钬铥双掺钨酸镱钾激光晶体光谱参数计算
毕 琳, 底晓强, 赵建平, 李 春, 刘景和
(1. 长春理工大学 计算机科学技术学院, 吉林 长春 130022; 2. 长春理工大学 材料科学与工程学院, 吉林 长春 130022)
1 Introduction
Ho3+, Tm3+ions can be commonly used as the activation ions at the range of 2-3 μm wavelength, which has the advantage of large stimulated-emission cross-section and long fluorescence lifetime. In the military, 2 μm laser has a strong ability to penetrate the smoke of the battlefield with good confidentiality. These two types of activation ions are suitable for military laser ranging, laser radar and electro-optical jamming,etc[1-4]. In the medical field, 2 μm laser can perform precise excision of biological tissues without damaging surrounding tissues, while reducing the anesthetic dosage[5]. In addition, it can be used to detect harmful gases such as formaldehyde with the magnitude of 10-9, as well as carbon monoxide in the environment[6].
Tm3+ions can be often used as the sensitizing agent of Ho3+ions since Tm3+ions have stronger absorption efficient than Ho3+ions at the range of 785-810 nm. It can achieve the energy transfer of Tm→Ho and then improve the absorption efficiency to pumping light[7-11]. Yb3+ions have been widely used as a sensitizing agent with the LD pumping popular. The absorption peak of Yb3+ions located near 980 nm and can be effectively coupled with InGaAs LD. Because the energy level structure of Yb3+ion is very simple without excitation state re-absorption process, Yb3+can be served as the sensitizing agent of Ho3+, Tm3+ions.
KYb(WO4)2is one of the self-activation laser crystals with a double tungstates structure. In the host of KYb(WO4)2,Yb3+ions have been served as not only an integral part of the host, but also the active ions. The absorption wavelength of KYb(WO4)2is 930-980 nm, which can be effectively coupled with the InGaAs LD pumping[12-14]. The laser output wavelength of KYb(WO4)2is in the range of 980-1 080 nm. Laser emission with multi-wavelength and tenability in this crystal can be achieved by doping Er3+, Tm3+, Ho3+,etc.Re∶KYb(WO4)2has several merits including high doping concentration, low fluorescence quenching, high conversion efficiency, low threshold value and well stability,etc. It can achieve all-solid-state, miniaturization and integration for lasers and widely used in the fields of human-eye safe, laser communication, medical treatment, remote sensing,etc[15-18]. In this paper, the growth, absorption and fluorescence spectra of KHo0.04Tm0.06-Yb0.9(WO4)2laser crystal were investigated, and the spectral parameters of this crystal were calculated as well.
2 Experiments
2.1 Crystal Growth
The chemicals included K2CO3(top-grade pure), Yb2O3(99.99%), Ho2O3(99.99%), Tm2O3(99.99%), and WO3(99.999%). The K2W2O7was served as solvent. They were mixed and reacted based on the equations below:
0.04Ho2O3+0.06Tm2O3+0.9Yb2O3+K2CO3+
4WO3→2KHo0.04Tm0.06Yb0.9(WO4)2+CO2,
(1)
K2CO3+2WO3→K2W2O7+CO2,
(2)
where the mole ratio of KHo0.04Tm0.06Yb0.9(WO4)2to K2W2O7was 1∶4. The crystal was grown by the Top Seeded Solution Growth (TSSG) method. The experimental equipment included a MCGE-furnace heated by resistance wire, a platinum crucible with a size ofΦ60 mm×50 mm, a Pt-Rh thermocouple, and an AI-808P thermal controller. The mixed materials was put in a platinum crucible and fully melted at a temperature of 80 ℃ above super-saturation for 24 h. The oriented crystallon was dipped at about 910 ℃, and grown gradually with a rotation rate of 15 r/min, a pulling rate of 2 mm/day and a cooling rate of 0.05/h. After 2 weeks, the crystal was removed from the crucible and cooled down to the room temperature at a rate of 20 ℃/h. Finally, the KHo0.04Tm0.06Yb0.9(WO4)2laser crystal with a dimension of 26 mm×11 mm×9 mm was obtained successfully. Then the crystal sample with a dimension of 3 mm×3 mm×1 mm was cut perpendicularly tobaxis for spectral measurement.
2.2 Measurement of Sample
The absorption spectrum of the crystal sample was measured using an ultraviolet (UV) spectrophotometer(Model UV360, Shimadzu Company, Japan) at room temperature. The fluorescence spectrum of the crystal sample was measured with an X-ray fluorescence spectrometer(FLUOROLOG-3, HORIBA Company, Japan) at room temperature.
3 Results and Discussion
3.1 Absorption Spectrum
The absorption spectrum of the crystal sample is shown in Fig.1. There are several absorption peaks at 378, 421, 450, 475, 538, 625, 645, 691, 805, 890, 916, 980, 1 000, 1 190, 1 223, 1 690, 1 712, 1 749, 1 804, 1 812, 1 924 nm. It can be seen that the absorption peaks at 378, 475, 805 and 1 690-1 812 nm are accounting for3H6→1D2(Tm3+),3H6→1G4(Tm3+),3H6→3H4(Tm3+) and3H6→3F4(Tm3+) transitions, respectively. Meanwhile, the absorption peaks located at 421, 450, 538, 625-691, 890, 916, 1 190 (1 223), 1 924 nm are due to5I8→5G5(Ho3+),5I8→5G6(Ho3+),5I8→5F4(Ho3+),5I8→5F5(Ho3+),5I8→5I4(Ho3+),5I8→5I5(Ho3+),5I8→5I6(Ho3+),5I8→5I7(Ho3+) transitions, respectively. The absorption peaks at 981 and 1 000 nm are attributed to2F7/2→2F5/2(Yb3+) transition. The full width at half maximum (FWHM) at the range of 890-1 000 nm amounts to 90 nm, which is more propitious for InGaAs LD laser pumping. In this absorption spectrum, the most interesting is a broader absorption band (Tm3+) at 1 690-1 812 nm with a FWHM of 118 nm. The effective energy transfer of Yb→Ho, Yb→Tm, Tm→Ho can be easily achieved to produce 2 μm around laser due to these two broader absorption bands at 890-1 000 nm(Yb3+) and 1 690-1 812 nm(Tm3+) in the crystal.
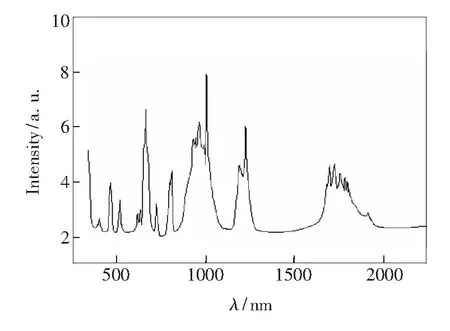
Fig.1 Absorption spectrum of KHo0.04Tm0.06Yb0.9(WO4)2 crystal
The absorption cross sectionσabsof the crystal can be defined as formula (3)[19]:
(3)
whereDis the optical density, which can be calculated asD=lg(I0/I), based on absorption spectrum measurements;I0andIrepresent the intensities of incident light and emergent light, respectively;Nrepresents the concentration of Ho3+, Tm3+or Yb3+ions, andLrepresents the thickness of the sample (L=1 mm). The absorption cross section of the crystal at the strongest peak of 1 000 nm was calculated as 16.92×10-20cm2. Tab.1 lists the main absorption peaks and absorption parameters of the crystal.
Meanwhile, the spectral intensity parameters in the crystal can be obtained based on the Judd-Ofelt theory[20-21]as follows:Ω2=10.24×10-20cm2,Ω4=2.37×10-20cm2,Ω6=1.43×10-20cm2.
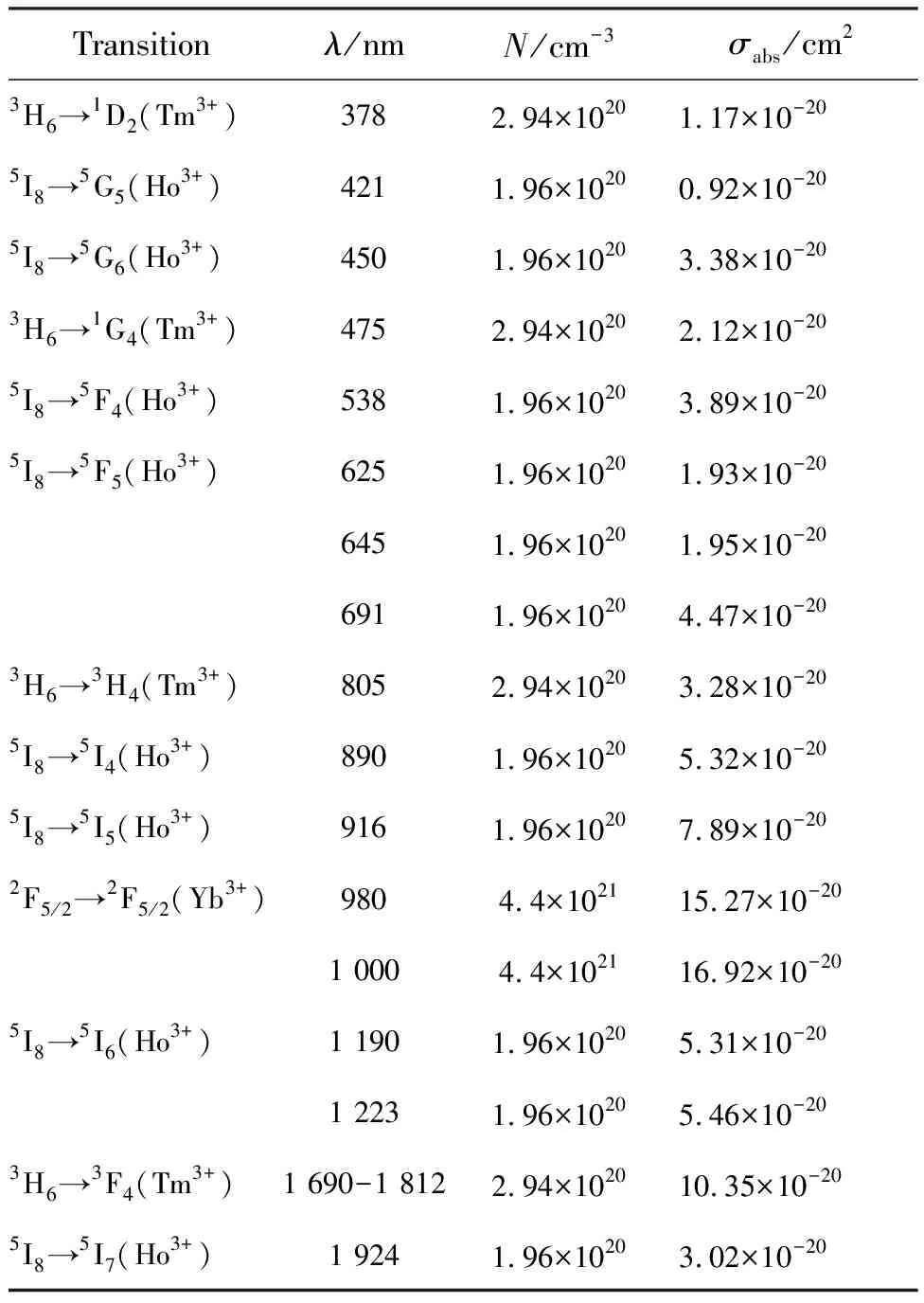
Tab.1 Main absorption peaks and absorption parameters of the KHo0.04Tm0.06Yb0.9(WO4)2 crystal
3.2 Fluorescence Spectrum
The fluorescence spectrum of the crystal sample under 980 nm pumping is shown in Fig.2. There are four obvious emission peaks at 950-1 050 nm, 1 100-1 250 nm, 1 400-1 470 nm, 1 750-2 200 nm, which are attributed to2F5/2→2F7/2(Yb3+),3H5→3H6(Tm3+) &5I6→5I8(Ho3+),3H4→3F4(Tm3+),3F4→3H6(Tm3+) &5I7→5I8(Ho3+) transitions, respectively. The emission line width at 1 750-2 200 nm amounts to 250 nm, which is due to the overlap between3F4→3H6(Tm3+) and5I7→5I8(Ho3+). It is indicated that the crystal can be used as a high quality tunable laser medium at 2 μm.
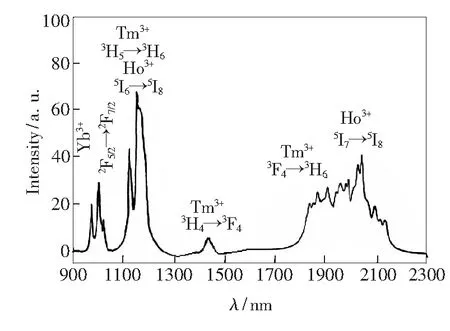
Fig.2 Fluorescent spectrum at 900-2 300 nm of the KHo0.04Tm0.06Yb0.9(WO4)2 crystal
The energy level diagram sketched in Fig.3 can describe the energy transfer among Tm3+, Yb3+, Ho3+ions in the crystal. The fluorescence emission at 1 750-2 200 nm is due to the following three energy transmission interaction.
(1)Energy transfer of Yb3+→Tm3+
Yb3+ions can uptake quantities of pumping light to generate2F7/2→2F5/2absorption transition under 980 nm excitation. Then the energy was transferred from2F5/2(Yb3+) to3H6(Tm3+) and3H6→3H5transition of Tm3+ions can be achieved. From3H5energy state(Tm3+), the emission at 1 700-1 900 nm (3F4→3H6) can be obtained by the non-radiative transition process from3H5to3F4energy state.
(2) Energy transfer of Yb3+→Ho3+
Yb3+ions generate a2F7/2→2F5/2absorption transition due to the 980 nm excitation. Then the energy is transferred from2F5/2(Yb3+) to5I8(Ho3+) and5I8→5I6transition of Ho3+ions can be achieved. From5I6energy state (Ho3+), the emission at 1 900-2 200 nm(5I7→5I8) can be obtained by the non-radiative transition process from5I6to5I7energy state.
(3) Energy transfer of Tm3+→Ho3+
This efficient resonant energy transfer happens due to the energy approximate overlap between the3F4(Tm3+) and5I7(Ho3+) energy levels. Therefore, the emission at 1 900-2 200 nm (5I7→5I8) can be achieved based on the energy transfer from3F4(Tm3+) to5I7(Ho3+).
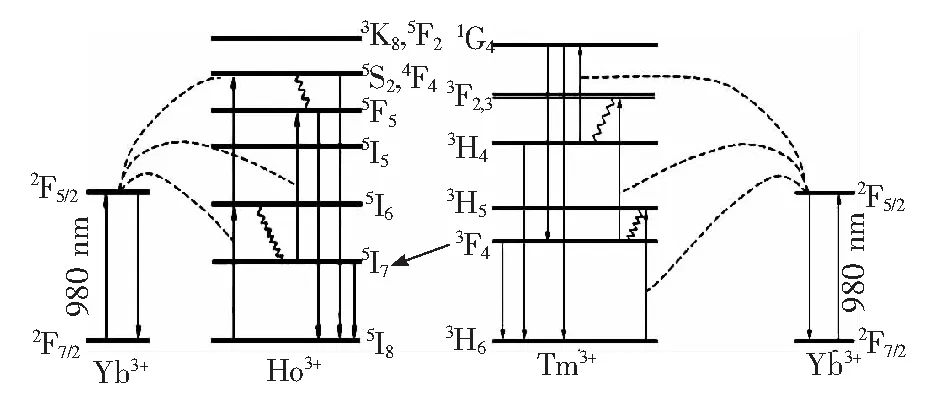
Fig.3 Energy level diagram of Tm3+, Yb3+, Ho3+ ions and emission channels.
According to the formula (4)[15], the stimulated emission cross section of main emission peak at 2 030 nm can be calculated as 3.47×10-20cm2.
(4)
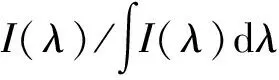
4 Conclusion
KHo0.04Tm0.06Yb0.9(WO4)2laser crystal with a dimension of 26 mm×11 mm×9 mm was grown by the TSSG method. The testing result of absorption spectrum shows that Yb3+ions have a wider absorption band at the range of 890-1 000 nm with a FWHM of 90 nm, and Tm3+ions have a broader absorption band at 1 690-1 812 nm with a FWHM of 118 nm. The effective energy transfer of Yb→Ho, Yb→Tm, Tm→Ho can be easily achieved to produce 2 μm around laser due to these two broader absorption bands at 890-1 000 nm (Yb3+) and 1 690-1 812 nm(Tm3+) in the crystal. The fluorescent spectrum measurement result indicates that the emission line width at 1 750-2 200 nm amounts to 250 nm, which is due to the overlap between3F4→3H6(Tm3+) and5I7→5I8(Ho3+). The spectral strength parameters of the crystal were calculated based on the Judd-Ofelt theory as follows:Ω2=10.24×10-20cm2,Ω4=2.37×10-20cm2,Ω6=1.43×10-20cm2。According to the energy level diagram of Tm3+, Ho3+and Yb3+ions, three kinds of energy transfer modes at 1 750-2 200 nm emission were discussed in the crystal. The stimulated emission cross section of main emission peak at 2 030 nm is 3.47×10-20cm2. It is indicated that KHo0.04Tm0.06- Yb0.9(WO4)2laser crystal can be widely used as an excellent laser gain medium at 2 μm around wavelength.
References:
[1] 袁云松, 吴从越, 李雨芬, 等. 铥、镱共掺可见光响应型纳米TiO2光催化剂的制备及性能表征 [J]. 发光学报, 2016, 37(11):1310-1315.
YUAN Y S, WU C Y, LI Y F,etal.. Preparation and characterization of TiO2∶Tm, Yb visible light responsive nano-photocatalyst [J].Chin.J.Lumin., 2016, 37(11):1310-1315. (in Chinese)
[2] 孙超, 朱忠丽, 张莹. 低温燃烧法制备的Ho∶YbGG多晶粉体及其发光性能 [J]. 发光学报, 2014, 35(9):1065-1070.
SUN C, ZHU Z L, ZHANG Y. Ho∶YbGG polycrystal powder synthesized by low temperature combustion method and its luminescent characteristics [J].Chin.J.Lumin., 2014, 35(9):1065-1070. (in Chinese)
[3] 付作岭, 董晓睿, 盛天琦, 等. 纳米晶体中稀土离子的发光性质及其变化机理研究 [J]. 中国光学, 2015, 8(1):139-144.
FU Z L, DONG X R, SHENG T Q,etal.. Luminescene properties and various mechanisms of rare earth ions in the nanocrystals [J].Chin.Opt., 2015, 8(1):139-144. (in Chinese)
[4] 任国光, 黄裕年. 用激光红外干扰系统保护军用和民航机 [J]. 激光与红外, 2006, 36(1):1-6.
REN G G, HUANG Y N. Laser-based IRCM system defenses for military and commercial aircraft [J].Laser&Infrared, 2006, 36(1):1-6. (in Chinese)
[5] VODOPYANOV K L, STAFSUDD R. Generation ofQ-switched Er∶YAG laser pulse evanescent wave absorption in ethanol [J].Appl.Phys.Lett., 1998, 72:2211.
[6] SRINIVASAN B, TAFOYA J, JAIN R K. High-power “watt-level” CW operation of diode-pumped 2.7 μm fiber laser using efficient cross-relaxation and energy transfer mechanisms [J].Opt.Experess, 1999, 4:495.
[7] REMSKI R, JAMES L T, GOOEN K H. Pulsed laser action in LiYF4∶Er3+,Ho3+at 77 K [J].IEEEQuant.Electron., 1969, 5(4):214.
[8] IMAI S, YAMADA T, FUJIMORI Y,etal.. A 20 W Cr3+, Tm3+, Ho3+∶YAG laser [J].Opt.LaserTechnol., 1990, 22(5):351-353.
[9] 姚宝权, 董力强, 王月珠, 等. 激光二极管抽运Tm,Ho∶YLF微片激光器的实验研究 [J]. 光学学报, 2004, 24(1):79-83.
YAO B Q, DONG L Q, WANG Y Z,etal.. Experimental study of microchip (Tm, Ho)∶YLF laser pumped by laser diode [J].ActaOpt.Sinica, 2004, 24(1):79-83. (in Chinese)
[10] 何晓敏, 张新陆, 王月珠, 等. 激光二极管泵浦室温Tm,Ho∶YLF微片激光器的实验研究 [J]. 激光与红外, 2005, 35(9):367-340.
HE X M, ZHANG X L, WANG Y Z,etal.. Experimental study of laser-diode pumped room temperature Tm, Ho∶YLF microchip laser [J].Laser&Infrared, 2005, 35(9):367-340. (in Chinese)
[11] 张兴宝, 姚宝权, 王月珠, 等. 准四能级双掺Tm/Ho钒酸钆激光器 [J]. 激光技术, 2006, 30(2):119-122.
ZHANG X B, YAO B Q, WANG Y Z,etal.. Quasi-four-level co-doped Tm/Ho gadolinium vanadate laser [J].LaserTechnol., 2006, 30(2):119-122. (in Chinese)
[12] BRENIER A. A new evaluation of Yb3+-doped crystals for laser applications [J].J.Lumin., 2001, 92:199-201.
[13] 张莹, 刘景和, 李春, 等. 高浓度铒离子掺杂钨酸镱钾激光晶体上转换发光研究 [J]. 激光与红外工程, 2012, 41(9):2322-2327.
ZHANG Y, LIU J H, LI C,etal.. Up-conversion luminescence of highly Er3+-doped potassium ytterbium tungstate laser crystal [J].InfraredLaserEng., 2012, 41(9):2322-2327. (in Chinese)
[14] KLOPP P, GRIEBNER U, PETROV V,etal.. Laser operation of the new stoichiometric crystal KYb(WO4)2[J].Appl.Phys. B, 2002, 74:185-189.
[15] MATEOS X, PUJOL M C, GÜELL F,etal.. Sensitization of Er3+emission at 1.5 μm by Yb3+in KYb(WO4)2single crystals [J].Phys.Rev. B, 2002, 66:214104.
[16] LOIKO P A, VILEJSHIKOVA E V, MATEOS X,etal.. Europium doping in monoclinic KYb(WO4)2crystal [J].J.Lumin., 2017, 183:217-225.
[17] LOIKO P, MATEOS X, DUNINA E,etal.. Judd-Ofelt modelling and stimulated-emission cross-sections for Tb3+ions in monoclinic KYb(WO4)2crystal [J].J.Lumin., 2017, 190:37-44.
[18] 王宇明, 张礼杰, 雷鸣, 等. 泡生法生长KYb(WO4)2晶体及其结构与光谱性能 [J]. 中国激光, 2006, 33(5):697-700.
WANG Y M, ZHANG L J, LEI M,etal.. Structure and spectra characteristics of KYb(WO4)2crystal grown by Kyropoulos method [J].Chin.J.Lasers, 2006, 33(5):697-700. (in Chinese)
[19] SARDAR D K, RUSSELL CC, YOW R M,etal.. Spectroscopic analysis of the Er3+(4f11) absorption intensities in NaBi(WO4)2[J].Appl.Phys., 2004, 95(31):1180-1184.
[20] JUDD B R. Optical absorption intensities of rare-earth ions [J].Phys.Rev., 1962, 127:750.
[21] OFELT G S. Intensities of crystal spectra of rare earth ions [J].J.Chem.Phys., 1962, 37:511.

