 8261.doi: 10.1039/C2EE22108J
(40) Frisch, M.; Trucks, G.; Schlegel, H.;, Revision A.01; Gaussian, Inc.: Wallingford CT,2009.
(41) Yanai, T.; Tew, D. P.; Handy, N. C.2004,, 51. doi:10.1016/j.cplett.2004.06.011
(42) Cossi, M.; Rega, N.; Scalmani, G.; Barone, V.2003,, 669.doi: 10.1002/jcc.10189
(43) Ding, W. L.; Wang, D. M.; Geng, Z. Y.; Zhao, X. L.; Yan, Y. F.2013,, 17382. doi: 10.1021/jp402645h
(44) Kresse, G.; Furthmüller, J.1996,, 11169.doi: 10.1103/PhysRevB.54.11169
(45) Thomas, A.; Bhanuprakash, K.2012,, 597.doi: 10.1002/cphc.201100565
(46) Lundqvist, M. J.; Nilsing, M.; Lunell, S.; Åkermark, B.; Persson, P.2006,, 20513. doi:10.1021/jp064045j
(47) Ambrosio, F.; Martsinovich, N.; Troisi, A.2012,, 1531.doi: 10.1021/jz300520p
(48) Ambrosio, F.; Martsinovich, N.; Troisi, A.2012,, 2622.doi: 10.1021/jp209823t
(49) McNamara, W. R.; Snoeberger, R. C., III; Li, G.; Richter, C.; Allen, L. J.; Milot, R. L.; Schmuttenmaer, C. A.; Crabtree, R. H.; Brudvig, G. W.; Batista, V. S.2009,, 1173.doi: 10.1039/B910241H
苯并噻二唑衍生物作为铆接基团提高染料敏化太阳能电池效率
李重杲1卢 天1高 恒2张 庆1李敏杰1,*任 伟2,*陆文聪1,*
(1上海大学理学院化学系,上海 200444;2上海大学理学院物理系,量子与分子结构国际研究中心,上海 200444)
我们设计了一系列苯并噻二唑衍生物作为铆接基团的染料分子,运用第一性原理方法研究了苯并噻二唑类染料分子的光电性质,并对氰基丙烯酸作为铆接基团的染料分子的性能进行了详细比较。结果表明,新设计的铆接基团的染料分子拥有更广的吸收光谱,甚至可以覆盖到1000 nm左右的近红外区域。更重要的是,通过界面电子转移(IET)的动力学研究发现:染料分子SPN和SPS能够更快速的将电子注入到TiO2表面,在15 fs之内,便可以将90%的电子注入到半导体TiO2上,并在100 fs之内完成电子注入过程。因此,SPN和SPS的铆接基团可以更加有效提高染料太阳能电池效率。我们的研究将为高效染料敏化太阳能电池非金属有机敏化剂分子的设计提供指导。
苯并噻二唑;铆接基团;吸收光谱;界面电子转移;染料敏化太阳能电池
O641
10.3866/PKU.WHXB201705082
March 30, 2017;
April 25, 2017;
May 8, 2017.
LI Min-Jie, Email: minjieli@shu.edu.cn; Tel: +86-21-66133513. REN Wei, Email: renwei@shu.edu.cn; Tel: +86-21-66133513.
LU Wen-Cong, Email: wclu@shu.edu.cn; Tel: +86-21-66133513.
The project was supported by the National Key Research and Development Program of China (2016YFB0700504) and Natural Science Foundation of Shanghai, China (16ZR1411500).
国家科技部“十三五”科技计划(2016YFB0700504)和上海市自然科学基金(16ZR1411500)资助项目

