发光碳纳米点的带隙调控及应用
张博涵,田 震,李 迪,周 鼎,鲍 鑫,周正杰,曲松楠,3*
(1.中国科学院长春光学精密机械与物理研究所发光学及应用国家重点实验室,吉林长春 130033;2.中国科学院大学,北京 100049; 3.澳门大学应用物理与材料工程研究所,中国澳门 999078)
1 Introduction
Carbon-based materials are known as the key materials for the continuation of silicon-basedmaterials in the post-Moore era.After fullerenes,carbon nanotubes and graphene,the carbon dots(CDots),as the latest form of nanocarbon,have received great attention in their own right.CDots are amorphous carbon-based nanoparticles whose sizes less than 10 nm.Usually they have various kinds of surface functional group,and this can help them maintain colloidal stability in a wide range of solvents.Generally,CDots have short wavelength emission in blue and green regions.Recently it has been reported that the CDots’emission range has been extended to nearinfrared(NIR)spectral ranges.Li et al.synthesized the CDots with the emission between 900-1 200 nm under the excitation of 808 nm laser by using watermelon juice as raw material,which is the longest emissive wavelength of CDots[1].As a new kind of fluorescentmaterial with promising expectation,CDots have a wide range of applications in many fields,in particular in bioimaging,gene transfer and drug delivery[2]due to its biocompatibility and good cell membrane permeability.CDots also exhibit tunable emission,high photostability,chemical stability,which renders them attractive applications in sensor[3-4],photocatalysis[5-8],lasing and optoelectronic devices.In addition,the luminescent CDots have the advantages of broad precursors,low cost and environmental protection,which have been regarded as another important carbon nanomaterial.
The origin of CDots can be traced back to 2004.Xu et al.isolated and purified single-walled carbon nanotube,found a carbonaceous material with fluorescent properties.Its sizewas confirmed by atomic forcemicroscopy(AFM)[9].In 2006,Sun et al.synthesized a carbon nanoparticle by using a multi-step process of laser ablation of carbon targets.The nanoparticle had good luminescence property and named carbon dot[10].From the first article about CDots to the present,there is an explosive growth in the number of reports devoted to thesematerials.Approaches for synthesizing CDots can be generally classified into two main groups:top-down and bottom-up methods.Usually top-down methods are used in the early days,including arc discharge[11-12],laser ablation[10],electrochemical oxidation[13-16]and ECT.The CDots are produced by breaking off bulk graphite precursors until nano size,which usually has low photoluminescence quantum yield(PLQY)and hard to control particle size.Thus more andmore researches are focused on the bottomup methods to synthesize CDots.Bottom-up approaches usually consist of heating,microwave assisted[17-20]or hydrothermal treatmentmethod,in which the CDots are formed from molecular precursors.Typically there aremany byproducts and need to be further purified by using centrifugation,dialysis or another separation techniques.The bottom-up method can utilize almost all the organic material as precursor and the synthesized CDots are easy to modification.Many studies focused on the bottom-up method to achieve optimization synthetic conditions for producing CDots with better characteristic.Among all these efforts,citric acid(CA)probably becomes the most widely used precursor to prepare CDots.
After unremitting research and exploration,several approaches have been found to produce CDots with improved luminescent behaviors.For instance,the PLQY of CDots has been enhanced by various methods as surface passivation,surface reduction and metal coating.It is reported that Liu et al.prepared CDots with the PLQY reaching to 94.5%by using folic acid(CA)as precursor via hydrothermal method,which is the highest PLQY of CDots at present[21].However, there are many issues remained.The high PLQYs were limited in blue or green blue spectral ranges,the inefficient emission of CDots in red and NIR regions limits the development and application.In addition,aggregation induced fluorescence quenching occurs in solid state of CDots.In order to solve these problems,it is very necessary to understand the PL origin of CDots.However,agreement can't be reached on the conclusions regarding to the luminescence mechanisms.Because CDots are synthesized by various precursors via different methods,this causes a huge variation among them.When the CDots with different synthesis conditionswere studied together,the PLmechanism was not convincing due to uncertain factors influencing the luminescence processes.
The accurate structures and photoluminescence(PL)mechanisms of CDots are complicated and blurred.It is generally accepted that the intrinsic(bandgap related)transitions of CDots(including GQDs)are driven by the graphitic core size composed of conjugated sp2-domains,which is the real domination of quantum confinement effect.Theoretical calculations and relative reported experimental results have demonstrated that the bandgap of CDots can be tuned by modulating the size of conjugated sp2-domains.At the same time,surface state and surface groups on CDots,especially oxygen related functional groups,such as carboxyl and hydroxyl,can also modify the bandgap.Through this method long wavelength emission CDots can be obtained,and it is also beneficial for determining accurate structures and PLmechanisms of CDots.
For the entire research field of CDots,the PL origin and bandgap modulation are essential for follow-up studies.The presence of bandgap modulation reveals amethod to in-depth study and promotes research progress.The determination of PL origin can maximum optimize the luminescence performance,make CDots have a broader application prospect.The first section of this review summarizes photoluminescence origins of CDots,and then analyzes bandgap modulation by particle sizemodification and surface structure engineering respectively.This is followed by introduction of the enhancement of PLQY,then introduces CDots’the most effective application in optoelectronic devices,information storage,bioimaging and cancer treatment.At last the review concludes with a prospect of the field.
2 Photoluminescence Origins of CDots
In recent years,with the deepening of research on CDots,their PL performances have been improved continuously.PLQY of the CDots emitting in the blue or green spectral range has reached 80%,and their emission range has been extended towards yellow/red and even near-infrared spectral region.However,the development of CDots is still hindered bymany issues,such as the CDots with high PLQY are usually limited in blue emissive region and aggregation induced luminescence quenching in solid state.To better address such issues,a clear understanding of the PL origins of CDots is needed.However,the complexity,large variation and substantial of precursor and different synthesis conditions result in varying properties of the prepared CDots,which make it difficult to define one commonly accepted mechanism of their PL origins.Initial studies claimed that the optical properties of CDots originate from core state(π-π*),edge state(n-π*),molecular fluorophores or their combination.We will analyze it successively in the following part.
2.1 Photolum inescence of CDots Originated from Core State(π-π*)
Jing et al.reported a microwave synthesized CDots with solvent dependent piezochromism.They observed the PL peak of CDots change from green to blue in water with increasing the pressure,while the PL peak of CDots in DMF is red-shifted.They proposed that the red-and blue-shift piezochromism of CDots originates from increased π-π*stacking and protic-solvent induced surface chemical structural changes under high pressure, respectively(Fig.1(c))[22].Chien etal.used themodified Hummers method to synthesize CDots with a broad prominent PL spectrum centered at longer wavelengths,due to the numerous disorder defect stateswithin the π-π*gap.After deoxygenation,the number of defect states within the π-π*gap decreases,and a small and isolated sp2domains is formed,exhibiting blue fluorescence at shorter wavelengths with a narrower bandwidth[23].
2.2 Photolum inescence of CDots Origin from Edge State(n-π*)
Yeh et al.used amidative cutting and ultrafiltration to synthesize Cdotswith excitation wavelength independent PL emissions.They demonstrated that the fluorescence change from red to blue upon reducing the CDots size,originated from the n-π*transition and the energy loss associated with phonon scattering.The excitation wavelength independent PL emissions are associated with electron transitions from the antibondingπ (π*)to oxygen nonbonding(n-state)orbitals.The observed quantum confinement is ascribed to the size change in the sp2domains,which leads to a change in the π-π*gap;the n-state levels remain unaffected by the size change[24].Bao et al.reported CDots by acid treatment and chemical exfoliation of carbon fibers,and proposed that the photoluminescence originated from the chemical oxidation of carbon fibers in the edge of CDots[25].
2.3 Photolum inescence of CDots Originated from M olecular Fluorophores
Xiong et al.studied the influence ofmolecular fluorophores on chemically synthesized CDots and revealed the co-existence of carbonized nanoparticles and molecular fluorophores[26].Ehrat et al.analyzed the luminescence mechanism when polycyclic aromatic hydrocarbon domains and molecular fluorophores co-existence by varying the hydrothermal synthesis time of CDs obtained from CA and ethylenediamine.They found that the initialmolecular fluorophores account for the blue luminescence of the CDots,and over time,aromatic domains form and grow,resulting in a second,faster decay channel at similar wavelengths and also creating additional lower energetic states[27].
2.4 Photolum inescence of CDots Originated from Combination
Xiao et al.observed dual fluorescence bands from CDots suggested the emission of CDots consists of intrinsic and extrinsic states respectively,from the sp2C conjugated domains and edge states(Fig.1(b))[28].Reckmeier et al.proposed that there are three emission bands belonging to the sp2-hybridized core(core state),the edge state,and the functional surface groups(molecular fluorophores)of CDots(Fig.1(a))[29].Charge transfer is assigned to the intrinsic processes within the edge state,while H-bonding interactions between CDots and solvent have themost influence on themolecular fluorophores.As a result,intrinsic edge state transitions are influenced by the solvent polarity,and molecular fluorophores transitions are influenced by hydrogen bonding between the CDots and the solvent.

Fig.1 (a)Possible PL emissionmechanism of N-doped CDot.(b)Intrinsic and extrinsic emission luminescencemechanism of CDots.(c)Calculated structures of CDot'smodelwith two watermolecules before(Status 1)and after(Status 2)structure relaxation.The charge distributions of LUMO and HOMO(left).(d)Energy levels of CD'smodel with two water molecules before(Status 1)and after(Status 2)structure relaxation(right).
Although there is no unified conclusion about the PL origins of CDots,with the deepening of research and the improving of theory,their luminescencemechanism will be revealed eventually.
3 Bandgap Modulation by Carbon Core
Commonly,CDots are consisted of sp2hybridized carbon domains or graphene flakes within the crystalline core,with an amorphous sp3carbon frame and a large variety of functional surface groups surrounded,such as carbonyl and amines.Theoretical calculations and experimental results have shown the intrinsic(bandgap related)transitions strongly depend on the conjugated sp2domains and effective conjugation length,which related to the particle size(Fig.2(a))[30].Modulating the particle size can get different sizes of conjugated sp2domain and HOMOLUMO levels,resulting in different emissions.

Fig.2 (a)Calculated emission wavelength by using TDDFTmethod in vacuum as a function of the diameter ofGQDs.The solid line shows the linear fitting of zigzag-edged GQDs.(b)Synthesis route of the CDots by solvothermal treatmentof PG triangulogen.(c)Preparation of CDots(top)and photographs of CDots under daylight and fluorescence images under UV light(bottom).(d)Schematic of a possible growthmechanism for CDots with different emission.
As a kind of CDotswithout doping heteroatoms,graphene quantum dots have been studied bymany researchers.Yeh et al.used GO sheets with mild oxidation as precursor to prevent the uncertain influence from oxygen functionalities'heterogeneities and got graphene oxide quantum dots(GOQDs)with excitation-wavelength-independent PL.As the GOQDs size was increased from 1 to 8 nm,the emission color changed from blue to red.The author pointed out that the different size of the carbon core led to a change of the sp2-domain size and π*-π level,resulting in a tunable emission[24].
Qu et al.developed blue,yellow,red emission CDots by using CA and diethylene-triamine(DETA)with different solvents via solvothermal approach.PLE spectra showed the PL was contributed from the π-π*transition of sp2C.The author indicated different sizes of the resulting CDots caused diverse dimensions of conjugated sp2-domains,determining different energy gap and emission colors[31].
It is reported that different degree of carbonization can affect the intrinsic emission.Tian et al.prepared full-color emissive(λpeak:448-638 nm)CDots from the same precursors CA and urea by tuning the solvents of water,glycerol,and dimethylformamide(DMF)in solvothermal conditions(Fig.2(d)).X-ray photoelectron spectroscopy(XPS)and energy dispersive spectroscopy(EDS)showed the CDotswith longwavelength emission had a large degree of carbonization.The author indicated that aprotic solvents could lead to a higher degree of dehydration,polymerization and carbonization during the high-temperature solvothermal reaction,resulting in a large dimensions of conjugated sp2-domains and thus CDotswith tunable emission colors[32].
Similarly,Miao et al.prepared a series of CDots with emission tunable from 430 to 630 nm through the reaction between urea and CA in DMF with different temperature.By regulating the thermal pyrolysis temperature and the ratios of CA/urea,the emission was shifted from blue to red.The author proposed the high molar ratio of CA/urea and high temperature can promote the graphitization of CA,this increased effective conjugation length and conjugated sp2domain size,resulting in a red shift emission of CDots[33].
Column chromatography is an approach used to isolate single compound from amixture.It separates substances by the differential adsorption of compounds to the adsorbent.Now it is often used to separate CDots synthesized by solvothermal method.Kang etal.developed alkali-assisted electrochemical fabrication of CDots and separated them by column chromatography.They found different-sized CDots yielded different emission colors of blue,green,yellow,and red respectively.Further theoretical calculations indicated that the conjugated sp2-domains increased with the CDots core's graphite sheet,resulting in decreasing of the HOMO-LUMO level and caused the red shift emission of CDots[34].
Phenylenediamine is a kind of common precursor for producing nanomaterials and polymers.Jiang et al.reported the preparation of red(604 nm,QY 26.1%),green(535 nm,QY 4.8%),and blue(435 nm,QY 17.6%)emissive CDots by using three phenylenediamine isomers as precursors in a solvothermalmethod in ethanol.The CDots can be easily purified by using a silica gel column chromatography.AFM and transmission electron microscopy(TEM)revealed the different size of CDots,indicating the emission red shiftwas ascribed to increased size[35].
Yuan et al.synthesized a kind of triangular CDots with narrow bandwidth(FWHM of 29-30 nm)and full color(from blue to red)emission.The CDotswere prepared by three-fold symmetric PG triangulogen in the solvothermal treatment with different reaction times,then being purified via silica column chromatography.Transmission electron microscopy(HAADF-STEM)observed bright multicolored emissions of blue,green,yellow,and red with gradually increasing sizes(Fig.2(b)).Theoretical calculations showed bandgap energy gradually decreased with the emission red-shifting when the size increased.This agreed wellwith the optical results,indicating the different size can cause a tunable emission[36].
In addition,Yuan and teammates also reported CDotswith tunable emission from blue to red,which were produced bymixing CA and DAN(2,3-diaminonaphthalene or 1,5-diaminonaphthalene)in solvent of ethanolwith different conditions such as controlling reaction time or adding appropriate amounts of concentrated sulfuric acid as catalyst(Fig.2(c)).TEM and AFM showed gradually increased size of CDots was consistent with the red-shifted PL emission,this clearly revealed the bandgap transitions which dominated by the quantum confinement effect[37].
4 Bandgap Modulation by Surface State
Except particle sizemodulation,surface state of the CDots can also be the dominant factor controlling the PL variations,such as the introduction of heteroatoms and sufficient surface groups on CDots.It is also revealed the surface oxidation introduced by surface defects could capture excitons and produce fluorescence related to the surface state[38].
Surface oxidation is an efficientmethod tomodulate bandgap and change emission color of CDots.Zhu et al.discovered CDots’varying degree of surface oxidation caused different emissions[39].Ding et al.reported CDotswith tunable PL from 440 to 625 nm via hydrothermalmethod in one pot.Urea and pphenylenedi-amine were selected as precursors and silica column chromatography was used to separate.Eight samples with different PL emissions were successfully collected due to different polarities.The TEM data clearly showed the CDots had similar particle size and demonstrated that the red shift of PL emission was not because of quantum size effects.XPS indicated the atomic ratio between oxygen and carbon gradually increased as the emission color was from blue to red,reflecting that the oxidation degree of CDots was related to the PL red shift.The author proposed the PL variations were controlled by the surface state.The bandgap reduced as an increasing number of oxygen atoms joined the structure,a PL red shift arose as a result of the increase in the degree of surface oxidation(Fig.3(c))[40].Bao et al.produced CDots with various fluorescence color by controlling the degree of surface oxidation.CDots were prepared through the carbon fibers'oxidation by nitric acid and separated via ultrafiltration.The degree of surface oxidation was controlled by temperature,reaction time and the concentration of nitric acid.The author indicated high degree oxidation of the CDots led to more surface defects,which narrowed the bandgap and caused the PL red shift of CDots[25].

Fig.3 (a)Energy level of surface and possible luminescencemechanism of CDots.(b)Illustration of the possible emission processed of the CDs in different solvents.(c)EightCDots samples under365 nm UV light(top)andmodel for the tunable PL of CDotswith different degrees of oxidation(bottom).(d)Schematic of structure and energy level alignments of nontreated CDs(left) and CDsmodified with S O /C O-richmolecules(right).
Wang et al.realized the tunable emission from 511 to 615 nm on a same CDotwith excitation-wavelength-independent in different solvents as carbon tetrachloride(CCl4),toluene,chloroform(CHCl3),DMF.Red shift emission was observed as the solvent polarity increased.The author regarded this phenomenon as surface electronic state changes.Nitrogen atoms provided by solventswere bonded to the edge of the sp2-hybridized carbon core,affecting the surface electronic structure and resulting in reduced of energy gap and thus the fluorescence wavelength red shift(Fig.3(b))[41].Yuan et al.prepared a kind of CDots with reversible switching ability between green and red PL with a high QY of both up to 80%by a surface electron-state engineeringmethod.The emission would change red from green by adjusting their surface electronic state via alkali addition(Fig.3(a)).The study also indicated the alkalinity of the added alkali caused an important influence on the surface electron state of CDots.XPS spectra showed the amounts of pyrrolic N of the CDotswere increased after interacting with alkali.The author proposed the use of alkali could lead to N doping,increasing the quinone structure and causing an electronrich property,which could lift the HOMO to higher energy levels,resulting in red shift absorption[42].
Based on the density functional theory,Chien et al.discovered carboxyl groups on sp2-hybridized carbon could cause local distortions,resulting in energy gap decrease and red-shift PL emission[23].Theoretical calculations and experimental studies also pointed out the emission of CDots could be tuned by modulating the number of amino group,the bandgap decreased as a result of the increased number of modifying groups when its number was between 1 and 6[43].Kwon et al.reported CDotswith changeable emission from yellow to red by surface engineering.The CDotswere synthesized from citric acid by solvothermal method and then modulated surface state with para-substituted anilines.The anilinemodified CDots could formatmore extrinsic energy levels and cause the red shiftemission[44].Li et al.tuned the CDots’emission from red to NIR by surface engineering.The synthesis of CDots was achieved by CA and urea in DMF via solvothermal method,and then sulfoxide/carbonyl groups were introduced onto surface by dispersing CDots in dimethyl sulfoxide(DMSO).Data from the study showed electron-acceptor groups can interact with CDot's surface and modulate optical properties.The author proposed the sulfoxide/carbonyl groups could interactwith the surface and cause increased oxidation of surface,which resulted in a decrease in the lowest unoccupied molecular orbital level and thus the NIR emission of the CDots(Fig.3(d))[45].
5 Enhancement of PLQY
The PLQY as an important parameter to assess the properties of fluorescentmaterials,has attracted wide attention for a long time.However,the photoluminescence origin of CDots may be different,includingmolecular fluorophores,core state(π-π*),edge state(n-π*)or their combination,and as a result,there is no fixed strategy to improve their PLQY.Many of the studies focused on the optimization of the synthetic conditions to produce CDotswith higher PLQY,which could be achieved through surface passivation,surface reduction,metal coating and/or heteroatom doping[46].
The PLQY of bare CDots usually is low due to the emissive traps on the surface.Thus,many researches focus on the surface passivation to improve the PLQY.Yuan et al.used CA and 2,3-diaminonaphthalene as precursor to synthesize blue emissive CDots with PLQY up to 75%,while the red emissive CDots from CA and 1,5-diaminonaphthalene is only 12%.They proposed that the decreased degree of surface-passivation of electron-donating amino groups at edge sitesmight result in the gradually decreased PLQY as the size of the CDots increased[35].Bao et al.prepared CDots through the carbon fibers’oxidation by nitric acid.They found the surface defects caused by surface oxidation can serve as capture center of excitons,thusmore surface emissive centers can be obtained through the increasing oxidation degree of surface.In their study the PLQY of CDots enhanced from 1.1%to 20.7%after a high degree of oxidation[25].
Wang et al.reported a microwave-assisted method to synthesize CDotswith PLQY of76%using CA and EDA as precursors.They analysized the effects of reaction temperature,reaction time and raw materials ratio on PLQY of CDs by orthogonal experiment,and assumed that the π-electron system could be forcefully combined with surface electronic states created by surface oxidation,consequently altering the whole electronic structure of the CDots to improve their PLQY[47].
Qu et al.demonstrated an orange emissive CDots in a solvothermal synthetic route using CA and urea as precursors.Through surface charges engineering by surface metal cation functionalization,enhanced orange emission with PLQY of 46%was realized.Interestingly,PLQY of the CDots decreased to 5%after removing the surface functionalized metal ions.They commentated that surface charges engineering by surface metal cation functionalization could decrease overlap between absorption and emission,and thus benefit efficient output emissions[48].
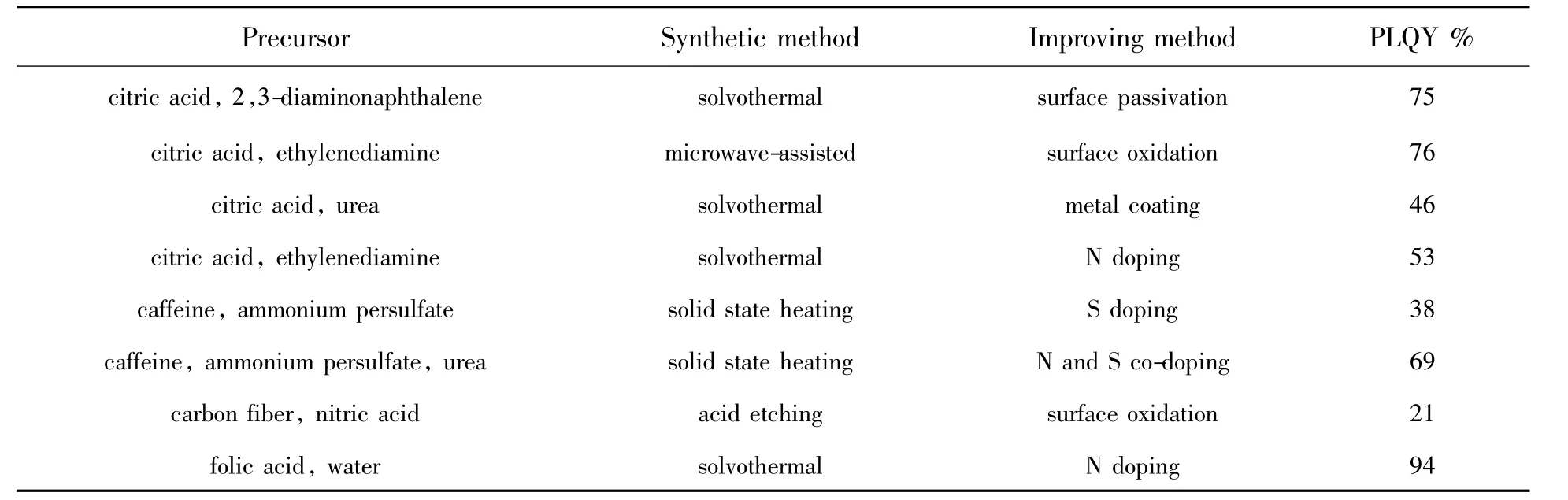
Tab.1 Im proving m ethod of PLQY
It has been shown that N-doping(mainly pyrrolic,possibly pyridinic,but not graphitic doping configuration)on CDots increases PLQY due to the electron-withdrawing ability of nitrogen atoms.Ding et al.used CA and ethylenediamine as precursors and obtained CDotswith PLQY of 53%through solvothermal synthesis.Changing the surface state by NaBH4,PLQY of the CDots is still as high as45%.Thus,they supposed that the nitrogen-derived structures in carbon cores,especially the pyrrolic N,had a great influence on the emission efficiency of the CDots.Another explanation is that the strong photoluminescent emission of the N and S co-doped CDots mainly result from the influence of surface-doped atoms onto the radiative recombination of electrons and holes trapped on the CDots surface[49].Dang et al.reported N and S co-doped CDots synthesized using caffeine as precursor via solid state heatingmethod.CDots could not be produced with caffeine alone,whereas PLQY of 38%was achieved when caffeine and ammonium persulfate were co-heated.Furthermore,the addition of urea could increase the PLQY of the CDots to 69%,probably due to the enhancement effect of N and S co-dopants on the surface of the CDots[50].Subsequently,doping with P,B,as well as co-dopingwith a combination of N,S,and P has also been revealed to lead to enhanced luminescence of CDots.In addition,some research has shown that the N-doped surface function group can enhance the PLQY.Liu et al.prepared high PLQY CDots by directly dissolving folic acid(FA)in deionized water under hydrothermal conditions at 240℃ for 6 h.The surface of CDots contained rich amino groups,which could enhance the conjugate degree,increase the probability of electronic transition from the ground state to the state of lowest excited singlet and cause an extreme high PLQY of the CDots to 94.5%[21].
6 Applications
Application of CDots will be discussed in this section from five aspects.Firstwe introduce the application about fluorescence imaging as multicolor patterning and information encryption.Then the CDots’application in white LED as color conversion layer and emissive layer will bementioned.The last part we highlight the application in the biological field,as cell imaging,in vivo imaging and cancer therapy.
6.1 Lum inescence Imaging and Information Encryption
Luminescence imaging is regarded as a powerful technique for data recording and information encryption.It greatly depends on the development of smart luminescent materials.Now CDots are attracting considerable attention owing to their high photostability and intriguing optical properties.They have been used as eco-friendly fluorescent ink aiming at luminescent printing patterns,multicolor patterning,and information encryption[51].
Lou et al.functionalized CDots with alkyl chains.The CDots can self-assemble into a supra-CD system in the form of agglomerates through amphiphilic interaction in toluene.Supra-CD exhibited weak luminescence due to aggregation-induced luminescence.Weak luminescent supra-CD-coated paper exhibited enhanced luminescence after a water-spray treatment,which ascribed to the decomposition of the supra-CDs in the presence of water.The supra-CD-coated paper can be used forwater-jet printing to realize optical information storage and information security protection.This low-cost,environmentally friendly CDots can also be applied in clinical diagnosis fields such as fingerprint sweat pore imaging(Fig.4(a))[52].
Jiang et al.prepared long afterglow materials by covalently fixing CDots onto colloidal nanosilica.Thematerial(named m-CDs@nSiO2)showed long afterglow emission in water dispersion and small effects of the dissolved oxygen.They dispersed mCDs@nSiO2in polyvinylalcohol(PVA)solution to prepare the corresponding PVA composite films,noticing that the afterglow alterations of m-CDs@nSiO2-PVA filmswere reversible when they were alternately desiccated and wetted.This material can be employed in border fields such as time-resolved sensing and bioimaging information protection(Fig.4(b))[53].
Lin et al.adopted the biuret/urea bonding encapsulating method to realize CDots’full-color ultralong phosphorescence and multiple-mode emissions.The CDots-based anti-counterfeiting ink showed triple emission modes and realized blue,green,and red emission when printed on the paper.Thismaterial can find broad applications in optical anti-counterfeiting and information protection(Fig.4(c))[54].
Tian et al.realized room temperature phosphorescence CDots by embedding different CDots into PVA matrix through post-synthetic thermal annealing at different temperature.The long afterglow controlled by thermal-treatment of CD@PVA composites can be ascribed to the decrease of oxygen which acted as quenchers and vibrational dissipation's suppression via chemical bonding.CD@PVA composites have different phosphorescence abilities under different process,thus can be utilized for design and development of novel compositematerials for anti-counterfeiting and data encryption(Fig.4(d))[55].

Fig.4 (a)Photographs of printed image of supra-CD-coated paper using water-filledand HP 46 tricolor cartridges under daylight and under UV and daylight.(b)Information protection applications ofm-CDs@nSiO2.(c)Schematic for optical anticounterfeiting applications by inkjet printing.(d)Three overlapping patternswritten with CD@PVA and applications in data encryption.
He et al.realized the phosphorescence at room temperature as well as thermally activated delayed fluorescence by embedding CDs into PVA with the help of electrospinning technologymethod.The triplet states of CDs@PVA electrospun nanofibers can be stabilized effectively due to ordered mesoporous structure.In addition,the PVA molecules rigidified carbonyls group on the CDot's surface with hydrogen bonds,which prevented the atmospheric oxygen quenching.The author developed CDs@PVA solution as ink,the written letters emitted blue fluorescence under the exicition of 365 nm and showed a long green phosphorescence after cutting off the light,this showed potential application in anti-counterfeiting and optical imaging[56].
6.2 Solid-state Fluorescence Devices
6.2.1 LED Pumped Lighting Devices
As next generation lighting sources,the white light-emitting devices(WLEDs)have been the subject of academic research.Combination of a blue LED chip and multicolor phosphors is commonly used to generate a warm white light.Traditional phosphors are based on non-renewable rare earth materials.Semiconductor QDs have been used in WLEDs in recent years.However,the QDs usually contain heavy metal elements,leading to toxicity concerns,so it is urgent to find new alternativematerials.Fluorescent CDots with high quantum yields have the advantages of tunable fluorescence emission,high photostability,low cost,and environmental friendliness,which can be used as color conversion layer for WLED preparation.
As a kind of nanoparticle,the aggregation of CDots in solid state can cause resonance between particles and this leads the formation of additional nonradiative channels.Under this condition,selfquenching of CDots is often observed because the energy is not transferred by light radiation[57].In order to handle this problem,one kind of solution is to prepare proper surface coatings and suitable matrix materials for CDots,holding them at distance from each other and prevent the self-quenching.Some researchers use polymer as precursor and utilize the polymer backbones to disperse the adjacent CDots in the aggregate state.In additon the surface statemodulatation by surface oxidation can also prevent the self-quenching.These methods can broader CDots'application in photoluminescence LED device.
Zhou et al.reported a fabrication method toward CDots-based hybrid phosphors by assembling SO24-and Ba2+ions onto the surface of CDots through electrostatic attraction.The author chose CDots with negative charges as nucleation centers and sequentially added BaCl2and Na2SO4.Firstly the Ba2+ions can be adsorbed on the surface of CDots by electrostatic interaction and likewise the SO24-ions can be adsorbed by Ba2+ions attached on CDots’surface.The CDots@BaSO4phosphors overcame the aggregation-induced luminescence quenching,obtaining thermal stability and high resistance to strong acid and alkali due to BaSO4's inorganic nature.Its luminescence exhibited good photostability,which used for fabricating white LEDs,and the LED's color temperature can be turned by changing the amount of the phosphors(Fig.5)[58].Another method reported by Zhou et al.indicated silica can also bematrixmaterials to overcome the aggregationinduced luminescence quenching of CDots and showed CDots@silica's applicability in LED device as color conversion layer[59].
Wang etal.prepared red emissive CDotswith a quantum yield of 53%by innovating a sequencial dehydrative condensation and dehydrogenative planarization(DCDP)approach.They overcame the aggregation quenching of CDots by dispersing in silicone uniformly,and combined the red emitting quantum phosphor with blue emissive CDots and green emissive CDots phosphors to realize a UV-pumped CDot phosphors-based warm WLED with good color chromatics and device stability.When the applied current was 20 mA,WLED showed warm white light characteristicswith the CIE coordinates,CRI and CCT of(0.392 4,0.391 2),97 and 3 875 K[60].

Fig.5 Photographs ofworking LED deviceswith different color temperatures(a - e),insets are the LEDs’corresponding emission spectra.(f)CIE chromaticity diagram of the five LEDs.The black curve shows the changes of color temperature with color coordinates.
Zhai et al.embedded CDots in NaCl crystals to get CDot@NaCl composite phosphors by evaporating saturated aqueous solution of CDots and NaCl and washed by ethanol to remove the CDots not embedded.Study showed the phosphors had UV resistant and thermal stability which ascribed to the protection of NaCl.Based on the phosphors they fabricated white LED with tunable CIE coordinates and color temperatures(3 944 - 5 478 K)[61].He et al.prepared the phosphors with double emission color by integrating a single emission CDots with different matrixmaterials as starch and Al2O3.The dual-fluorescence CDots based phosphors can realize tunable photoluminescence by adjusting the ratio and the author developed its application for WLED and oxygen sensing[62].Tian et al.chose aqueous solution of sodium silicate as a transparent matrix and surface functionalizing agent for fabrication of inorganic CDot phosphors and used microwave assisted heating to accelerate dehydration processes in sodium silicate.The WLEDs fabricated by the CDot phosphors had CIE of(0.34,0.31)and the color rendering index of 82.4(Fig.6)[32].
Chen et al.reported the production of quenching-resistant CDots by using polyvinyl alcohol and EDA as precursor via one-pot hydrothermal treatment.The CDots emitted blue fluorescence in isolated condition and had green or yellow emission in aggregation state.The author proposed the branched chains and polymer backbones can disperse the adjacentgraphitizing cores in the aggregate state homogeneously,preventing interparticle surface electron transitions and π-π interactions of luminescence centers and thus aggregation quenching.The author prepared the composite with white light emission by combining the CDots with epoxy resin.It showed good photostability under the UV irradiation.The LED device based on the CDots emitted white light with CIE of(0.285,0.341),indicating promising use in organic LEDs or white LED devices[63].

Fig.6 (a)Emission spectra of operating devices on 440 nm emissive InGaN chips.(b)Emission spectra of operating CDotbased WLEDs.(c)Photograph of pen caps and fruitswith different colors under CDot-based WLEDs array and(e)photograph of the same pen caps and pen fruits under sunlight.(d)Photograph of working CDot-based WLEDs array and performance parameters.
By surface engineering,Zhou et al.prepared quenching-resistant CDots by oxidizing with H2O2and obtained green emissive solid state CDots phosphor(ox-CDs phosphor)with PL quantum yield of 25%and short luminescence lifetime of 6 ns.The author indicated due to the H2O2treatment,the CDots obtained awider bandgap of the surface structure and inhibited the non-radiative transition process of the surface state in aggregation state.They used ox-CDs phosphors as color conversion layer to fabricateWLEDs emitted pure,cool,and warm white light with CIE coordinate of(0.34,0.37)and CT of 5 240 K.Because of its short PL life time,data communication based on the ox-CDs phosphor as a fast color converterwas demonstrated,which had a high modulation bandwidth(≈120 MHz)and high data transmission rate(using OOK≈350 Mbps).This also showed the CDots’potential application in Visible Light Communication[57].
6.2.2 Electroluminescent(EL)Devices
In the beginning,the application of CDots for electroluminescent LEDs is hard to realize because the surface defects and molecule states which dominant the fluorescence limit the carrier injection's efficiency.Now great progress has been made to solve this problem.Ma et al.synthesized high PLQY(60%)CDots by using CA as precursor and 1-hexadecylamine as passivation agent via hydrothermal method.The CDots were dissolved in toluene and spun-casted until forming a 20 nm thick film and used as emissive layer in a LED device.The LED had a tri-layer EL devices architecture and realized white light emission with CIE of(0.40,0.43)and CRIof 82.Its turn-on voltage was about 6 V,with maximum brightness output of 35 cd/m2and maximum external quantum efficiency(EQE)of 0.083%.This showed the CDots potential application as emissive layer for high performance white LED[64].
Itwas reported Zhang et al.used CDots as emissive layer and realized carbon-dotbased LEDswith driving current controlled color change.By tuning the current injecting density,multicolor emission can be obtained.The EL device with white emitting can reach the maximum luminance to 90 cd/m2,and show its turn-on voltages at 4.6 V.It was the first time to observe a switchable EL behavior with white emission in single emitting layer structured nanomaterial LEDs.And this facilitated CDots’practical application in next-generation color displays and solid state lighting[65].
Yuan et al.prepared multi-color emission CDotswith high surface passivation and high crystallinity.The LEDs emitted from blue to red was fabricated by using the CDots blendingwith polyvinylcarbazole(PVK)as an active emission layer which showed high photostablity and great performance.The LEDs’emission can be ascribed to the energy transition from PVK host to CDots guest as well as the charge injection to CDots.The white LED based on the CDots emitted white light with CIE coordinates of(0.30,0.33),with maximum luminance at2 050 cd/m2and a low turn-on voltage about3.9 V.This indicated the CDots’ability and great potential in electroluminescent LEDs(Fig.7)[37].

Fig.7 (a)Picture of luminance and current voltage characteristic.(b)Current density versus currentefficiency ofWLED.(c)Output EL spectra ofWLED.The inset picture shows the surface white light emission from WLED.(d)CIE coordinates of theWLED.
6.3 Bioimaging and Cancer Therapy
Traditional quantum dots(QD)and organic polymers have been used for bioimaging and cancer therapy. However, release of toxic metals from quantum dots can cause irreversible damage to organisms and environment,organic polymers have poor stability and poor biocompatibility.CDots have attractive properties such as excellentbiocompatibility,water solubility and good cellmembrane permeability.Now they can serve as nontoxic substitutes for QDs and organic polymers.
The potential application of CDots in the biological field has been discovered and first applied in cell experiment.Cao et al.first reported the use in cell imaging.They incubated the MCF-7 cells for 2 h in a cell culture medium containing CDots,and observed bright fluorescence of the cells under a fluorescencemicroscope.They noticed CDots can enter the inside of the cells but hardly exist in the nucleus[66].Liu et al.used glycerol and TTDDA as precursor to prepare CDots via microwave method.The CDots’cytotoxicity was assessed via MTT method by incubating with human hepatoma cell line HepG-2.Emitted color of blue,green,and red was observed at the excitation wavelengths of 405,488,and 543 nm,respectively(Fig.8).The study indicated the CDots showed a light biotoxicity when the concentration was less than 240 μg/mL,which demonstrated good biocompatibility[67].Bhunia et al.synthesized a series of fluorescent emission CDots from blue to red and modified with FA on the surface to achieve self-targeted ability of cancer cell[68].Liu etal.modified the CDots with PEI to prepare a transgenic vectorwith fluorogenic imaging ability,good transfection ability and low cytotoxicity.The CDots could enter the nucleus through nuclearmembrane by conjugating with pDNA and realize intranuclear fluorescence imaging,which can effectively track the transfection process[19].Li et al.coupled amine group-modified CDots with the carboxyl group and incubated with HeLa cells.The author found intracellular fluorescence was significantly enhanced,which indicated the conjugation of transporter enhanced cell membrane transport of CDots,caused increased intracellular PL intensity[69].

Fig.8 Photographs of cells as negative controls(a)and cells incubated with CDots(b)under laser scanning confocalmicroscopy,with the excitation at405,488,543 nm.
At first,most CDots only had short emission and excitation wavelengths,which limited their application in in vivo imaging due to poor tissue penetration and a high background signal.To date,increasing kinds of CDots with red or NIR luminescence and high PLQY are prepared to reduce the background signal and increase tissue penetration,which led a better use in in vivo fluorescence imaging.Lu et al.chose dopamine and o-phenylenediamine as precursor to fabricate CDots with NIR emission at710 nm by hydrothermalmethod.MTT assays suggested the CDots showed a low cytotoxicity and can be used a high concentration for in vivo imaging or other biological experiments.The CDots were injected into a nude mouse's back and observed a strong fluorescence emission with good signal to noise ratio,which showed promising application in bioimaging(Fig.9(a))[70].Li et al.synthesized CDots with NIR emission via surface engineering.The CDotswas sent into a nude mouse by various injection methods as tail intravenous,subcutaneous injection and gavage injection.The NIR fluorescence appeared under the excitation of 671 nm laser in all the three situations and showed the distribution of CDs in vivo in real time,which demonstrated the CDots’potential application in bioimaging(Fig.9(d))[45].
Zheng et al.reported fluorescent CDots(CDAsp)with self-targeting ability and tunable full-color emission synthesized by using D-glucose and L-aspartic acid as startingmaterials.The quick accumulation of CD-Asp in the brain site after intravenous indicated the good ability of CD-Asp to pass through the blood brain barrier(BBB)and reach the brain tissue.The imaging reconstructed by three dimensional(3D)after injection indicated the glioma's fluorescent intensity was much stronger compared to that in normal brain,which showed CD-Asp's selftargeting ability to glioma site.The author proposed CD-Asp could be used to diagnosis brain cancer cells as targeting agent[71].
Yang et al.developed tumor targeting CDots(iRGD-CDs)by conjugating a tumor-homing penetration peptide(iRGD)to red shift emissive CDots.Stained tumor slices showed the iRGD-CD could penetrate the tumor until deep tumor site where far away from the new vessels.This showed iRGD-CD's enhancement of tumor penetration and tissue permeability compared with CDotswhich was not conjugated with iRGD.In addition,Hemocompatibility research indicated iRGD-CDs had low hemolysis potential,demonstrated its adaptability in vivo application(Fig.9(b))[72].

Fig.9 (a)In vivo fluorescence images of nudemouse treated with subcutaneous injection.(b)Living imaging ofmouse after intravenous injection with iRGD-CDs and control CDots.(c)In vivo fluorescence images ofmice at different time intervals after intravenous injection of pCDPIand control.Yellow lines circled the cancer area.(d)In vivo NIR fluorescence images of amouse with excitation by 732 nm.
Xie et al.prepared a new CDots with strong NIR emission via solvothermal process.Mice were injected intravenously with CDots and the whole body fluorescence images can be obtained.The fluorescence intensity became stronger progressively as time elapses and became highest at 6 h,indicating an increasing accumulation of CD in the tumor region.Then themouse was sacrificed at 48 h postinjection,major organs(heart,liver,spleen,lung,and kidneys)and the tumor were excised and subjected to ex vivo fluorescent imaging.The fluorescence intensity showed CDots’plenty accumulation in kidneys,liver,and the tumor.Enrichment of CDots in tumors showed prominent passive targeting ability,which ascribed to the EPR effect. The kidney's high fluorescence intensity indicated mice used kidney to metabolize CDots.The CDots achieved high tumor uptake through passive targeting and renal clearance[73].
Cancer is themost serious disease facing human being.It is hard to treat and causes a high mortality.Traditional treatmentmethods usually have side effects and can hardly eradicate cancer.In addition,the high costs of anticancer drugsmake itmore difficult for cancer patients to afford.Thus,effective treatmentmethods and drugs with few side effects and low cost are urgent to develop.CDots,as a new kind of nanomaterial with characteristic of biocompatibility,nontoxicity and strong fluorescence,has been used in cancer therapy.
Many CDots were conjugated with anticancer drugs to treat cancer as drug carriers.Tang et al.modified FA and doxorubicin on the surface of carbon dots to achieve specific recognition,drug trafficking and fluorescence imaging of cancer cells[74].Pan et al.conjugated fluoresceinisothiocyanate(FITC)with abundant amino functional groups’CDots and evaluated their potential to serve as a carrier for drug delivery.They incubated MCF-7 cells with CDots-FITC conjugate and then subjected to confocal imaging.Both deep-red(from CDots)and bright-green(from fluorescein)emissionswere clearly observed,indicating CDots can bring FITC into living cells effectively and serve as a promising carrier for delivery of drugs[75].Wang et al.conjugated the CDotswith I6P8(cys-I6P7)to construct a simple nanocarrier for augmenting the drug delivery(pCDPI),which can overcome the BBB,realizing specific bindingwith blood vessel of tumor and show deeply penetrate into glioma.Study showed under physiological condition the DOX can be stable encapsulated into pCDPI and be released in acidic solutions which was good for cancer therapy(Fig.9(c)).In vivo experiments in mice suggested pCDPI can obviously inhibit the growth of tumor and induce glioma apoptosis,which showed potential application in cancer therapy[76].
Photodynamic therapy(PDT)is a phototherapy method for cancer treatment with minimally invasive and low toxic.The photosensitizing chemical substance can conjunction with molecular oxygen to kill cancer cells under the excitation of laser.Ge et al.prepared red light-emitting graphene quantum dots via hydrothermal approach,which can be used for in vivo imaging.The GQD exhibited high1O2yield in visible light irradiation,which can be served as photosensitizer for PDT[77]. Huang et al. modified CDots’surface with PEG2000 and successfully used for imaging and photodynamic therapy in mice.CDotswere covalently coupled with chlorin e6(Ce6)by surface group(amine group)to obtain compound CDots(CDs-Ce6)and the PDT effect of Ce6 was enhanced by fluorescence resonance energy transfer.CDs-Ce6 was effective for fluorescent labeling and PDT of tumors in living organisms,which showed potential application for clinical treatment of gastric cancer or other tumors[78].
Photothermal therapy(PTT)is a treatmethod aiming at using electromagnetic radiation in NIR wavelengths for the treatment of cancer in recent years.The photosensitizer used by PTT is required to have high photothermal conversion,low toxicity and often on the nanoscale.It is reported the CDots have been utilized in PTT as photosensitizer for cancer therapy.By using thiophene-propionic acid as precursor,Ge et al.prepared CDots with red emission by hydrothermal method.The CDots were successfully used in PA imaging and PTT in living mice[79].Xu et al.used supra-CDots to realize PTT and photoacoustic(PA)imaging,which was assembled by surface charge-confined CDots through hydrogen bonding and electrostatic interactions.Study showed the supra-CDot had a NIR absorption maximum at 670 nm and a high photothermal conversion over50%.After intravenous injection intomicewith a tumor on the back,the supra-CDots’accumulation in tumor can be observed by in vivo PA imaging,and realized tumor PTT under the exicition of 655 nm laser.The tumor was inhibited and finally eradicated,demonstrating the supra-CNDs’application as biomedical agents[80].Bao et al.synthesized CDots with strong NIR absorption and photothermal conversion efficiency of 59.1%.It was prepared from the precursors CA and urea in DMSO in solvothermal conditions.The author ascribed its high photothermal conversion efficiency to the dope of S heteroatom and the CDots had been used for FL imaging,PA imaging,and PTT(Fig.10).CDots showed light cytotoxicity through MTT method and can be excreted by renally.In vivo experiments suggested the tumor was ablated after PTT which used the CDot as photosensitizer,demonstrated CDotswas an excellent nanomaterial with highly efficient and had a broader application in biomedicine[81].

Fig.10 (a)NIR fluorescence images ofmice bodies after intravenous injection of CDots at various times.(b)Tumors’NIR fluorescence thatwere dissected from mice at various postinjection time points.(c)NIR fluorescence of themajor organs and tumors thatwere dissected from mice at3 h postinjection.NIR fluorescence images ofmice bodies,major organs,and tumors with excitation by 655 nm laser.(d)PA MAP images and B-scan PA images of tumors inmice after intravenous injection with CDots at various times.
7 Conclusion and Outlook
In this review,we introduce the photophysical properties and applications of the CDots.So far,the scientific field dedicated to this luminescentnanomaterial is still developing rapidly,and CDotswith high fluorescence quantum efficiency in visible and NIR spectral regions have been synthesized and applied in many fields.However,the accurate origin of CDots emission is still controversy even contradictory due to diversity of the recognized PLmechanisms.In this review,we summarized and clarified several possibilities for the PLmechanisms of CDots and ascribed to several categories.Then analyzed the method tomodulate bandgap by particle sizemodification and surface structure engineering respectively,indicating the graphitic core size,the surface state as degree of oxidation and surface functional group were all critical factor to dominate the bandgap and thus control the color of the emitted light.We also concluded several effective method to enhance the PLQY.
With continuous improvement,CDots has been applied in many fields.The appearance of long afterglow phosphorescent CDots expands their applications in information encryption and data storage.The CDots overcoming aggregation-induced fluorescence quenching show great potential in white LED devices.In addition,because of biocompatibility and good cell membrane permeability,significant progress has been achieved in the application of the biological field.The CDotswith NIR absorption and emission have been applied in bioimaging.In cancer treatment,CDots can be used as nano-drug-carrier as well as photosensitizer for PTT and PDT.These all exhibit broad application space of CDots.
However,more efforts are needed for the researching of CDots.The accurate structures and PL mechanisms of CDs are still unclear,more research should be concentrated on CDots properties.Compared to commercial phosphors,the low PLQY of CDots becomes themain reason for its limited application in LED pumped lighting devices,so CDots with higher PLQY are urgent to develop.In addition,the application of CDots’in electroluminescent LED device is notmature and needs further development.Now CDots have shown a strong adaptability in the biological field,which hopes to develop more effective applications in this area.

