生物活性配体喹啉-2-羧酸根锰ギ配位聚合物的晶体结构及生物活性
王仁舒 冯 静 雷以柱 杨 丹 朱明昌 邢家领 连明磊
(1六盘水师范学院化学与材料工程学院,六盘水 553004)
(2沈阳化工大学配位化学研究室,辽宁省无机分子基化学重点实验室,沈阳 110142)
0 Introduction
Owing to their wide and potential applications in the fields of adsorption,catalysis,magnetism,luminescent,anti-cancer activity,and so on,great attention has been drawn to metal-organic coordination polymers[1-5].These extended coordination polymers have molecular topological structures,which are mainly based on N,N-bidentate bridging ligands such as 1,2-bis (2-pyridyl)ethylene and 4,4′-trimethylenedipyridine[6].Heteroaromatic N,N-bidentateligands is a kind of molecule with rigid structure[7],which means the effects of π…π and C-H…π are involved in the synthesis of the coordination polymers[8].More recently,attractive forces of π…π and C-H…π effect have been proved to play significant roles in a variety of biological phenomena[9-10].On the other hand,the flexible structure that connected with the nitrogencontaining heterocyclic of the aliphatic chain owns various lengths,which means,the carbon chain of the N,N-bidentate ligands exhibit various efficiencies in DNA-binding rate for DNA of biological cell and the cytotoxic activity[11].
N-O chelate ligands are widely-used and strong chelating agents coordinated to transition metal cations.2-Quinolinecarboxylic acid,has three potential coordination sites,including two oxygens of carboxylate groups and one pyridinic nitrogen[12-13].Meanwhile,as the quinaldic acid is a bioactive molecule in regard forthe metabolism oftryptophan,itshould be conducive to applications in pharmaceutical[14].
Conventional small molecule of metal-based anticancer drugs applied to clinical trials,such as species Ruバcomplexes are restricted for demerits of general toxicity and drug resistance[15-17].Due to their small sizes,small molecule complexes cannot accumulate efficient enough at tumor sites. Similarly, the complexes may get lost rapidly from the blood stream due to their poor biocompatible[18].
As an ingredientofglycosyltransferase and phosphoenolpyruvate carboxylase,manganese is necessary for human beings[19-21].Additionally,some special Mnギcomplexes could imitate as catalase,to decrease the transporting channels with ATP-related Ca2+under certain conditions.Thus special Mnギcomplexes can induce the apoptosis of tumour cells[22-24].
Consequently,the researchers have focused on developing low-toxic anti tumor agents,thus the metal complexes cultivated by constituents of Mnギ,2-quinolinecarboxylic acid and 4,4′-trimethylenedipyridine are selected.In this paper,the synthesis and characterization of the coordinator polymer{[Mn(Qina)2(Bpp)]·2/3H2O}nwill be presented specifically,as well as the evaluation for ct-DNA-binding abilities by using fluorescence spectroscopy.Its cleavage behavior toward pBR322-DNA and cytotoxicity of HeLa and MCF-7 in vitro will also be investigated.
1 Experimental
1.1 Materials and instruments
All reagents are purchased through commercial channels and directly used without any treatment.As for analysis,a model Finnigan EA 1112 is employed for Elemental analyses (including C,H and N);and a Thermo Nicolet 380 FT-IR spectrophotometer for IR analysis by KBr disks method.And simultaneous thermogravimetry-differential scanning calorimetry(TG-DSC)was measured on a NETZSCH STA 449 F5 instrument under a dynamic argon atmosphere,and the sample was heated from room temperature to 600℃ at a heating rate of 10℃·min-1.As well as a Perkin-Elmer LS55 fluorescence spectrofluorimeter for emission spectrum and a JS-380A gel electrophoresis spectrometer for gel electrophoresis.
1.2 Synthesis of{[Mn(Qina)2(Bpp)]·2/3H2O}n
For all reagents,the concentration was prepared as 15 mmol·L-1.Firstly,an ethanol-water mixtures (1∶1,V/V,10 mL)containing 1.5 mmol of 2-quinolinecarboxylic acid was added into 10 mL of aqueous solution containing 1.5 mmol of MnCl2dropwise under stirring.After reacting for 6 h at room temperature,another 10 mL of mixture prepared by 1.5 mmol of 4,4′-trimethylenedipyridineand ethanol-water mixtures(1 ∶1,V/V)was added and continued the reaction for another 10 h.Subsequently,the pH value of the mixture was adjusted to 8.37 using KOH aqueous solution to generate yellowish-brown sediment after filtration,and then the sediment was dissolved with acetonitrile-water mixtures (1∶1,V/V)and stored in the open air.A few days later,the desired yellow transparent crystals were generated and then sequentially filtered and rinsed with water.Anal.Calcd.for C33H25N4O4Mn·2/3H2O (%):C 65.13,H 5.48,N 9.21;Found(%):C 63.61,H 4.79,N 9.76.IR (KBr,cm-1):3 237(m),3 080(w),2 925(w),1 708(s),1 601(m),1 571(m),1 405(m),1 372(m),1 213(m),805(m),520(w).
1.3 Single crystal X-ray crystallography
The single-crystal X-ray diffraction analysis for complex was conducted by a Bruker SMART APEX2 CCD diffractometer with Mo Kα irradiation source (λ=0.071 073 nm)at 296 K,and intensity data were collected within 2θ range of 3.786°~50.842°by a ω scan technique.Data indexing,integration and absorption correction were done with APEX 2 software package.Structure solution and refinement were done by using SHELXL-2014 software[25-26].There is an alert level B because the unordered object water molecules are unable to be added the hydrogen atoms.To facilitate the analysis of crystal structure,the highly disordered solvent molecules in the structure were squeezed and refined by used the PLATON and the SHELXL.All non-hydrogen atoms of complex were determined by successive difference Fourier syntheses and refined by full-matrix least squares on F2.It is found that the locations of all hydrogen atoms are theoretically correct.Selected crystal data of the complex is listed in Table 1.
CCDC:1550803.

Table 1 Crystal data and structure refinement for the complex
1.4 Hirshfeld surface calculations
The analytical cif file of the complex molecule was carried out by the CrystalExplorer software[27].And all the Hirshfeld surfaces were output at the highest resolution.Moreover,the translated command whose coordinate range is from 0.08 to 0.3 nm was selected.And it was applied to all the fingerprint plots.
1.5 Fluorescence spectrum
The molecule of ethidium bromide (EtBr)for fluorophore labeling,has a conjugate planar,the fluorescence intensity of which is extremely weak.But fluorescence intensity is significantly increased if EtBr is specifically intercalated among the base pairs of double strands.The effects of complex difference in concentration are competitive binding with DNA-ethidium bromide (cDNA=5.0 μmol·L-1,cEtBr=1 μmol·L-1,ccomplex/cDNA=1.0,2.0,3.0,4.0).The buffer of 50 mmol·L-1Tris-HCl,pH 7.4,which contains 10 mmol·L-1NaCl,was used in the binding studies.The sample was firstly incubated at temperature of 20℃for 4 h before spectral analysis[28].The excitation wavelength for fluorescence measurement was 615 nm,while the emission range was set in scope of 550 and 750 nm.
1.6 Gel electrophoresis experiments
As regards to the gel electrophoresis experiments,the pBR322 DNA (0.33 mg·mL-1)was mixed with the complex and then the mixture was incubated at room temperature for 1.5 h.The samples were electrophoresed at 90 V for 1.5 h on 0.8%agarose gel in Tris-acetate buffer (50 mmol·L-1Tris-acetate,18 mmol·L-1NaCl buffer,pH=7.4).Finally,the gels were stained with 1.0 mg·mL-1EtBr and then photographed under UV light[29].
1.7 Cytotoxicity assay
The MTT assay was used to evaluate the growth inhibitory effect of metal complex on the HeLa and MCF-7 cells.Briefly,cells cultured in RPMI-1640 containing 10%fetal bovine serum,was placed in 37℃and 5%CO2incubator.The 96-well culture plate was accurately inoculated with 3×104cells suspension in 100 μL and then incubated for 24 h.RPMI-1640 was used to dilute the solution of complex and 150 μL solution was joined to each well.Besides,the highest concentration of DMSO concentration was not higher than 0.1%and each concentration was under three replication settings.After 72 h,the old solution was cleaned by using 200 μL of saline for each well.MTT solution was diluted for 10 times with RPIM1640.In addition,100 μL of MTT solution (0.5 mg·mL-1)was added into each well and then was cultivated for 3 h.Subsequently,the media containing MTT was removed,and the formazan crystals were dissolved with 150 μL of DMSO under ambient temperature for 0.5 h.The absorbance value of each hole was measured respectively at 492 and 630 nm[30].However,the effects of the cisplatin on cell growth were studied in another experiment which was treated as a control team.
2 Results and discussion
2.1 Crystal structure determination
Results of single-crystal X-ray analyses indicated that complex was monoclinic system with P21/n space group.The single crystal X-ray diffraction analysis showed that each manganeseギion is six-coordinated by two nitrogen atoms originated from two molecules of 4,4′-trimethylenedipyridine,as well as two oxygen atoms and two nitrogen atoms that separately from two respective quinoline-2 carboxylic acid ligands(N1-O2,N2-O3).The base structure of polymer complex is shown in Fig.1,and the chosen bond lengths and bond angles are tabulated as Table 2.Besides,along c axis,the fragments of[Mn(Qina)2]formed from centric Mnギions were in turn bridged by Bpp nitrogen atoms,resulting in a 1D chain,as shown in Fig.2.

Fig.1 Structure of the complex
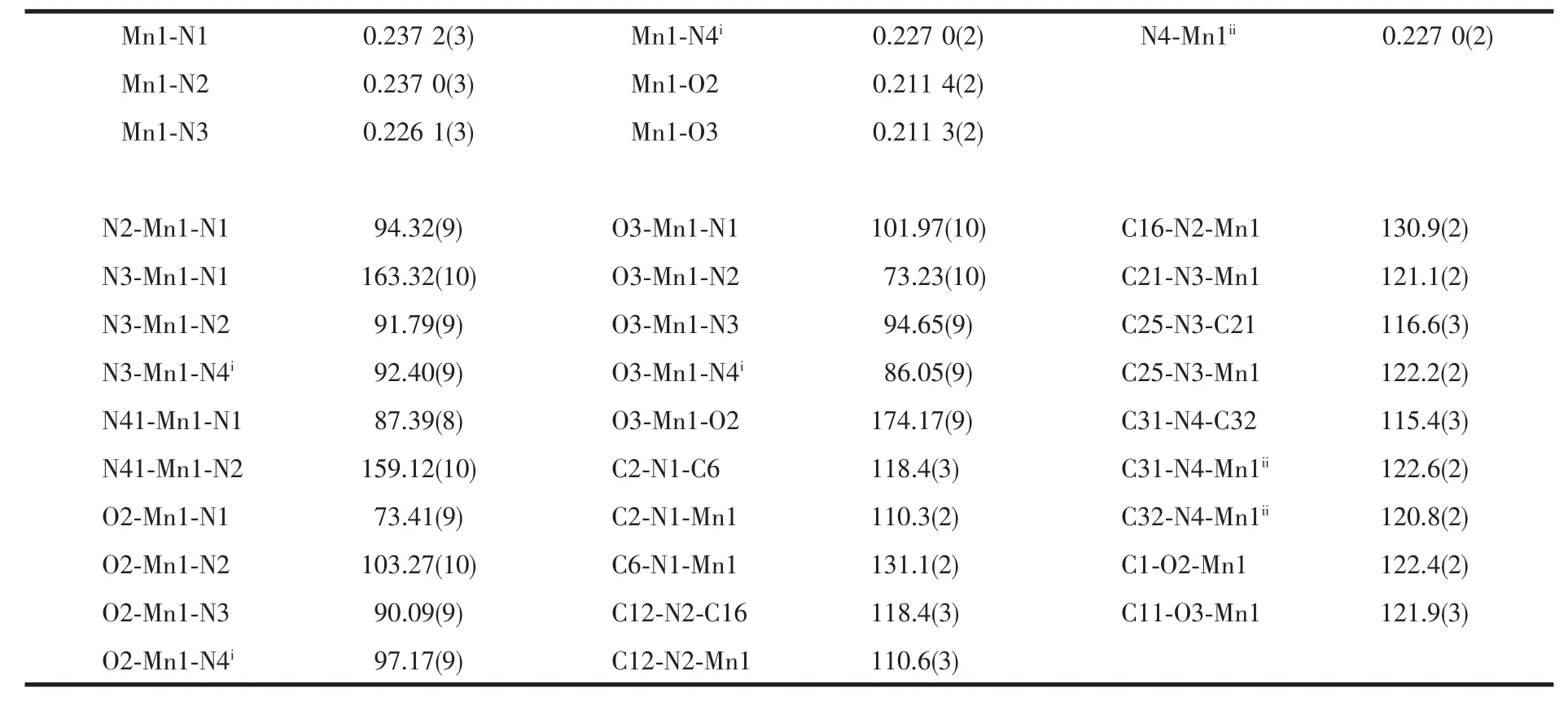
Table 2 Selected bond lengths (nm)and angels (°)
Additionally,stacking interactions of C-H…π and π…π between the two one-dimension chains was found in the complex.The H30 from pyridine ring of Bpp molecule,will produce the stacking interactions of C-H…π by an aromatic ring from Qina in the different chain.The distances between H30 from the Bpp molecule and the benzene ring of the Qina molecule are 0.371 9 nm,which is shorter than the general one of 0.38 nm that was observed in intermolecular C-H…π interactions[31],as shown in Fig.3a.Meanwhile,the H20 from benzene ring of Qina molecule,will also produce the stacking interactions of C-H…π by a pyridine ring from Qina in the different chain,as shown in Fig.3b.The centroid-to-centroid distance between a pair of Qina ligands and Bpp ligands possessing π … π stacking interactions is 0.363 0 and 0.379 6 nm,respectively,which is also within normal ranges[32],as shown in Fig.3c.Finally,the 3D supramolecular architecture is directed by 1D-interchains via weak stacking interactions of C-H…π and π…π (Fig.4).

Fig.2 One dimensional polymeric chain of coordination polymer

Fig.3 C-H…π and π…π stacking interactions in the complex:(a)C-H…π stacking interactions between Bpp molecule and Qina molecule;(b)C-H…π stacking interactions between two Qina molecules;(c)π…π stacking interactions between multiple 1D molecular chains

Fig.4 Three dimensional structure of coordination polymer:(a)3D structure is composed by multiple 1D chains;(b)weak effect superimposed in the supramolecular structure
2.2 Hirshfeld surface analysis
Hirshfeld surface isa way to analyze the intermolecular interactions in a crystal[33].As shown in Fig.5,there is Hirshfeld dnormsurfaces,shape index and curvedness of complex.The red spots or regions on the 3D Hirshfeld surfaces indicate the close-contact interactions, the blue regions represent longer contacts,and the white and green regions represent the distance of contacts.In order to illustrate the weak forces in the crystal better,there are two different observation directions in each type ofanalysis diagram.
The red parts of the dnormsurfaces diagram represents strong intermolecular interactions which represent the C-H…π and π…π interactions.The red concave with the corresponding positions in shape index and the blue convex ofthe surrounding receptors complement each other, which has confirmed the existence of such effects[34].In addition,the white part of the dnormsurfaces diagram represents the weak intermolecular interaction which represents the H…H interactions[35].The curvedness diagram also shows the same information expressed in the previous two graphs.
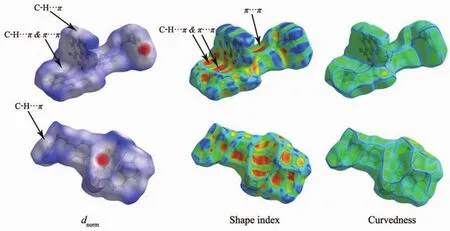
Fig.5 Hirshfeld surface of the complex
Furthermore, the quantitative relationship between the intermolecular interactions of the crystals has also been measured by the 2D fingerprint plots of the CrystalExplorer program.As shown in Fig.6,H…H interactions account for the largest proportion of the 2D fingerprint plots.Additionally,the C…H interactions are shown on the finger print to represent the symmetrical wing shape,which illustrate that the main intermolecular force between the complex is the C-H…π interactions.Moreover,the C…C interactions are the evidences that there are weak π…π effects in the intermolecular of the crystal[36-37].Besides,it can be obtained from the fingerprint plots that the N…H interaction comprises 2.1%,and H…O comprises 7.9%of total Hirshfeld surfaces.All Hirshfeld analysis was consistent with single crystal structure analysis.

Fig.6 Two dimensional fingerprint plots of the complex
2.3 Thermogravimetric analysis

Fig.7 TGA curve of the complex
As shown in the thermogravimetric analysis curve(Fig.7),the complex exhibits three steps of weight losses.The first weight loss corresponding to the removal of lattice solvent is 4.54% (Calcd.3.94%)from 190 to 290℃,the second weight loss corresponding to the removal of Bpp ligand is 27.07%(Calcd.32.58%)from 190 to 300℃,and the third weight loss correspondingtotheremovalofQinaligand is 52.14% (Calcd.56.92%)from 300 to 550℃.The final formation of the complex is metal oxide MnO(Calcd.11.66%).
2.4 Fluorescence spectroscopic properties
Since the complex molecule binds to DNA more strongly than EB,its addition would quench the DNA-induced EB emission[38].The fluorescence spectra of EB and DNA-bound EB in the absence and presence of the complex is provided as Fig.8.

Fig.8 (a)Fluorescence spectra of DNA-EB in the absence and presence of the complex;(b)Stern-Volmer plot for the complex
In the classical Stern-Volmer equation:I0/I=1+Ksqr,I0and I separately represent the fluorescence intensity with and without the complex;r stands for the total concentration ratio of the complex to that of DNA (ccomplex/cDNA);Ksqis the linear Stern-Volmer quenching constant depending on the ratio of the bound concentration of EB to the concentration of DNA,which is generated as the slope of linear curve of I/I0against r[39].The fluorescence quenching curves of DNA-bound EtBr by the complex is also provided in Fig.8.The Ksqvalue for the complex is 0.046 6,which indicates that the complex displaced DNA-bound EB and was bound to DNA with a weak bound at the intercalative mode,due to certain partial weak effect intercalation of the aromatic ring. And the spectrophotometric studies show that the main mode of complex interacting with DNA was electrostatic interaction[40-41].
2.5 Cleavage of extracted pBR322-DNA
The behavior of complex in DNA cleaving was evaluated by monitoring the conversion of the tertiary structure of pBR322 DNA.When circular plasmid DNA issubjected to electrophoresis,the intact supercoiled form (Form Ⅰ)shows a relatively fast migration.While,when scission occurs on one strand of supercoil,it will generate an open circular form(Form Ⅱ )which exhibited a slower-moving migration,and when both strands were cleaved and migrated between FormⅠand FormⅡ,it will form a linear form (Form Ⅲ)[42].The complex can significantly induce the cleavage of the pBR322 DNA at the concentration of 3.23,6.46 and 12.92 μmol·L-1.As shown in Fig.9,with the increase of concentration of complex,the pBR322 supercoiled DNA (Form Ⅰ )decreases gradually,and the open circular(Form Ⅱ)comes into existence.However,the characteristic of linear(FormⅢ)was not obviously changed.The complex has the moderate intensity cleavage of pBR322 DNA,which is in agreement with the trend found in fluorescence quenching experiment.

Fig.9 DNA strand break in HeLa cells treated with the complex
2.6 Cytotoxicity in vitro study
The prepared complex was measured in term of the in vitro cytotoxic activity by MTT assay.IC50values estimated for two human tumor cell lines of HeLa and MCF-7 are summarized in Table 3.The complex showed a certain level of anti-tumor activity and cytotoxicity[43].

Table 3 Cytotoxicity of the complex against selected human tumor cells after 72 h of incubation
3 Conclusions
A coordination polymer of{[Mn(Qina)2(Bpp)]·2/3H2O}nwas synthesized and characterized in aspects of FT-IR spectroscopy,elemental analysis as well as single-crystal X-ray diffraction.The properties of the complex in DNA binding were tested via fluorescence spectra,and resultsindicated thatthe complex behaves varied binding affinities in DNA interaction.The cleavage capability of the complex with respect to pBR322 DNA was reviewed by agarose gel electrophoresis,and the results showed that the complex is efficient in DNA cleaving.Through in vitro cytotoxicity experiment,it was testified that the complex possesses a level of toxicity to inhibited Hela and MCF-7 cell lines.
:
[1]Noro S I,Fukuhara K,Kubo K,et al.Cryst.Growth Des.,2011,11:2379-2385
[2]Zhou S B,Wang X F,Du C C,et al.CrystEngComm,2017,19:3124-3137
[3]DENG Qing-Feng(邓青峰),YU Liang-Min(于良民),LI Xia(李霞).Chinese J.Inorg.Chem.(无机化学学报),2017,33(9):1561-1567
[4]Zhang X,Wang N,Liu P F,et al.Polyhedron,2017,129:149-156
[5]Etaiw S E H,Amer S A,Bendary M M E.J.Inorg.Organomet.Polym.Mater.,2011,21:662-672
[6]Taleghani S,Mirzaei M,Eshtiagh H H,et al.Coord.Chem.Rev.,2016,309:84-106
[7]Haque A,Ilmi R,Busaidi I J A,et al.Coord.Chem.Rev.,2017,350:320-339
[8]Guo Y,Xu L,Liu H,et al.Adv.Mater.,2015,27:985-1013
[9]Gao E J,Lin L,Zhang Y,et al.Eur.J.Med.Chem.,2011,46:2546-2554
[10]Singh M,Butcher R J,Singh N K.Polyhedron,2009,28:95-100
[11]Gao E J,Zhu M C,Liu L,et al.Inorg.Chem.,2010,49:3261-3270
[12]Ye J W,Wang Q Q,Gao H Z,et al.Inorg.Chim.Acta,2012,384:1-7
[13]Gao E J,Zhu M C,Zhang W Z,et al.Russ.J.Coord.Chem.,2009,35:621-627
[14]Zhou P,O′Hagan D,Mocek U,et al.J.Am.Chem.Soc.,1989,111:7274-7276
[15]Casolaro M,Cini R,Del B B,et al.Biomacromolecules,2009,10:944-949
[16]Sullivan S T,Ciccarese A,Fanizzi F P,et al.Inorg.Chem.,2009,39:836-842
[17]Caruso F,Rossi M,Benson A,et al.J.Med.Chem.,2012,55:1072-1081
[18]Sun W,Li S,Haupler B,et al.Adv.Mater.,2017,29:1603702[19]Yang P,Klimistavantzis D J.Biol.Trace Elem.Res.,1998,64:275-288
[20]Miller R S,Mildvan A S,Chang H C,et al.J.Biol.Chem.,1968,243:6030-6040
[21]Rico H,Gómez Raso N,Revilla M,et al.Eur.J.Obstet.Gynecol.Reprod.Biol.,2000,90:97-101
[22]Miguel L L.Cancer Lett.,2007,252:1-8
[23]Zhou D F,Chen Q Y,Qi Y,et al.Inorg.Chem.,2011,50:6929-6937
[24]Chen Q Y,Zhou D F,Huang J,et al.J.Inorg.Biochem.,2010,104:1141-1147
[25]Sheldrick G M.Acta Crystallogr.Sect.A:Found.Crystallogr.,2015,A71:3-8
[26]Sheldrick G M.Acta Crystallogr.Sect.C:Cryst.Struct.Commun.,2015,C71:3-8
[27]Liu Q L,Yang L J,Luo Y H,et al.Res.Chem.Intermed.,2016,42:6947-6957
[28]Gao E J,Zhang Y,Lin L,et al.J.Coord.Chem.,2011,64:3992-4005
[29]Rossiter C S,Mathews R A,Morrow J R.J.Inorg.Biochem.,2009,101:925-934
[30]Hsu C P,Kao T K,Chang W L,et al.Eur.J.Surg.Oncol.,2011,37:140-147
[31]Tsuzuki S.Annu.Rep.Prog.Chem.Sect.C:Phys.Chem.,2012,108:69-95
[32]Oszajca M,Nitek W,Rafalska L A,et al.Cryst.Res.Technol.,2015,50:781-790
[33]FENG Chao(冯超),ZHANG Duo(张舵),ZHOU Shi-Yan(周士艳),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(7):1215-1222
[34]Seth S K,Sarkar D,Kar T.CrystEngComm,2011,13:4528-4535
[35]Seth S K.J.Mol.Struct.,2014,1064:70-75
[36]Spackman M M,McKinnon J J.CrystEngComm,2002,4:378-392
[37]McKinnon J J,Jayatilaka D,Spackman M A.Chem.Commun.,2007:3814-3816
[38]Zhu M C,He W X,Gao E J,et al.Life.Sci.,2012,90:519-524
[39]WANG Hui(王慧),GAN Guo-Qing(甘 国 庆),QU Yang(瞿阳),et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28(6):1217-1221
[40]Satyanarayana S,Dabrowiak J C,Chaires J B.Biochemistry,1993,32:2573-2548
[41]YANG Hao(杨浩).Chem.J.Chinese Universities(高等学校化学学报),2007,28(5):872-876
[42]Rajendiran V,Karthik R,Palaniandavar M,et al.Inorg.Chem.,2007,46:8208-8221
[43]SHI Lei(史蕾),YANG Wen-Cong(杨文聪),ZENG Shu-Ying(曾淑莹),et al.Chem.J.Chinese Universities(高等学校化学学报),2016,37(6):1059-1068

