Next-Generation Materials for Cutting Tools: Superhard Materials
ZHANG Taiquan
(1.China National R&D Center for Tungsten Technology, Technology Center of Xiamen Tungsten Co ., Ltd ., Xiamen 361009, China)(2.Xiamen Golden Egret Special Alloy Co ., Ltd ., Xiamen 361021, China)
1 Introduction
Superhard materials are highly imcompressible solid materials with a hardness value exceeding 40 GPa when measured by the Vickers hardness test[1], at the same time, usually also show high thermal conductivity and good wear resistance. As a result of their unique properties, these materials are of potential great interest in many industrial areas including, but not limited to, abrasives, polishing, cutting tools, wear-resistant and protective coatings, etc. For now, diamond and cubic boron nitride (cBN), which are widely used, are mainly two categories of superhard materials.Especially for diamond with a dazzling appearance and an extremely high hardness has always attracted people to study how to synthesize. It is more than half a century from the first particle of synthetic diamond to now[2,3], and the family of superhard materials has been expanded continuously, and their application fields have expanded from the initial abrasives to the current advanced cutting tools and even some precision parts. In this paper, development of superhard materials is detailedly overviewed along to the main line of synthetic diamond and related materials and tools, and the advantages and potential limitations of superhard materials seen as next generation materials for cutting tools are discussed by comparing the characteristics and applications of traditional cutting tools.
2 History of superhard materials
Diamond is the hardest known material to date, with a Vicker’s hardness in the range of 70~150 GPa, and coupled with its dazzling appearance, which has always attracted people to study how to synthesize. Therefore, history of superhard materials is originated from synthesizing diamond, and can be roughly divided into six stages or classified into five kinds of products according to the extending of application fields, as shown in Figure 1.
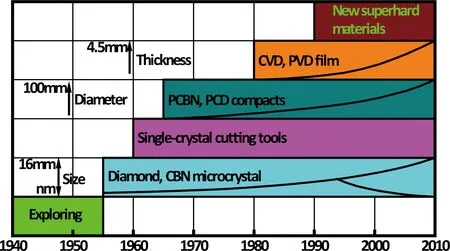
Fig.1 History of superhard materials
(1)Exploringstage. Humans have began to explore the synthesis journey of artificial diamond since 1797, in which Tennant[4]firstly revealed that diamond is made of only carbon element by a famous experiment of burning natural diamond. Many reports of synthetic diamonds had been published in 1879~1928, but after careful analysis, it can’t be sure that their synthetic crystals were a real diamond, more like a spinelle[5]. It was not until the graphite-diamond equilibrium phase diagram, which is a key to open synthetic diamond, had been obtained by Simon and Berman[6]according to experimental results and reasoning in the 1950s.
(2)Syntheticdiamondbyhigh-pressureandhightemperature(HPHT)technology. The HPHT synthesis of diamond in 1953 in Sweden[2]and in 1954 in the US[3]became a milestone in synthesis of artificial superhard materials. Four years after the first synthesis of artificial diamond, cubic boron nitride (cBN) which cannot be found in nature was synthesized and found to be the second hardest solid[7]. Up to date, the size of the synthesized diamond crystals by HPHT has been covered from a nanoscale (5 nm in diameter[8]) to a gem grade (>16 mm in diameter and 34.8 ct in weight[9]).
(3)Singlecrystalcuttingtools. The single crystal diamond cutting tools had been fabricated by adhering or welding large natural or artificial single crystal diamond (usually >3 mm in diameter) on a cutter body, and used for machining since 1960s. The development of single crystal cBN cutting tools[10]is slower due to probably difficult growth of a large single crystal cBN and its lower toughness.
(4)Polycrystallinediamond(PCD)compactandpolycrystallinecubicboronnitride(PcBN)compact. Because there are the potential shortages of easy cleavage and anisotropy for single crystal used as the cutting tools, materials researchers have studied the preparation technology of PCD and PcBN since about 1965, and the commercial products of PCD and PcBN cutting tools appeared in the 1970s. PCD is fabricated by sintering individual diamond crystals (which are direct diamond powders orinsitugenerated by carbon materials with a non-diamond structure) together with or without binders under HPHT or other conditions, and it is a similar process for the preparation of PcBN. And the further, PCD and PcBN compacts are formed by bonding PCD or PcBN to a substrate (WC/Co alloy is commonly used, but it can also be other materials[11,12]). Now PCD and PcBN compacts have been widely used in cutting tools, coal mine, oil drilling, wear resistant parts, and wire drawing die fields, etc., and the size in diameter of the compact can be beyond 100 mm.
(5)SyntheticdiamondbyChemicalVaporDeposition(CVD)technology. Although a diamond film had been fabricated by using C-H compound at an atmospheric pressure in 1962[13], the innovation has not attracted people’s attention due to the widespread influence of HPHT technology and a slower growth rate for CVD. Until 1982, the commercialization of CVD diamond became possible because a faster growth rate than 20 μm/h was broken[14]. Subsequently, CVD diamond technology has been widely and quickly developed in the US, Russian, Japan, England, etc. Now diamond coated cutting tools have been widely used, the size in diameter of flat with a thick diamond film of 2 mm grown by CVD method is larger than 100 mm[15], and the size and weight of a gem diamond fabricated by three-dimensional growth CVD technology[16]can reach 1 inch and 300 ct, respectively.
(6)Newsuperhardmaterials. Many simple substances and compounds composed of non-metallic elements (such as, B, C, N, Si, O), and many compounds composed of these non-metallic elements and transition elements (such as, Re, W, Mo, Ti, Zr, Cr, Ir, Pt, Os, etc.), which have already been synthesized or indirectly extrapolated some possible crystal structure and composition by theoretical calculation, have also very high hardness due to their high electron density and high bond covalency. The synthetic technologies and properties for these new superhard materials have been widely studied since the 1990s, and some of them have been attempted to use as cutting tools, wear resistant parts, and protective equipments, such as, B6O[17,18], BxCyNz[19,20], and B12-nC3Men(Me is a general designation of above transition metal elements)[21], etc.
3 Advantages of superhard materials used as cutting tools
3.1 Mechanical, physical and chemical properties
During cutting, cutting tools will withstand the test of high temperature, high load, strong friction, thermal shock, etc., so cutting tool materials must have the following basic properties: ①high hardness; ②good wear resistance; ③sufficiently high strength and toughness; ④good heat resistance; ⑤good thermal conductivity; ⑥low thermal expansion.
Figure 2 shows a schematic distribution for four commercial cutting tool materials according to hardness and toughness. Diamond and cBN materials have absolute advantage in hardness and thermal conductivity, but their toughness is lower than that of cemented carbides. The disadvantages of low toughness for diamond and cBN materials are greatly weakened by designing a multilayer gradient structure (PCD and PcBN compacts are made of WC/Co layer and diamond or cBN layer) or a coating. Under the premise of without damaging other properties, the toughness (K1C) of PCD can also reach 10~14 MPa·m1/2by optimizing its microstructure[22,23]. Another disadvantage of diamond is that its strength and hardness will sharply decrease due to diamond-to-graphite phase transformation at the temperature of higher than 700 °C and atmospheric pressure, as shown in Figure 3, but the resistance temperature of PCD, which is defined as the thermal stability polycrystalline diamond (TSPCD), can be improved up to 1200 ℃ by using ceramic binder[23-26]instead of Co binder.
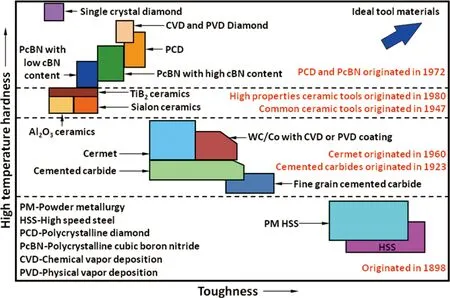
Fig.2 Schematic distribution for four cutting tool materials according to hardness and toughness
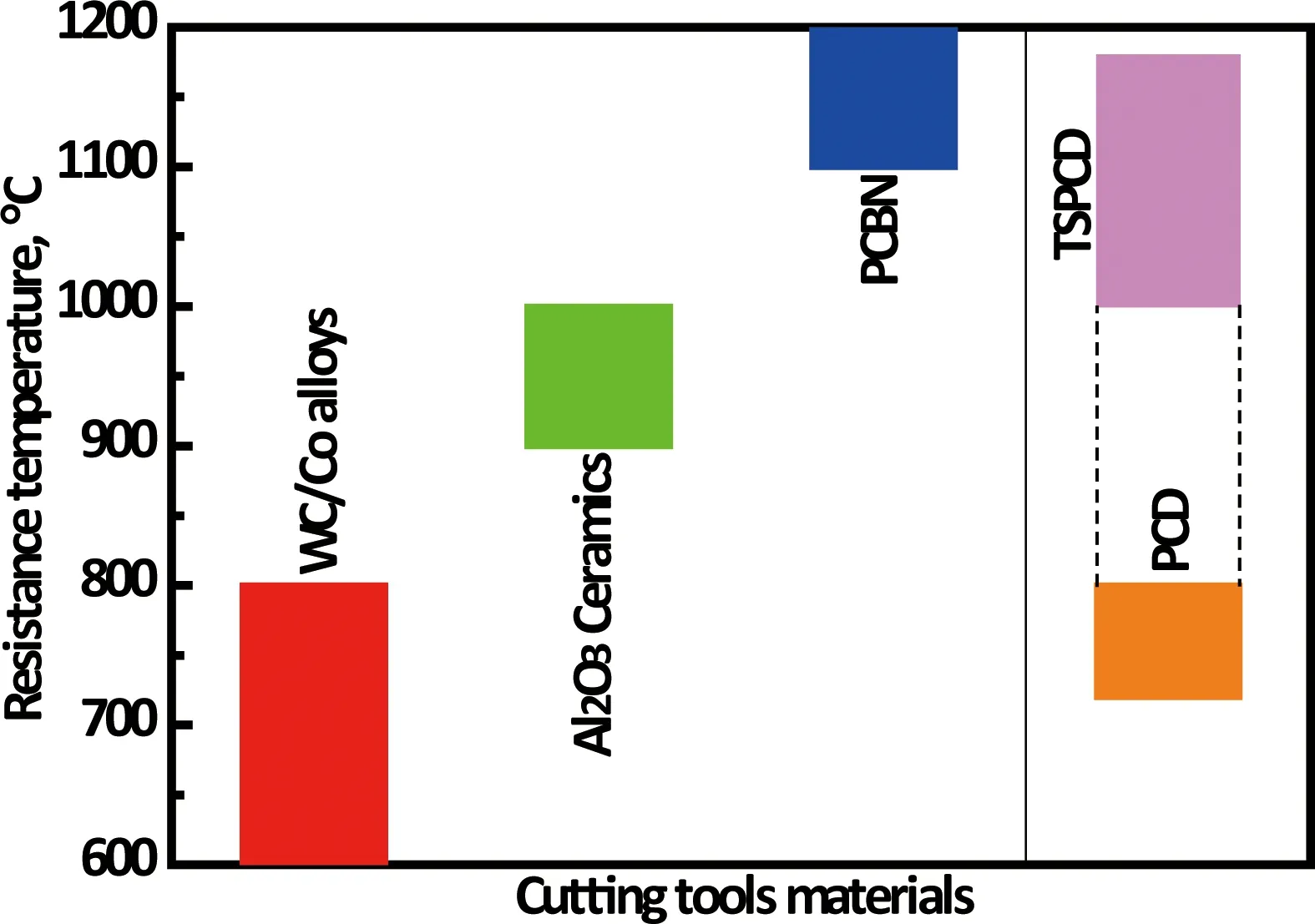
Fig.3 The resistance temperatures of four cutting tools materials
3.2 Development of high performance materials

Fig.4 Evaluation of main materials use in aero-gas turbines
With the development of aero-space technology, the requirements for the performance of materials and the machining accuracy of workpieces, especially for aero-engine, are higher and higher. Many novel nickel, titanium and aluminium super-alloys, high strength refractory metal alloys, metal-matrix composites, ceramic-matrix composites, and Cf/C composites, etc., have been developed. Figure 4 shows the weight percentages for main materials in a typical aero-gas turbine[27]. The above alloys and composites with good high temperature properties, good thermal fatigue and shock resistance, good ablation resistance, good corrosion resistance, and high strength-to-weight ratio can ensure the efficient fuel consumption with low cost and longer service life. However, high performance materials will also bring many difficulties in manufacturing and machining of the corresponding work-pieces. The difficult-to-machine characteristics are mainly shown in the following aspects:
Forsuper-alloysandmetal-matrixcomposites: ①Strong work hardening;②High strength and good plasticity; the sharp and proper shaped cutting tools are required to ensure a smooth cutting action and a chip with a proper shape and size.③Low thermal conductivity; heat generated by the cutting action cannot be dissipated quickly, and will be directly gathered at the cutting edge of the tools. High thermal load at the cutting edge will damage its strength, wear-ability, and sharpness.④Good chemical activity to easily cause adhesive and chemical wear;⑤Easy to cause machining deformation due to cutting heat and stress for some large or irregular work-pieces with a large difference in the three-dimension.
Forceramic-matrixcompositesandCf/Ccomposites: ① Good abrasive resistance; to realize the machining method of turning instead of grinding.②High hardness;③Low thermal conductivity;④Burr generation due to fracture and pulling out of carbon fiber; the sharp cutting edge with good roughness is required.
Although the ultrafine-grained cemented carbides with high properties[28]have been developed in recent year, they are very difficult to reach the requirements of the modern cutting techniques in many cases while cutting the above high performance materials and some materials with a hardness of higher than HRC55. The modern cutting techniques must have the characteristics of high speed, high accuracy, high efficiency, low cost, and low pollution (green). Therefore, superhard cutting tools with good performances (see the section 3.1) are a potential suitable choice. With extending application fields of superhard cutting tools, many advanced machining concepts and theories have been proposed, such as, high speed cutting, hard turning, turning instead of grinding, dry cutting, green cutting technology, etc.
3.3 Cutting efficiency and cost
Although the price of superhard cutting tools is 5~10 times higher than that of WC/Co cutting tools, the total cutting cost is saved more than 15% due to the increase (more than 25%) of cutting efficiency and the decrease (more than 15%) of power consumption while using superhard cutting tools. Machining cost is saved more than 30% by turning instead of grinding, as shown in Figure 5, and now some high cobalt cemented carbides and even ceramic materials can be machined by turning instead of grinding. In addition, the dimensional accuracy and surface roughness of the work-piece machined by superhard cutting tools is better than those by the traditional cutting tools because superhard cutting tools have the low thermal expansion and the sharp edge retention.
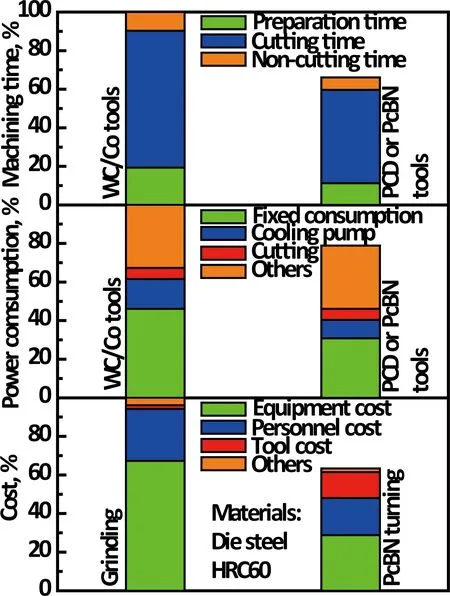
Fig.5 Comparison of superhard cutting tools and traditional cutting tools for the cost, power consumption and efficiency
4 Limitations and some possible solutions
Comparing PCD and PcBN cutting tools with the traditional cutting tools, although the superhard cutting tools have many obvious advantages, as analyzed in the section 3, some limitations need to be improved or overcome.
(1)Complexandlongmanufacturingprocessesleadtohighcost. PCD and PcBN compacts, which are one of the most important raw materials used to fabricate superhard cutting tools, are usually fabricated by HPHT sintering with a cubic or belt type press, and it is very difficult to directly fabricate a near-forming cutting tool. Therefore, the fabricating process of PCD and PcBN cutting tools is very complex and long, as shown in Figure 6, which results in a relatively high cost. With the progress of synthesis technology and ultra-high pressure equipments, the diameter of PCD and PcBN compacts has improved from the initial about 10 mm to the current more than 100 mm and the number of products in a single synthetic block is also greatly increased, so the unit cost is greatly reduced.

Fig.6 A schematic expression for the main fabricating processes of PCD and PcBN cutting tools
(2)LowtoughnessforcBNanddiamond. The edge chipping resistance of PCD and PcBN cutting tools is very weak due to low toughness (3~5 MPa·m1/2) of diamond and cBN materials, so PcBN and PCD cutting tools are generally considered unsuitable for heavy cutting. Many studies have focused on improving the toughness of PcBN and PCD materials. The toughness (K1C) of PCD can be improved to reach 10~14 MPa·m1/2by using a nano-size SiC binder[22]. The toughness of PcBN can be improved to reach 7~10 MPa·m1/2by using Y-ZrO2and Si3N4tough binders[29], and should be improved byinsitureacting cBN and, such as Ti, Zr, etc., to form a nano-columnar boride grain with a diameter of 100~200 nm[30-31]. Coated technology is also a good way to improve the toughness of PcBN inserts[32]. In order to improve the properties, similar to cemented carbides or other cutting tools materials, the effect of micro-alloying elements (for example: Boron[33-37], Rare earth[37,38], etc.) on the properties of PCD and PcBN have been researched.
(3)Thermalstabilityfordiamond. As shown in section 3.1, the diamond-to-graphite phase transformation will occur at temperatures of higher than 700 °C and at atmospheric pressure and it is also oxidized at temperatures of above 800 °C. In addition, diamond dissolves in iron and forms iron carbides at high temperatures and therefore is inefficient in cutting ferrous materials or even materials containing iron element. Therefore, many recent researches of diamond materials have been focusing on doped compounds or composites which would be thermally and chemically more stable than pure diamond. As mentioned above, the resistance temperature of TSPCD can be improved up to 1200 °C by using ceramic binders[23-26]instead of Co binder. The oxidation temperature of diamond can be increased to more than 900 °C by doping boron element[33-35], and even PCD cutting tools boron doped can be used for turning of hardened steel.
(4)Difficultmachiningprocess. The edge of superhard cutting tools is usually machined by grinding method, and in order to ensure grinding precision and efficiency, the grinding machine must have a good rigidity and an online wheel dressing device, which will give many difficulties for the design and manufacture of the grinding machine. Recently, the laser and electric discharge machining (EDM) methods with high efficiency have been well developed for machining of superhard cutting tools.
(5)Difficulttomanufacturelongedgedrillingorendmillingtoolsorinsertswith3Dchipbreaker. As mentioned above, it is very difficult to directly fabricate a near-forming superhard cutting tool, especially for a long edge drilling or end milling tool because of difficult welding and an insert with 3D chip breaker because of difficult machining. PCD or PcBN inserts with 3D chip breaker can be fabricated by an electro-erosion technology in LaCH Diamond Inc.[39]or a further advanced laser engraving technology. An integral sintered PCD drilling tool (PCD-veined drill) in Precorp Inc.[40]or a superhard cutting insert with 3D chip breaker E6[41]can be fabricated by HPHT sintering, which provides the possibility for making long edge PCD or PcBN drilling or end milling tools.
5 Conclusion
Compared with the traditional cutting tool materials, superhard materials used as cutting tools have obvious advantages in many aspects, such as, high cutting efficiency, long life, low cutting cost, high machining accuracy, etc. After half a century of technological progress, the manufacturing cost of superhard materials has been also continually reduced, and their limitations are being gradually solved, and their applications are invading the market of traditional cutting tools, as shown in Figure 7. Even their application ratio in metal machining fields is seen as an index by some authorities to measure the level of advanced manufacturing technology for a country. You admit it or not, the age of superhard cutting tools is coming to us. In addition, cemented carbides have been threatened by the depletion of raw materials (W and Co), and the raw materials (C, B, N) used to fabricate diamond and cBN are almost inexhaustible.

Fig.7 Application percentage of PCD and PcBN cutting tools in all cutting tools, and three inserted pie figures are the actual statistical application percentages of various cutting tools in 1998, 2005, and 2008 in the world, respectively.
[1] Wentorf R H, De Vries R C, Bundy F P.Science[J], 1980, 208(4446): 873-882.
[2] Liander H, Lundblad E.ASEAJournalofEngineeringforIndustry[J], 1955, 28(5-6): 97-98.
[3] Bundy F P, Hall H T, Strong H M,etal.Nature[J], 1955, 176(4471): 51-54.
[4] Tennant S, Esq F R S.PhilosophicalTransactionsoftheRoyalSocietyofLondon[J], 1797, 87(5): 123-127.
[5] Desch C H.Nature[J], 1928, 121(3055): 799-800.
[6] Berman R, Simon S F.ZeitschriftfürElektrochemie[J], 1955, 59(5): 333-338.
[7] Wentorf R H.JournalofChemicalPhysics[J], 1957, 26(4): 956-957.
[8] Iakoubovskii K, Baidakova M V, Wouters B H,etal.DiamondandRelatedMaterials[J], 2000, 9(3-6): 861-865.
[9] Kanda H.BrazilianJournalofPhysics[J], 2000, 30(3): 482-489.
[10] Nishiguchi T, Masuda M.JournaloftheJapanSocietyofPrecisionEngineering[J], 1988, 54(2): 384-389.
[11] Lee M Y, Szala L E, DeVriesR C. U.S., US4234661 [P]. 1980.
[12] Lee M Y, Szala L E, DeVries R C. U.S., US4241135 [P]. 1980.
[13] Eversole W G. U.S., US3030187, US3030188 [P]. 1962.
[14] Matsumoto S, Sato Y, Tsutsumi M, et al.JournalofMaterialsScience[J], 1982, 17(11): 3106-3112.
[15] Linares R C, Doering P J. U.S., US6858080 [P]. 2005.
[16] Hemley R J, Mao H K, Yan C S. U.S., US7594968B2 [P]. 2009.
[17] He D W, Zhao Y S, Daemen L,etal.AppliedPhysicsLetters[J], 2002, 81(4): 643-645.
[18] Johnson O, Sigalas L.BoronSuboxise(B6O)MaterialswithImprovedProperties[M]. LAP LAMBERT Academic Publishing, 2012.
[19] Zhao Y S. Novel Superhard Materials and Nanostructured Diamond Composites for Multiple Industrial Applications [R]. Los Alamos National Laboratory.
[20] Li F, Hui Q, Ao J,etal.AppliedMechanicsandMaterials[J], 2013, 302: 165-169.
[21] Zachary Z. New Superhard Ternary Borides in Composite Materials [R]. Institute of Polymers, Bulgarian Academy of Sciences, Bulgaria, 2011.
[22] Zhao Y S, Jiang Q, Daemen L L,etal.AppliedPhysicsLetters[J], 2004, 84(8): 1356-1358.
[23] Shulzhenko A A, Bochechka O. Diamond Polycrystal Nano-Composite for Industry [R]. Institute for Superhard Materials of the National Academy of Sciences of Ukraine, 2006.
[24] Szutkowska M, Jaworska L, Rozmus M,etal.ArchivesofMaterialsScienceandEngineering[J], 2012, 53(2): 85-91.
[25] Jaworska L.HighPressureResearch[J], 2002, 22: 531-533.
[26] Bellin F. U.S., US8522900B2 [P]. 2013.
[27] Miller S.InterdisciplinaryScienceReviews[J], 1996, 21(2): 117-129.
[28] Wu Chonghu (吴冲浒), Nie Hongbo (聂洪波), Xiao Mandou (肖满斗).MaterialsChina(中国材料进展), 2012, 31(4): 39-46.
[29] Zang Jianbing (臧建兵).ThesisforDoctorate(博士论文) [D]. Qinhuangdao: Yanshan University, 2002.
[30] Benko E.DiamondandRelatedMaterials[J], 1997, 6(9): 1192-1197.
[31] Benko E, Morgiel J, Czeppe T,etal.JournalofEuropeanCeramicsSociety[J], 1998, 18(4): 389-393.
[32] Katsuhisa Ohtomo. U.S., US20110014426A1 [P]. 2011.
[33] Wentorf R H, Bovenkerk H P. U.S., US3148161 [P]. 1964.
[34] Elyutin A V, Ermolaev A A, Laptev A I,etal.DokladyPhysics[J], 2002, 47(9): 651-653.
[35] Sun Yanlong (孙延龙).DissertationforMaster(硕士论文) [D]. Beijing: Iron and Steel Research Institute, 2012.
[36] Brookes K.MetalPowderReport[J], 2010, 65(7): 7-10.
[37] Huang Changgeng (黄长庚).MaterialsChina(中国材料进展) [J], 2014, 33(8): 492-496.
[38] Xue Yong (薛 勇).DissertationforMaster(硕士论文) [D]. Qinhuangdao:YanshanUniversity, 2007.
[39]EugenMaurer. U.S., US6315502B1 [P]. 2001.
[40] Bunting J A, Hanks K H. U.S., US4762445 [P]. 1988.
[41] Olofsson Bo C, Jonker C R, Nilen R W N,etal. E.P., WO2014005834A1 [P]. 2014.

