具有同质多晶现象的一维和二维锌Ⅱ配合物的水热合成及对苦味酸的可循环荧光检测性能
李欣書 王 倩*, 丁 斌*,
(1天津市功能分子结构与性能重点实验室,无机-有机杂化功能材料化学教育部重点实验室,天津师范大学化学学院,天津 300387)
(2南开大学先进能源材料化学教育部重点实验室,天津 300071)
0 Introduction
During the last two decades,rational design and syntheses of novel coordination polymers have received great interest because these materials can be widely utilized in gas storage/separation,drug delivery,photoluminescent materials and magnetic materials[1].In general,the successful preparation of coordination polymers can be correlated with judicious selective of multi-functionalligands,secondarybasicbuilding units(SBUs)and various different reaction conditions[2].Generally,flexible links can give rise to materials that demonstrate crystal-to-crystal breathing and complex adsorption effects such as gating,and sensing,where metal-organic frameworks respond to the nature and/or pressure of the adsorbate[3].Link-derived dynamic behavior carefully controls the flexibility of the ligand.Thus,links with limited degrees of freedom have most successfully been employed in the synthesisof flexible metal-organic frameworks[4].The integration of materials displaying advanced framework flexibility with photoluminescent responsive SBUs underlies one approach to sense and respond to small molecule adsorbents[5].
With the development of modern society and industry,hazardous chemicals,like toxic organic small molecules,are increasingly released from industrial facilities and other anthropogenic activities,which cause adverse effects on human health and the environment[6].As a class of toxic and hazardous chemicals,nitro explosives should not be neglected.Picric acid(PA),as one of the common nitro explosives,is extensively applied in dyes,fireworks,and the pharmaceutical and leather industries[7].Due to its frequent use,a large quantity of PA is released to the environment,which may cause serious health problems,such as gene mutation,injury to respiratory organs,anemia,and male infertility.Besides,PA has stronger explosive power than other common nitro explosives.Previously,some studies on luminescence-based detection of PA have been reported,especially with luminescent sensors,indicating the current topic has received great interest[8].Consequently,it is necessary todevelop convenient,fast,and highly efficient methods to detect PA in various samples with regard to environmental monitoring,homeland security,forensic science,and military applications[9].
We also are interesting in the construction of novel metal organic frameworks,which can be utilized as functional materials with intriguing magnetic and photoluminescent properties[10].1,2,4-Triazole,especially its derivatives,have the potential to bridge in bi-dentate and tri-dentate bridging fashions,which are expected to construct novel functional coordination polymers with intriguing structural motifs and novel functional properties[11].On the other hand,tetrazolebased ligands are also a splendid option of N-donor ligands,and the four nitrogen atoms provide enough coordination sites when coordinating to metal centers with certain coordination geometries[12].However,it is noted that the asymmetric semi-rigid bridging N-donor ligands,namely,the ligand possessing different N-containing coordination groups (such as the bifunctional ligands simultaneously containing triazole and tetrazole groups)have rarely been investigated.5-(4-((1H-1,2,4-triazol-1-yl)methyl)phenyl)-1H-tetrazole(HL)simultaneously contains one triazole group,one aromatic benzene group and one tetrazole group.As a matter of fact,HL has six potentially coordinated N atoms in a molecule,which may exhibit various bridging modes.Additionally there also exist flexible dihedral angles between triazole,phenyl and tetrazole aromatic rings within HL (Scheme 1).The conformational freedom of these semi-rigid flexible ligands should provide more possibilities for the construction of coordination polymers with interesting structures and appealing functional properties.
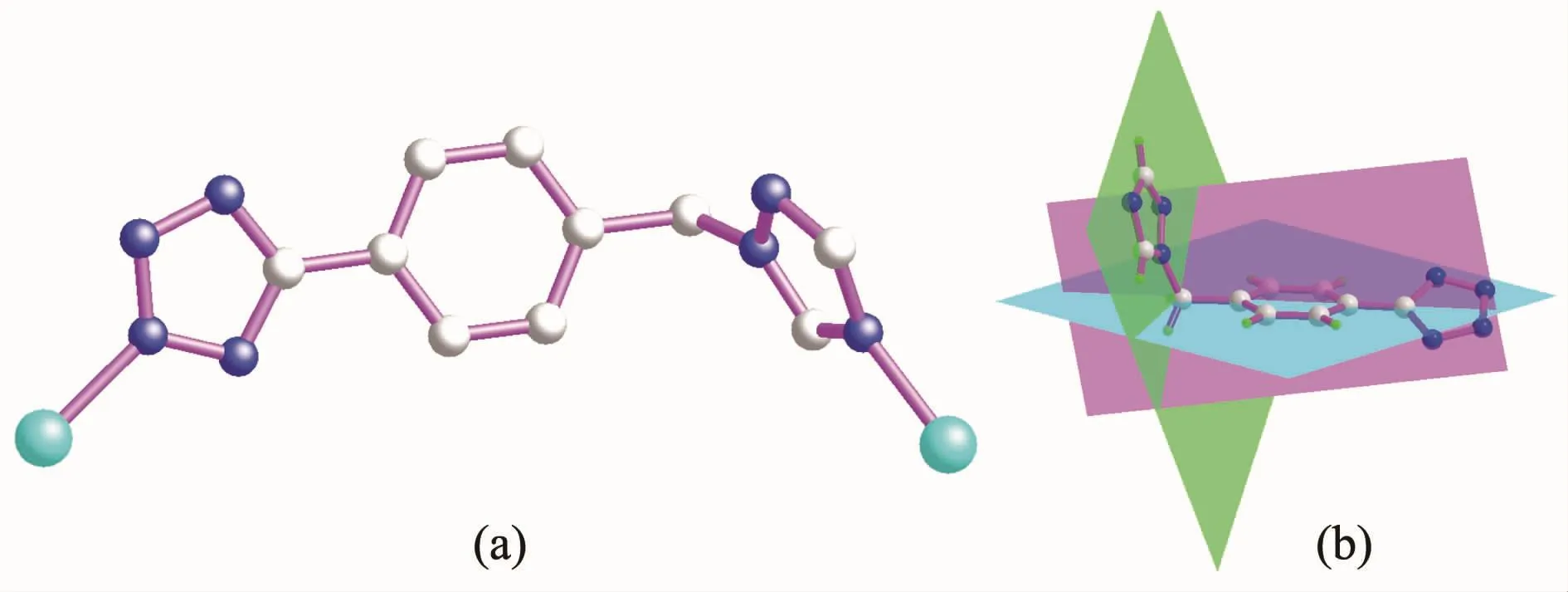
Scheme 1 (a)Bi-dentate coordination mode of flexible multi-dentate HL ligand;(b)Flexible dihedral angles between triazole,phenyl and tetrazole moieties within HL
In this work,the semi-rigid HL containing bifunctional triazole and tetrazole moieties has been employed,and two polymorphic zincⅡcoordination polymers,namely[Zn(μ2-L)2]n(1)and[Zn(μ2-L)2]n(2),have been isolated under the hydrothermal conditions.1 and 2 present temperature induced polymorphic zincⅡ-L 1D (1)and 2D (1)coordination polymers.Further photo-luminescence experiment illustrate that complex 1 exhibits highly sensitive luminescence for PA in aqueous solutions with high quenching efficiency(KSV=3.65×103L·mol-1)and low detection limit(3.004 μmol·L-1,S/N=3),which make it a promising candidate for sensing PA in the measurement process.The detection platform based on 1 has its own advantage in the detecting process of PA since it possess high KSVvalue and low detection limit,additionally the detection method is simple,rapid,cost effectiveness and recyclable.The polymorphic phenomena about the sensing coordination polymer 1 also are scarcely reported.
1 Experimental
1.1 General
Ligand L was prepared according to the literature methods[13].All the other reagents were commercially available and utilized without further purification.Perkin-Elmer 240 elemental analyzer was ulitilized to perform C,H and N microanalyses.FT-IR spectra(4 000~500 cm-1)were recorded by utilizing a NICOLET 6700 FT-IR spectroscope with KBr pellets(NICOLET,USA).Powder X-ray diffraction analysis was performed on a D/Max-2500 X-ray diffractometer using Cu Kα radiation (λ=0.154 1 nm,U=40 kV,I=40 mA)with a 2θ range of 5°~50°.Photoluminescent emission fluorescence spectra were recorded on a RF-5301 spectrophotometer(Shimadzu,Japan).
1.2 Preparation of complexes 1 and 2
A mixture of Zn(NO3)2·6H2O(29.8 mg,0.1 mmol)and HL(44 mg,0.2 mmol)in 10 mL H2O was placed in a Teflon vessel in a steel autoclave,heated at 120℃for 12 h and then cooled to room temperature over 72 h.The resulting colorless block-shaped crystals of 1 were washed several times by water and diethyl ether.Elemental analysis Calcd.for C20H16N14Zn(%):C 46.39,H 3.11,N 37.87;Found(%):C 46.65,H 3.31,N 37.98.FT-IR(cm-1,KBr):3 387(m),3 085(w),2 924(w),1 604(m),1 532(w),1 369(w),1 280(m),1 134(s),999(m),863(m),744(m),700(w),673(w),633(w),569(w).
Complex 2 was synthesized by the same methods as1 exceptthatdifferenthydrothermalreaction temperature 160℃was used.The resulting colorless block-shaped crystals of 2 were washed several times by water and diethyl ether.Elemental analysis Calcd.for C20H16N14Zn(%):C 46.39,H 3.11,N 37.87;Found(%):C 46.68,H 3.38,N 37.96.FT-IR(cm-1,KBr):3 780(m),3 691(w),3 445(s),2 921(m),2 356(s),1 594(m),1 382(m),1 063(w),670(m).
1.3 X-ray crystallography
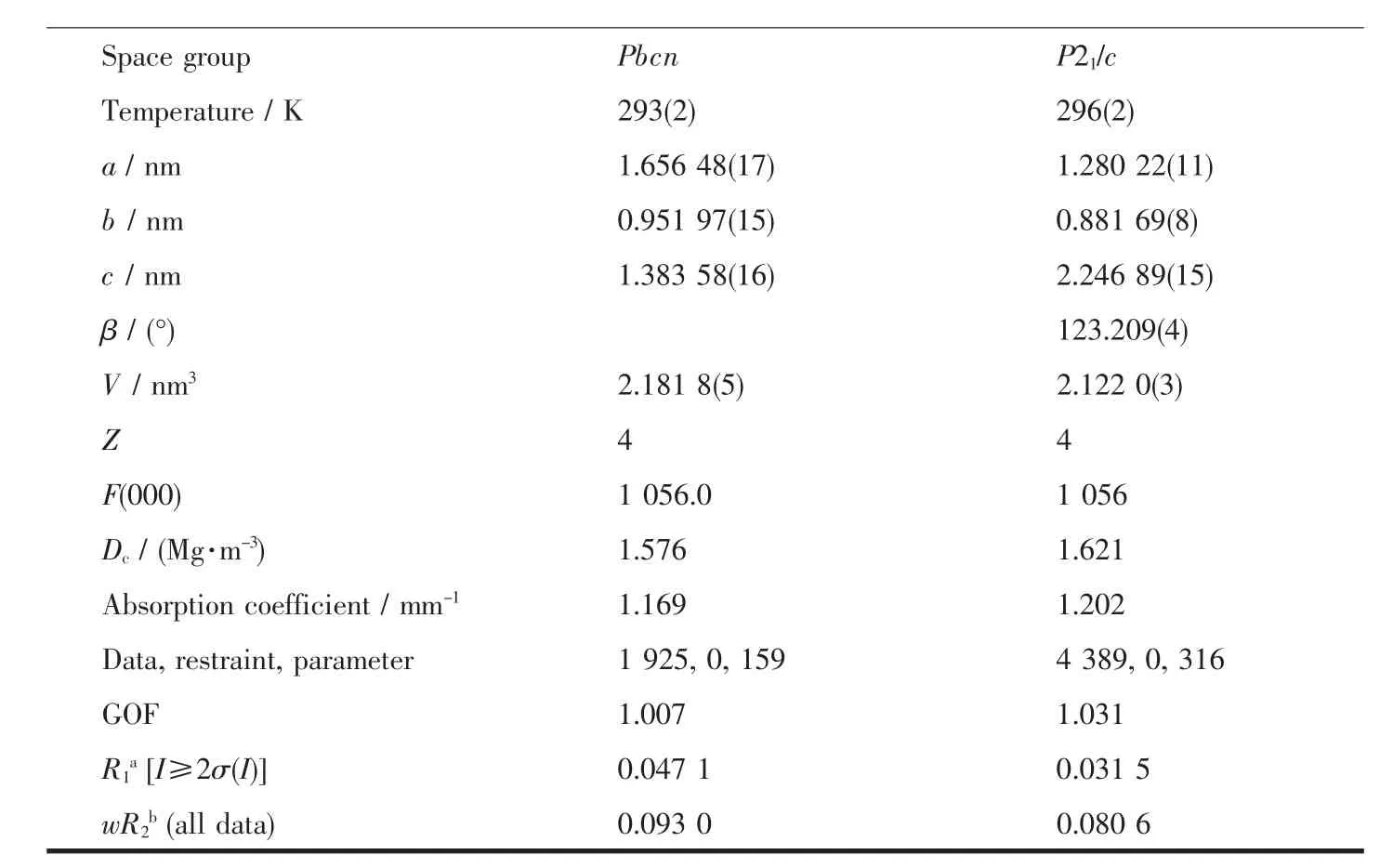
Diffraction intensities for complexes 1 and 2 were collected on a Bruker SMART 1000 CCD diffractometer with graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)by using the ω-φ scan technique.Lorentz polarization and absorption corrections were applied.The structures were solved by direct methods and refined with the full-matrix least-squares technique using the SHELXTL-2013 program[14-15].Anisotropic thermal parameters were assigned to all non-hydrogen atoms.The hydrogen atoms were generated geometrically.The crystallographic data and details of refinements for complexes 1 and 2 are summarized in Table 1.Selected bond lengths and angles are listed in Table 2.Corresponding hydrogen bonds lengths and angles for 1 and 2 are listed in Table 3.
CCDC:1865772,1;1486544,2.

Table 1 Crystal data and structure refinement information for complexes 1 and 2
Continued Table 1
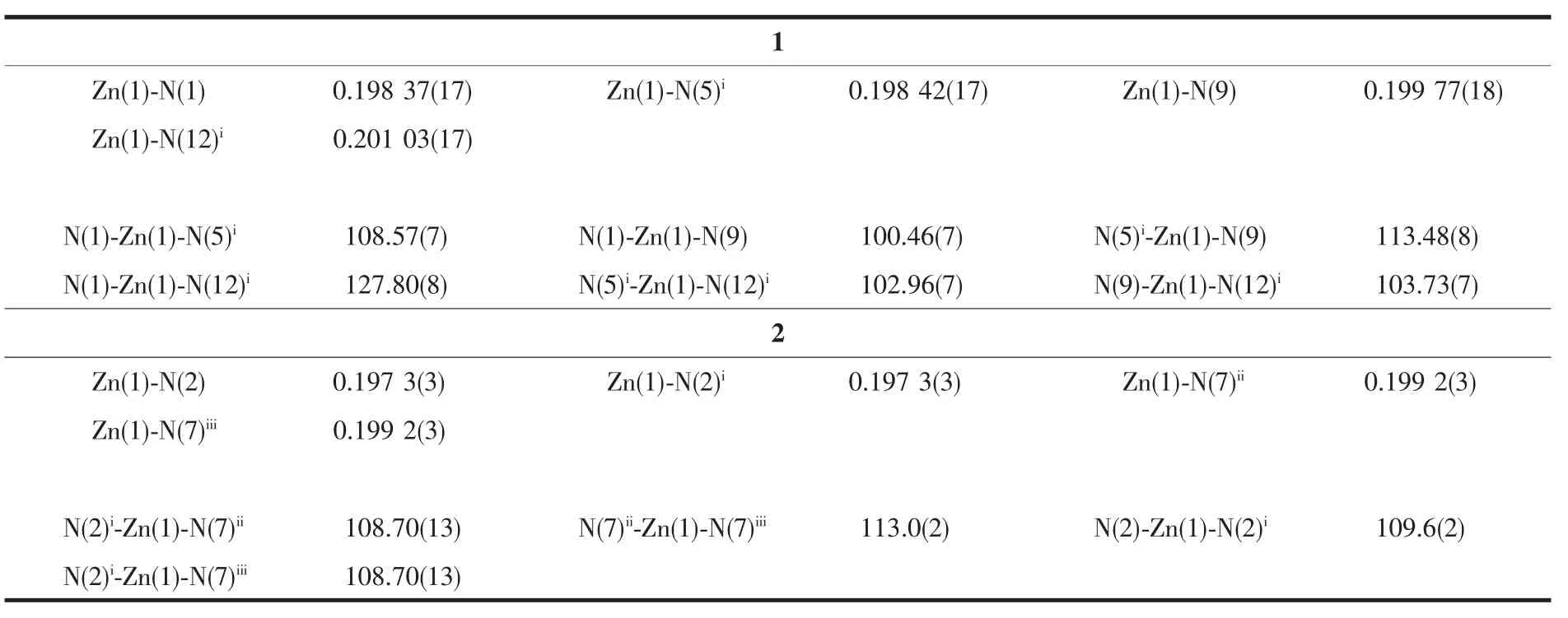
Table 2 Selected bond lengths(nm)and angles(°)of 1 and 2
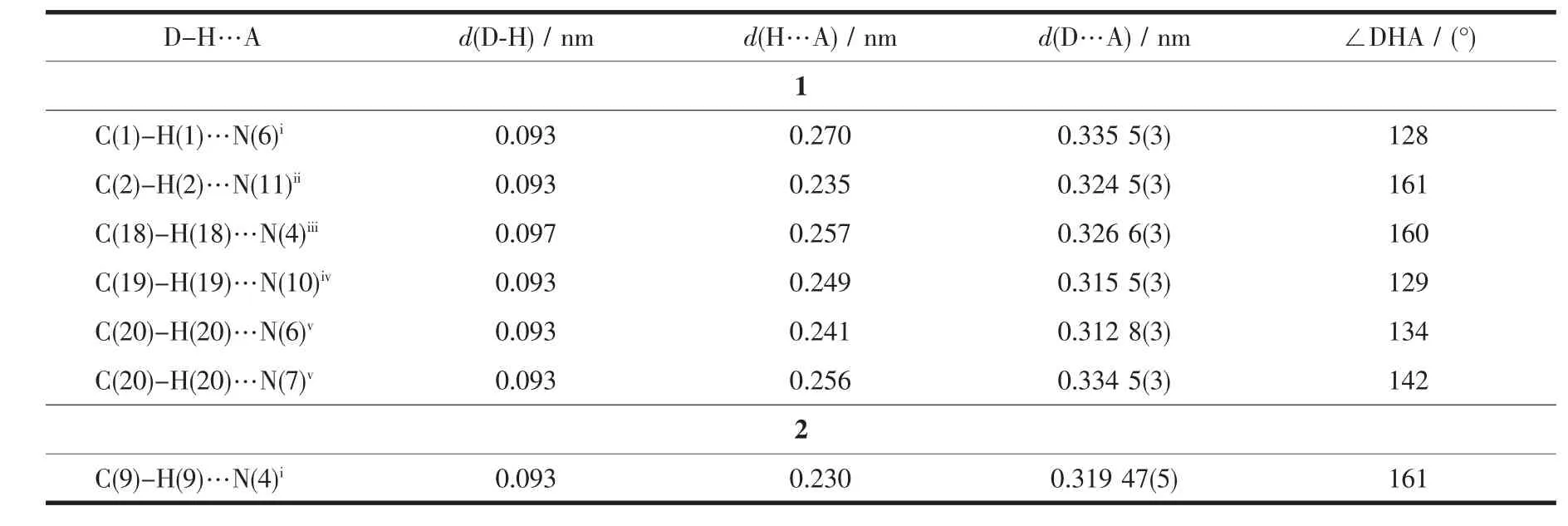
Table 3 Hydrogen bond parameters for 1 and 2
2 Results and discussion
2.1 Syntheses of polymorphic complexes 1 and 2
Complexes 1 and 2 are air-stable and can retain their structural integrity at room temperature for a considerable length of time.Notably,hydrothermal reaction conditions are essential for preparing 1 and 2.HL ligand contains rich nitrogen atoms,and two nitrogen atoms of triazole moieteis and four nitrogen atoms oftetrazole moieteis can participate in coordination.As shown in Scheme 1,the L-ligands in these coordination polymers contain the bi-dentate bridging coordination mode.For 1 and 2,bidentate coordination modes draw the self-assembly of coordination polymers to form 1D and 2D coordination polymers.On the other hand,it is also noted that dihedral angles between triazole,phenyl and tetrazole aromatic rings are also flexible.For example,as listed in Table 4,these dihedral angles between triazole and aromatic benzene rings are 81.01(2)°in 1,while these dihedral angles in the polymorphic complex 2 are 72.08(1)°indicating great change of these flexible dihedral angles.Flexible dihedral angles together with diverse coordination modes make the fact that we could not succeed to anticipate the self-assembly results of L-ligand and metal ions.Therefore the building block of HL has great potential in the construction of these flexible dynamic coordination frameworks[16].

Table 4 Different flexible dihedral angles between triazole,phenyl and tetrazole aromatic rings within the multi-dentate HL ligand for 1 and 2
2.2 Structure of polymorphic zincⅡcoordination polymers[Zn(μ2-L)2]n(1)and[Zn(μ2-L)2]n(2)
Colorless crystals of polymorphic complexes 1 and 2 can be obtained at different hydrothermal reaction temperature (120℃for 1 and 160℃for 2).1 crystallizes in orthorhombic Pbcn space group while 2 crystallizes in monoclinic P21/c space group.Complex 1 is a 1D chain zincⅡ-L coordination framework.As shown in Fig.1 and Fig.2,each symmetric unit of the 1D framework[Zn(μ2-L)2]n(1)contains one ZnⅡ ion in tetrahedral geometry and two bridging L-ligand.The central ZnⅡion is four-coordinated by four nitrogen atoms(N(1),N(5)i,N(9)and N(12)i)from four L-forming ZnN4donor set.The Zn-N distances are 0.198 37(17)~0.201 03(17)nm,and all the N-Zn-N angles are in a range of 100.46(7)°~127.80(8)°.Such coordination geometry is in accordance with a tetrahedral coordinated ZnⅡ center[17].ZnⅡ…ZnⅡdistances across the bridging L-ligand in 1 are 1.213(4)nm.These bridging L-ligands connect the adjacent ZnⅡions forming 1D left-and right-handed helical chains.The helical pitch for these 1D helical chains is 2.302 7(8)nm.It is noted that the C-H…N nonclassical hydrogen bonding interactions(C(1)…N(6)i0.335 5(3)nm,C(2)…N(11)ii0.324 48(3)nm,C(18)…N(4)iii0.326 6(3)nm,C(19)…N(10)iv0.315 5(3)nm,C(20)…N(6)v0.312 72(3)nm,C(20)…N(7)v0.334 39(3)nm)also can be found in 1(Symmetry codes:ix+1,-y+1/2,z+1/2;ii-x+2,-y+1,-z+1;iii-x+1,y-1/2,-z+1/2;ivx-1,-y+1/2,z-1/2;v-x+1,-y,-z),which also further stabilized the 1D coordination chain of 1.
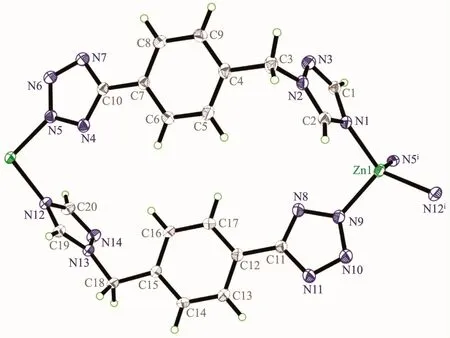
Fig.1 Structure of[Zn(μ2-L)2]n(1)
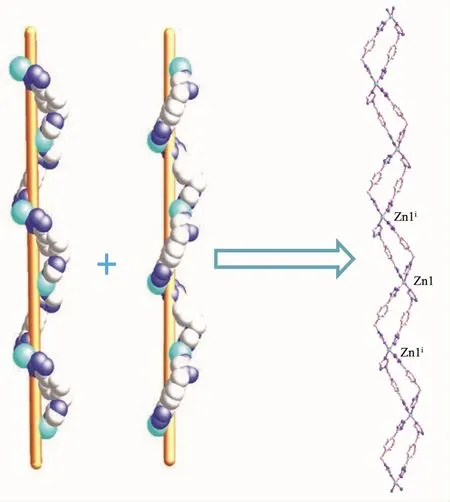
Fig.2 Left-and right-handed single helical chains in[Zn(μ2-L)2]n(1)
Complex2isapolymorphic2D zincⅡ-L framework,in which central ZnⅡions are also linked by the bridging L-ligands(Fig.3).As shown in Fig.3 and 4,The fundamental structural unit of 2 contains one ZnⅡion in tetrahedral geometry and two bridging L-ligands.The central ZnⅡion is four-coordinated by four nitrogen atoms(N(2),N(2)i,N(7)iand N(7)ii)from four L-forming ZnN4donor set.The Zn-N distances are 0.197 3(3)~0.199 2(3)nm,and all the NZn-N angles are in a range of 108.43(13)°~113.0(2)°.Such coordination geometry is in accord with a tetrahedral coordinated ZnⅡ centers.The ZnⅡ…ZnⅡ distance across the bridging L-ligand in 2 is 1.173 0(1)nm.All the N-Zn-N and N-Zn-O angles are in the normal range,such coordination geometry is in accord with a four-coordinated ZnⅡcenters.
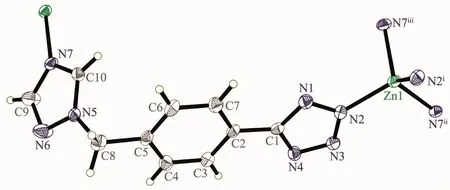
Fig.3 Fundamental structural unit of[Zn(μ2-L)2]n(2)
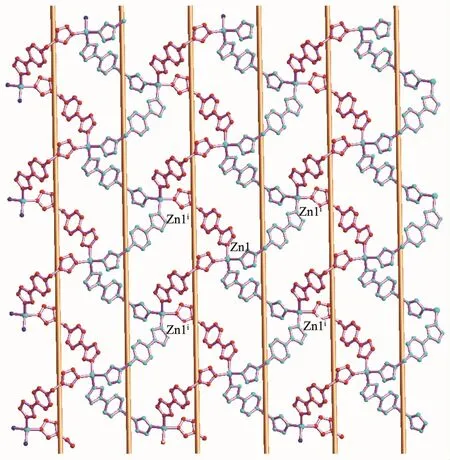
Fig.4 Two dimensional coordination framework of[Zn(μ2-L)2]n(2)containing alternative 1D righthanded and left-handed helical chains
As shown in Fig.4,the 1D left-and right-handed helical chains can also be observed,which are further interlinked via central zincⅡions forming a twodimensional(2D)coordination framework.The helical pitches for these 1D helical chains are 1.382 6(2)nm.It is noted that the C-H…N non-classical hydrogen bonding interactions(C(9)…N(4)i,0.319 47(5)nm)also can be found in 2(Symmetry codes:i-x+1/2,-y+1/2,z+1/2),which also further stabilized the 2D coordination framework of 2.
2.3 Photoluminescent emission spectroscopy of 1 and 2
Inorganic-organic hybrid coordination polymers have been investigated for fluorescence properties and for potential applications as luminescent materials,such as light-emitting diodes (LEDs)[18].Owing to the abilityofaffectingtheemission wavelength and strength of organic materials,syntheses of inorganicorganic coordination polymers by the judicious choice of conjugated organic spacers and transition metal centers can be an efficient method for obtaining new types of electroluminescent materials,especially for d10or d10-d10systems.In the present work,we have explored the luminescentpropertiesofHL and organic/inorganic coordination polymers 1 and 2 based on the ligands in aqueous solutions.
As shown in Fig.5,at ambient temperature,the freeligandsHL in theaqueoussolutionswere luminescent and showed the broad emission maximum at 303 nm (λmax=260 nm).The chromospheres are the aromatic rings and the observed emission is ascribed to π-π*transition.The fluorescence spectra of 1 and 2 at room temperature have been determined.In comparison with that of free HL,the main emission bands of complexes 1 and 2 also almost were located at the same position exhibiting fluorescence(λmax=260 nm)with slightly different band shape,which also should be ascribed to intra-ligand fluorescent emissions.The syntheses of new polymorphic ZnⅡcomplexes with the combination of triazole and tetrzole bifunctional groups can be an efficient method for obtaining new types of luminescent materials.On the other hand,in order to examine the stability of 2 in aqueous solutions,the resulting materials were dispersed in waterfollowed bythe fluorescence intensity measurement at different time intervals.The fluorescence intensity ratio was independent of time and almost remained constant within 12 h indicating 2 can behavegood fluorescentstability.Thegood fluorescent stability results from good solvent stability and dispersibility of complex 2 in aqueous solution.Therefore,2 can be employed asa prominent candidate for fluorescent detection in aqueous solution.

Fig.5 Photoluminescent spectra of HL and complexes 1 and 2
2.4 Photoluminescent sensing PA by complex 2
To explore the potential of 2 toward the sensing ofPA,itsluminescence propertieswere further investigated.The solventsuspension of2 were prepared by placing its finely ground samples(3 mg)into 4 mL of H2O,then PA was further added into the resulting aqueous solutions.As shown in Fig.6a,it is noted that the emission intensities of solvent suspensions were largely dependent on the addition of PA,which exhibits a significant quenching effect,resulting in nearly photoluminescence quenching.These results indicated that complex 2 can be used as a luminescent probe for detecting PA molecules.Moreover,powder XRD analysis of 2 after immersing it in different analytes revealed that the original framework structure is retained.
To further investigate the quenching effect of PA molecules on the luminescence intensity of 2,complex 2 was dispersed in aqueous solutions as the standard emulsion,then the analyst PA gradually increased while the emissive response was monitored.It is obvious that the photoluminescent intensity of2 gradually decreases with the addition of PA(Fig.6b and 6c)[19].For 2,with the addition of 1 mmol·L-1PA to the aqueous emulsion,the corresponding emission intensity is attenuated by approximately 42.15%,and the emission spectra shows 74.3%photoluminescent quenching after the addition of 1.4 mmol·L-1PA.
In order to further display the detection sensitivity,the photoluminescent quenching efficiency can be rationalized using Stern-Volmer(SV)equation:I0/I=KSVcA+1,where I0and I are the suspension luminescence intensities of complex 2 without and with the addition of the analyte,respectively,cAis the concentration of analyte,and KSVis the quenching coefficient.The Stern-Volmer plot for PA is typically linear at low concentrations,and the KSVvalue for PA can be calculated (For 2:3.65×103L·mol-1).The high sensitivity of the photoluminescent response of 2 to PA shows that these ZnⅡcoordination frameworks could be used as the excellent sensors for identifying and quantifying these PA molecules.As we all know,the practical applications of sensors are always restricted,since luminescent probes are costly and can be hard to reuse.Hence,fast and simple regeneration methods are important for luminescent probe applications.Herein,to investigate the recyclable performance of 2,we attempted to immerse 2 in an aqueous solution of 1 mmol·L-1PA for 20 s to completely form 2-PA,and then 2-PA was washed with water several times.Five runs were performed(Fig.6d),while the luminescence intensity and the PXRD pattern of the recycled 2 were wellconsistentwith the original2 (Supporting Information,Fig.S1).The results clearly showed that 2 can be recycled by a fast and simple method,and the framework of 2 still remains intact,indicating that 2 is a recyclable probe for detecting PA[20-21].

Fig.6 (a)Photoluminescent spectra of 2 in the absence and presence of PA with different concentrations;(b)Photoluminescent intensity of 2 at 303 nm in the presence of PA with different concentrations;(c)Linear correlation of luminescence intensity vs PAconcentration;(d)Recyclable photoluminescent detection of PA by 2
Although various analytical and spectroscopic methods have been developed for the detection of PA,a simple and rapid technique using ZnⅡcoordination framework 2 might be an ideal photoluminescent sensing platform,since it seems more attractive by virtue of its high sensitivity,simple operation,rapid response time and cost effectiveness.
3 Conclusions
In conclusion,a flexible bi-functional multidentate 5-(4-((1H-1,2,4-triazol-1-yl)methyl)phenyl)-1H-tetrazole(HL)has been employed,to obtain two novel polymorphic one-and two-dimensional zincⅡcoordination polymers,namely[Zn(μ2-L)2]n(1)and[Zn(μ2-L)2]n(2).1 and 2 present temperature induced polymorphic zincⅡ-L 1D(1)and 2D(2)coordination frameworks.Furthermore,the luminescence properties of 1 and 2 have been investigated,indicating strong photoluminescent emissions.Additionally,photoluminescent measurements illustrate that complex 2 exhibits highly sensitive luminescence for PA in aqueous solution with high quenching efficiency(KSV=3.65×103L·mol-1)and low detection limit(3.004 μmol·L-1,S/N=3).The results also reveal great potential in the construction of these flexible frameworks employing these semi-rigid multi-dentate ligands as basic building blocks.On the basis of this work,the syntheses,structures and properties studies of these coordination polymers using HL as basic building blocks are also under way in our laboratory.
Supporting information is available at http://www.wjhxxb.cn

