铜Ⅱ和镍ⅡSalamo型配合物的合成、晶体结构、Hirshfeld 表面分析、热稳定和荧光性质
苏 琼 赵 青 安晓欣 王彦斌*, 李肖妍 董文魁*,
(1西北民族大学化工学院,甘肃省高校环境友好复合材料及生物质利用省级重点实验室,兰州 730030)
(2兰州交通大学化学与生物工程学院,兰州 730070)
More recently,the preparation of a series of metal complexes has attracted considerable attention in modern coordination chemistry.Salen (R-CH=N-(CH2)n-N=CH-R)and its derivatives have been widely studied by many organic as well as inorganic chemists[1-3],because Salen-type ligands containing tetradentate [N2O2]coordination site can form stable alkaline earth,rare earth,and transition metal complexes for several decades[4-7].Moreover,Salen-like ligands and their corresponding metallic complexes have excellent potentialapplicationsin many aspects,such as biological systems[8-15],electrochemical conducts[16-18],supra-molecular buildings[19-26],luminescence properties[27-33],catalysis[34-36],magnetic materials[37-44],nonlinear optical materials[45]and ions recognition[46-51].
Recently,a new class of Salamo(-CH=N-O-(CH2)n-O-N=CH-)ligands containing[N2O2]donor have been exploited[52-56]. The studies previously showed that Salamo-type complexes are relatively stable at least 10 000 times than Salen-like complexes[57].Based on our previous reported,Salamo-type metal complexes also have been applied in supramolecular architectures[58-59],organic reaction catalysts and luminescence properties[60-63].Coumarin,as important materials and intermediate product of organic chemicals,has been widely used in medicine,agriculture,photosensitive materials and other fields.It is noteworthy that coumarin has good characteristics of luminescence[64-65].
Based on the above research,we have devised and prepared a ligand H2L containing coumarinskeleton and its corresponding mononuclear CuⅡand trinuclear NiⅡSalamo-type complexes.Furthermore,thefluorescentproperties,thermalstabilitiesand Hirshfeld surface analyses of complexes 1 and 2 were also studied in detail.
1 Experimental
1.1 Materials and physical measurements
4-(N,N′-Diethylamino)salicylaldehyde(99%)and 7-hydroxyl-4-methylcoumarin (98%)were purchased from Alfa Aesar and used directly without purification.All other solvents and chemicals were analytical grade and obtained from Tianjin Chemical Reagent Factory.ElementalanalysesdataofC,H and N were performed on a GmbH VarioEL V3.00 automatic elemental analysis instrument.Elemental analyses for copperⅡ and nickelⅡ were detected with an IRIS ER/S-WP-1 ICP atomic emission spectrometer.Melting points were measured with a microscopic melting point apparatus made by Beijing Taike Instrument Limited Company and were uncorrected.Fourier transform IR spectra were recorded with a VERTEX70 FT-IR spectrophotometer,with samples prepared as KBr(500~4 000 cm-1)and CsI(100~500 cm-1)pellets.UVVisible absorption spectra were collected on a Shimadzu UV-3900 spectrophotometer.Fluorescent spectra were recorded on F-7000 spectrophotometer.Single crystal X-ray structure determinations were determined by a SuperNova Dual,Cu at zero,Eos four-circle diffractometer.Thermal stabilities analyses were carried out at a heating rate of 10℃·min-1on a ZRY-1P thermoanalyzer.Hirshfeld surface analyses and two-dimensional fingerprint plots were calculated using CrystalExplorer program.
1.2 Synthesis of ligand H2L
1,2-Bis(aminooxy)ethane and 8-formyl-7-hydroxy-4-methylcoumarin were prepared in accordance with the previously literature[36,61,66].Major synthetic route of the ligand H2L are presented in Scheme 1.

Scheme 1 Synthetic route to H2L
4-(N,N′-Diethylamino)salicylaldehyde(483.1 mg,2.5 mmol)dissolved in ethanol solution (25 mL)was added slowly to a colorless ethanol solution(50 mL)of 1,2-bis(aminooxy)ethane(322.4 mg,3.5 mmol)more than 3 h.Subsequently,the above solution was heated at 52~55℃ about 5.5 h.The solution was concentrated by vacuum distillation.Then,the column chromatography isolation for reaction mixture was carried out with ethyl acetate/chloroform (1∶20,V/V)and gained 550.2 mg expected intermediate product 2-(O-(1-ethyloxyamide))oxime-5-(N,N′-diethylamino)phenol.
8-Formyl-7-hydroxy-4-methylcoumarin(306.3 mg,1.5 mmol)was dissolved in the ethanol solution(15 mL).The above solution was added to the stirred colorless ethanol solution (15 mL)of the obtained intermediate product (393.1 mg,1.5 mmol).The mixture was refluxed and stirred at 80~82 ℃ about 6 h before being allowed to cool to room temperature.Then the suspension solution was filtered and washed with ethanol/hexane(1∶4,V/V).After dried under vacuum,the resulting white solid ofH2L was collected.Yield:61.6%.m.p.148~149 ℃ .Anal.Calcd.for C24H27N3O6(%):C 63.56;H 6.00;N 9.27.Found(%):C 63.62;H 6.09;N 9.18.
1.3 Synthesis of complex 1
A methanol solution (2 mL)of copperⅡacetate dihydrate (1.99 mg,0.01 mmol)was mixed to an acetonitrile solution (2 mL)of H2L (4.53 mg,0.01 mmol).The mixture was stirred further at room temperature for 30 min,and the color of the solution turned dark green.After that the solution was filtered and the filtrate was left undisturbed at room temperature for approximately one week.Clear dark brown blockshaped crystals suitable for X-ray measurement were obtained.Yield:40.5%.Anal.Calcd.for C24H25CuN3O6(%):C 55.97;H 4.89;N 8.16;Cu 12.34.Found(%):C 56.08;H 4.96;N 8.09;Cu 12.27.
1.4 Synthesis of complex 2
A methanol solution (2 mL)of nickelⅡacetate tetrahydrate (7.47 mg,0.03 mmol)was completely added to an acetonitrile solution(2.0 mL)of H2L(9.07 mg,0.02 mmol).Then the color of the mixed solution became light green and was stirred for 20 min at room temperature.After that the mixture was filtered,and the resulting filtrate was stand undisturbed for about two weeks at room temperature.Clear light green block-like single crystals for X-ray crystallographic analysis were collected.Yield:45.2%.Anal.Calcd.for C56H72Ni3N6O20(%):C 50.75;H 5.48;N 6.34;Ni 13.29.Found(%):C 50.82;H 5.56;N 6.26;Ni 13.19.
1.5 Crystal structure determinations of complexes 1 and 2
Suitable crystals of complexes 1 and 2 with approximate dimensions of 0.15 mm×0.18 mm×0.21 mm and 0.17 mm×0.21 mm×0.23 mm,respectively,were mounted on glass rod for determining single crystal structures.X-ray diffraction data of complexes 1 and 2 were performed on a SuperNova(Dual,Cu at zero,Eos)diffractometer using a graphite monochromated Mo Kα radiation source(λ=0.071 073 nm)at 293.53(10)K, respectively. Multi-scan absorption corrections were applied.The crystal structures were solved using direct methods and refined anisotropically by the full-matrix least-squares techniques based on F2with SHELX-2014 program[67].The positions for hydrogen atoms were fixed on geometrically idealized positions and refined via a riding model.The non-hydrogen atoms were refined anisotropically with displacement parameters.Selected collection parameters and structure refinementofcomplexes1 and 2 are summarized in Table 1.
CCDC:1869995,1;1869996,2.

Table 1 Crystal and refinement parameters for complexes 1 and 2

Continued Table 1
2 Results and discussion
2.1 Crystal structure of complex 1
X-ray diffraction analysis demonstrated that complex1 possessesan asymmetricmononuclear structure and crystallizes in the monoclinic system with P21/c space group.Asymmetric unit of complex 1 is composed of one CuⅡion and one fully deprotonated L2-unit.The molecular structure with atomic labeling of complex 1 is depicted in Fig.1.Selected bond lengths and angles of complex 1 are listed in Table 2.

Fig.1 (a)Molecular structure of complex 1 with 50%probability displacement ellipsoids;(b)Coordination polyhedrons for the CuⅡion
The CuⅡ ion in the[N2O2]donor coordination sphere from full deprotonated L2-unit is four-coordinated,which is bonded to two oxygen atoms(Cu1-O1 0.192 6(2)nm,Cu1-O6 0.189 4(2)nm)of the phenolic groups and two nitrogen atoms(Cu1-N1 0.199 5(3)nm,Cu1-N2 0.194 5(3)nm)of the oxime groups.Consequently,the coordination geometry of CuⅡ ion could be best described as a distorted square planar geometry with[CuN2O2]coordination and deduced by using τ4index(τ4=0.169)[68](Fig.1b).Moreover,the coordination planes of N1-Cu1-N2 and O1-Cu1-O6 have a dihedral angle of 17.593°,which has a nonplanar configuration.Based on the above data,the distances of Cu1 atom and phenolic oxygen(O1 and O6)atoms of the fully deprotonated L2-unit are clearly shorter than those of the nitrogen (N1 and N2)atoms from Salamo-type moiety of H2L.The angle of N-Cu1-O is in a range of 88.45(10)°~173.65(11)°,and the angle of O1-Cu1-O6 and N1-Cu1-N2 are 87.03(9)°and 94.73(11)°,respectively(Table 2).
It is worthy to note that important supramolecular interactions exist in complex 1.The major hydrogen bonds and C-H…π interactions in complex 1 are listed in Table 3.Two intramolecularhydrogen bonding(C10-H10…O3,C11-H11A…O2#1)and one intermolecular hydrogen bonding(C11-H11B…O6#2)interactions form an infinite 1D chain structure[24,58](Fig.2).There is only one C-H…π (C21-H21A…Cg1#1,Cg1:C1-C2-C3-C4-C8-C9)interaction.A complicated 3D supramolecular architecture is formed through the interplay of hydrogen bonding and C-H…π interactions(Fig.3)[25,59].

Fig.2 View of the hydrogen bonding interactions of complex 1

Table 2 Selected bond distances(nm)and angles(°)for complexes 1 and 2

Table 3 Hydrogen bonding and C-H…π interaction of complexes 1 and 2

Fig.3 Three dimensional supramolecular architecture of complex 1 constructed by hydrogen bonding and C-H…π interactions
2.2 Crystal structure of complex 2
The molecularstructure ofcomplex 2 was determined by X-ray crystallographic and atom numbering ispresented in Fig.4.Selected bond lengths and angles of complex 2 are listed in Table 2.
Single crystal X-ray diffraction analysis indicated that the crystal structure of complex 2 is very different from complex 1,forming a new 2∶3 (molar ratio of L2-to NiⅡ)trinuclear structure.Complex 2 crystallizes in the triclinic system space group of P1 and is made up of three NiⅡ ions,two μ-acetate anions,one coordinated methanol molecule,two fully deprotonated L2-units and one lattice methanol molecule.
As depicted in Fig.4,two symmetrically equivalent terminal six-coordinated NiⅡions(Ni1 or Ni1#1)in[N2O2]cores of the two Salamo-type ligands,are bound by four donor atoms(N1,N2,O1,O6 or N1#1,N2#1,O1#1,O6#1)from the unsymmetrical fully deprotonated L2-units(Ni1-N1 0.207 6(4)nm,Ni1-N2 0.206 7(3)nm,Ni1-O1 0.204 8(3)nm,Ni1-O6 0.202 0(3)nm),one oxygen atom(Ni1-O9 0.208 7(3)nm)from coordinated methanol molecule and one oxygen atom(Ni1-O7 0.202 4(3)nm)from a μ-acetate anion.Therefore,the terminal NiⅡions (Ni1 or Ni1#1)form the slightly distorted octahedral geometries.Meantime,the central Ni2 ion is surrounded by six oxygen atoms:four phenolic oxygen atoms(Ni2-O1 0.210 4(3)nm,Ni2-O6 0.204 4(3)nm,Ni2-O1#1 0.210 4(3)nm,Ni2-O6#1 0.204 4(3)nm)arising from two fully deprotonated L2-units and two oxygen atoms(Ni2-O8 0.210 7(3)nm,Ni2-O8#1 0.210 7(3)nm)of two μ-acetate anions.Consequently,the central Ni2 ion is also six-coordinated possessing the geometry of a slightly distorted octahedron.The angle of N1-Ni1-N2 is 106.53(16)°,and Ni1-O-Ni2 is in a range of 96.27(12)°~99.09(11)°.The Ni1…Ni2 distance is 0.309 2(4)nm and the length of two terminal Ni1…Ni1#1 is 0.618 4(4)nm(Table 2).
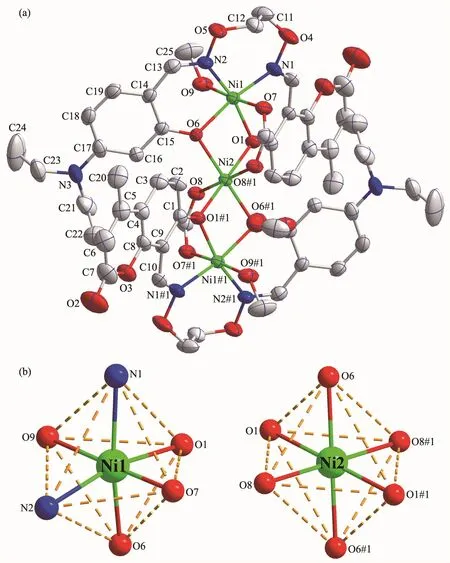
Fig.4 (a)Molecular structure of complex 2 with 50%probability displacement ellipsoids;(b)Coordination polyhedrons for the NiⅡions
Supramolecular architecture of complex2 is connected by extensive hydrogen bonding and C-H…π interactions,which plays an important role in the crystal packing modes.Three intramolecular hydrogen bonding interactions(C10-H6…O3#1,C12-H12A…O7#1 and C16-H16…O8#1)are observed(Fig.5)[24,58].In addition,2D supramolecular architecture is built by one C-H…π (C20-H20A…Cg2#3,Cg2:O3-C7-C6-C5-C4-C8)interaction (Fig.6).With the help of hydrogen bonding and C-H…π interactions,adjacent molecular units are linked together to form an infinite 3D supramolecular structure shown in Fig.7[25,59].

Fig.5 View of the intramolecular hydrogen bonding interactions of complex 2

Fig.6 View of 2D supramolecular architecture of complex 2 constructed by C-H…π interactions

Fig.7 Three dimensional supramolecular architecture of complex 2 constructed by hydrogen bonding and C-H…π interactions
2.3 FT-IR spectra
Infrared spectra were measured at various bands within a region of 4 000~500 cm-1(Fig.8),which confirms the presence of H2L and its complexes 1 and 2.Major infrared bands are delineated in Table 4.It can be seen from the IR spectra,the phenolic O-H stretching vibration band of H2L exhibited a characteristic absorption at 3 231 cm-1.However,this band was not emerged in complex 1,which indicated that the phenolic hydroxyl groups of H2L are fully deprotonated.This band disappeared,and a new O-H stretching vibration band of the coordinated methanol molecule was seen at around 3 443 cm-1[27].The expected Ar-O vibration band of H2L was observed at ca.1 286 cm-1;nevertheless,this band occurred at 1 241 and 1 244 cm-1in complexes 1 and 2,respectively,shifting to low frequencies[32].The observed band at 1 613 cm-1in H2L is assigned to a characteristic C=N vibration.Meanwhile,the C=N vibration bands of complexes 1 and 2 displayed at 1 596 and 1 595 cm-1,respectively.The frequencies in complexes 1 and 2 moved towards lower frequencies region due to the coordination between nitrogen atoms of the C=N group and the metal ions[40].A typical C=O vibration band in the H2L emerged at 1 731 cm-1,and those of complexes 1 and 2 were obtained at approximately 1 715 and 1 724 cm-1,respectively[65].Upon complexation,M-N/O interactions were formed in the IR spectra.Most important of all,the appearance of new medium intensity bands for complexes 1 and 2 at approximately 529 and 539 cm-1are attributed to M-N stretching vibration band and the bands at 456 and 462 cm-1are assigned to M-O vibration band[65].The facts mentioned above are in accordance with the results of crystal X-ray diffractions.
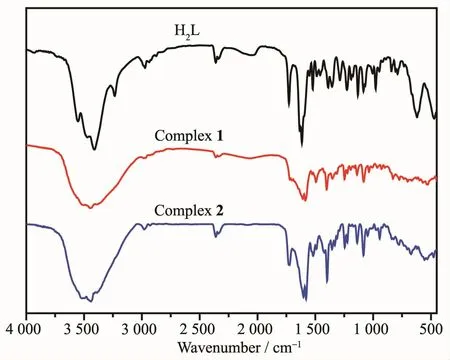
Fig.8 FT-IR spectra of H2L and complexes 1 and 2

Table 4 Main data of IR spectra of H2L and complexes 1 and 2
2.4 UV-Vis absorption spectra
UV-Vis spectrum of H2L in methanol solution(25 μmol·L-1)and its corresponding complexes 1 and 2 in methanol solution (1 mmol·L-1)were measured within a range of 220~550 nm (Fig.9).Clearly,the UV-Vis spectrum of H2L had three relatively intense absorption peaks at 289,328 and 356 nm,respectively.The former peak at 289 nm could be assigned to the π-π*transition for the benzene rings;the second peak at 328 nm could be appointed to π-π*transition for the oxime group;and the third peak at 356 nm could be assigned to the n-π*transitions for carbonyl group[32,64].
In the titration experiment of complex 1,the gradual addition of Cu(OAc)2to H2L solution caused the color variation from colorless to dark brown.In contrast to H2L,the absorption peaks were bathochromically shifted[64].The phenomena above are owing to the reaction of H2L with CuⅡ ions.When CuⅡ ions were added in excess (cCu2+/cH2L>1),the absorbance remained relatively stable.Quite evidently,the spectroscopic titration of H2L and CuⅡshows the formation of a 1∶1 mononuclear CuⅡ complex(Fig.9a).
Similar change was also seen in the titration experiment of complex 2.Obviously,the spectroscopic titration of H2L and NiⅡdisplayed the formation of a 2∶3 trinuclear NiⅡ complex,giving the similar results and presented in Fig.9b.

Fig.9 (a)UV-Vis spectral changes of complex 1 of H2L in methanol by the addition of Cu(OAc)2in methanol;(b)UV-Vis spectral changes of complex 2 of H2L in methanol by the addition of Ni(OAc)2in methanol
2.5 Fluorescence properties
The fluorescence behaviorsofH2L and its corresponding complexes 1 and 2 were measured in methanol solution (25 mmol·L-1)at room temperature in a wavelength range of 350~600 nm and displayed in Fig.10.The spectra of H2L exhibited an intense emission peak at 416 nm upon excitation at 350 nm,which should be attributed tointra-ligand π-π*transitions owing to the existence of lone pair electrons of the donor atoms (N,O-donor)[30,61].While the spectra of complexes 1 and 2 displayed two relatively weak emission peaks at 422 and 435 nm upon excitation at 350 nm,respectively.The emission peaks of complexes 1 and 2 were bathochromically-shifted compared to those of H2L,which can be attributed to L-M charge-transfer transitions(LMCT)resulting from the coordination of nitrogen and oxygen atoms to metal(Cu and Ni)ions[31,62].

Fig.10 Emission spectra of H2L and its complexes 1 and 2 in methanol solution
2.6 Hirshfeld surface analyses
The Hirshfeld surfaces of H2L and its complexes 1 and 2 were analyzed using Crystal Explorer.The surface analyses of H2L and its complexes 1 and 2 mapped with dnorm(standard high resolution),curvedness and shape index,are illustrated in Fig.11[69].
When mapped with the function of dnorm,the surface ofH2L produces several sphericalred depressions indicating that there are mainly C-H…O interactions,and other visible depressions correspond to H…H and C…H interactions.For complexes 1 and 2,more intense red depressions are seen on the Hirshfeld surfaces,which marks the O…H/H…O interactions persisting.Two dimensional fingerprint plots are used to explain the atom pair contacts of the crystal, which can quantify the intermolecular interactions.Meantime,it could be decomposed to highlight contributions from different interactions.The 2D fingerprint plot of H…H,C…H/H…C and O…H/H…O interactions in H2L and complexes 1 and 2 are depicted in Fig.12.For each point on the Hirshfeld isosurface,two distances,de(the distance from the point to the nearest nucleus external to the surface)and di(the distance to the nearest nucleus internal to the surface),are defined.The proportions of C…H/H…C,O…H/H…O and H…H interactions in H2L cover 10.3%,11.7%and 48.1%of the Hirshfield surfaces,respectively.However,the proportions of C…H/H…C,O…H/H…O and H…H interactions in complexes 1 and 2 cover 10.6%,13.5%,44.1%and 11.0%,12.1%,54.1%of the Hirshfield surfaces,respectively.It is due to the existence of hydrogen bonds that complexes 1 and 2 can be more stable[70].
2.7 Thermal properties
TG curves of complexes 1 and 2 are shown in Fig.S1(Supporting Information).The TG analysis of complex 1 indicated that a weight loss occurred in a temperature range of 168~179 ℃,which is due to the breakdown of ligand H2L.Between 198 and 650℃,TG curve of 1 displayed overall estimated weight loss of 83.7% (Calcd.84.6%),indicating the completely decomposition of ligand molecule and the formation of CuO(Calcd.15.4%,Obsd.16.3%)[62].
Complex 2 was thermally decomposed in three steps.An initial weight loss of 3.1% (Calcd.2.4%)was observed in a temperature range of 70~85 ℃,which corresponds to the loss of one lattice methanol molecule.The second step of decomposition was noticed in a temperature range of 150~230 ℃,which is due to the elimination of two μ2-acetate ions and two coordinated methanol molecules.Meanwhile,the weight loss obtained in this step was 13.1%against the calculated loss of 13.7%.Finally,the third step was between 245 and 650℃ with approximately 66.2%weight loss (Calcd.67.0%),indicating the

Fig.11 Hirshfeld surfaces of H2L,complexes 1 and 2 mapped with dnorm,shape index and curvedness

Fig.12 Two dimensional fingerprint plots of H2L,complexes 1 and 2 with the major decomposition plots
decomposition of ligand molecule and the remaining product weight seems likely to correspond to NiO with a value of 17.6% (Calcd.16.9%).The overall estimated weight loss amounts to 82.4%[62].
3 Conclusions
In summary, the syntheses and structural characterization ofa new containing coumarinskeleton ligand H2L and its two new mononuclear CuⅡand trinuclear NiⅡSalamo-type complexes,with the general chemical formulae of[CuL](1)and[Ni3(L)2( μ-OAc)2(CH3OH)2]·CH3OH(2),were described in the present paper.Single-crystal X-ray diffractions have established their structures. Abundant hydrogen bonding and C-H…π supra-molecular interactions exist in complexes 1 and 2.The fluorescent results of complexes 1 and 2 showed relatively weak emission peaks compared to H2L.The Hirshfeld surfaces and 2D fingerprintplotscan explain the atom pair contactsofthe crystal,which can quantifythe intermolecular interactions.
Acknowledgements:This work was supported by the National Natural Science Foundation of China (Grants No.51563022,21761018)and the Program for Excellent Team of Scientific Research in Lanzhou Jiaotong University (Grant No.201706),which are gratefully acknowledged.
Supporting information is available at http://www.wjhxxb.cn

