茶树CsGPX基因全基因组鉴定和表达分析
王赞 曹红利 岳川 郭雅玲
摘 要 为明确茶树谷胱甘肽过氧化物酶(CsGPX)基因与非生物胁迫的关系,本研究利用生物信息学手段在茶树基因组中筛选得到3条CsGPX基因,对其编码蛋白理化性质、基因结构、系统进化树、顺式作用元件进行了分析。结果表明,CsGPX包含完整GPX结构域和3段保守序列,都具6个外显子。预测启动子上有参与植物激素和非生物胁迫响应的顺式作用元件。使用实时荧光定量PCR测定该基因的表达谱,发现它们在不同组织中的表达有显著差异。进一步分析表明,CsGPX被低温、ABA、盐以及干旱处理上调表达,但CsGPX2和CsGPX3的表达受低温抑制。本研究为茶树CsGPX家族基因克隆和功能验证提供基础。
关键词 茶树;谷胱甘肽过氧化物酶(GPX);非生物胁迫
中图分类号 S571.1 文献标识码 A
Abstract To reveal the possible involvement of glutathione peroxidase genes (CsGPX) in the responses of tea plants to abiotic stresses, three CsGPX genes were identified by bioinformatics tools from the tea genome data, and the gene structures, the cis-elements on their promoters, the physiochemical characteristics of the encoded proteins and the phylogenetic trees were also studied. The results showed that all the CsGPX genes contained 6 exons and shared the gene family-specific GPX domain in addition to three other conserved domains. Cis-elements for the responses of plants to hormones and abiotic stresses were identified on the promoters. Quantitative real-time PCR analysis of the expression profiles revealed that they were significantly different in expression in different tissues. Further analysis showed that the three CsGPX genes were, in general, up-regulated by treatments of low temperature, ABA, salt and drought except that CsGPX2 and CsGPX3 were down-regulated by treatment of low-temperature. The results should have laid down a basis for further characterization of CsGPX family genes in tea plant.
Keywords tea plant (Camellia sinensis); glutathione peroxidase (GPX); abiotic stress
DOI 10.3969/j.issn.1000-2561.2019.06.014
生长环境造成的非生物胁迫是农作物减产、品质下降的重要因素[1]。植物在低温、干旱、高盐、重金属和农药等非生物胁迫下产生大量活性氧(reactive oxygen species, ROS)[2-4],导致脂质过氧化、DNA和蛋白质氧化以及光合器官的损伤[5]。而酶促和非酶促抗氧化防御机制是调节植物体内ROS稳态,提高抗氧化能力的重要途径[6-7]。
谷胱甘肽过氧化物酶(glutathione peroxidase, GPXs)作为重要的酶促抗氧化剂,依赖于硫氧还蛋白(thioredoxin, Trx)还原分解H2O2,避免植物细胞氧化损伤,提高植物的抗逆性[8-10]。已经在拟南芥[11]、水稻[12]、棉花[13]等植物中鉴定得到谷胱甘肽过氧化物酶基因家族,也验证其在非生物胁迫氧化应激响应中的重要性。Martinez等[14]检测番茄叶绿素荧光参数发现,LeGPX表达增加了对高盐和热胁迫抗性,保护光反应系统功能。大豆GPX减少脂质过氧化对细胞的损伤,缓解干旱胁迫带来的危害[15]。转录组分析表明拟南芥AtGPX参与了低温胁迫导致的氧化应激响应,通过维持ROS平衡而减少氧化损害[16]。Wang等[17]发现水稻OsGPX突变体萌发活力低,对胁迫敏感并且发育不良。Zhou等[18]利用蛋白质组学方法发现非生物胁迫导致ROS积累,而GPX等抗氧化酶活性的增加则有利于植物对抗ROS胁迫。
随着茶树栽培面积的扩大,由环境胁迫导致的茶芽萌发迟、长势差,营养积累困难等问题同步增长,对茶产量和品质的影响更加严重[19-21]。虽然植物GPX基因在胁迫氧化应激响应中十分重要,但茶树CsGPX在多种非生物胁迫下的表达模式的报道还少。茶树基因组测序的完成使挖掘功能基因资源成为可能[22-23]。本研究通过生物信息学方法,基于已公开的基因组数据,对茶树CsGPX家族基因进行了全基因组鉴定,并分析了它們在非生物胁迫下的表达模式,为CsGPX基因功能的进一步研究打下初步基础。
1 材料与方法
1.1 植物材料与非生物胁迫处理
2018年5月中旬,在福建农林大学园艺学院教学实验基地采取以下供试材料:
(2)按照Yue等[24]的方法,选取2年生、健壮的盆栽铁观音茶苗,分别进行如下处理:通过叶片喷洒浓度为100 μmol/L ABA溶液;将植株移入并保持在4 ℃人工气候室中;将茶苗从盆中小心取出,用清水洗净根部泥土后放置在10% (w/V) PEG-6000溶液中;用250 mmol/L NaCl溶液灌溉。所有处理分别在0、3、9、24 h取样,取顶端第2和第3片成熟叶片,用锡箔纸分装后迅速投入液氮速冻,然后移至?80 ℃保存备用。各样品均设置3次生物学重复。
1.2 方法
1.2.1 茶树基因组CsGPX基因鉴定方法 从Pfam数据库(http://pfam.xfam.org)下载GPX基因典型保守结构域隐马可夫模型HMM(Hidden Markov Modelle)文件Pf00255[8],利用HMMER3.0软件分别在中国科学院昆明植物研究所公布的阿萨姆种[Camellia sinensis var. assamica (CSA; Assam type)]茶树基因组数据库中(http://www.plantkingdomgdb.com/tea_tree/)进行比对搜索[22]。同时从TAIR数据库(https://www. arabidopsis.org/)下载拟南芥AtGPX蛋白序列,作为检索序列使用本地BLAST在茶树基因组数据库中检索候选蛋白(E-value≤10?10)。利用Pfam和SMART(http://smart.embl-heidelberg.de/)检测候选蛋白结构域,获得CsGPX蛋白序列。
2 结果与分析
2.1 茶树CsGPX基因鉴定
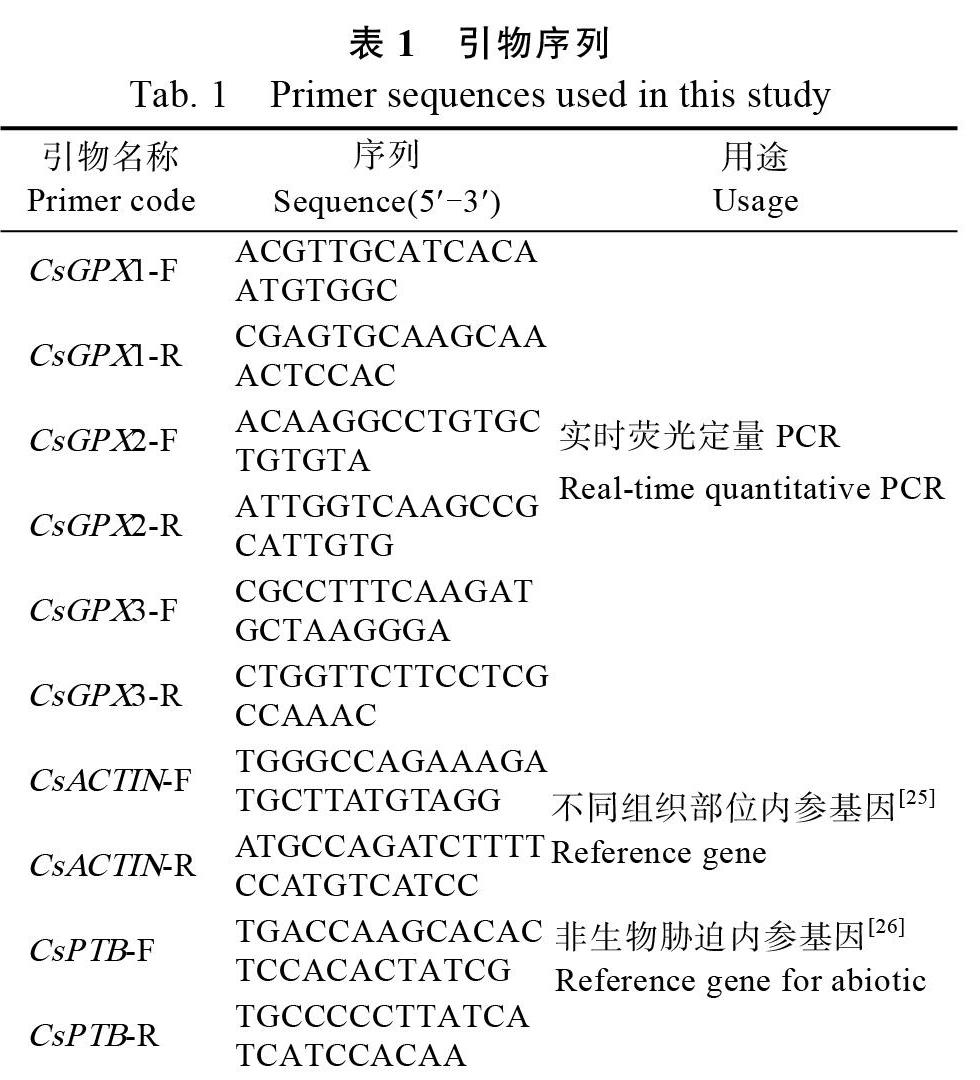
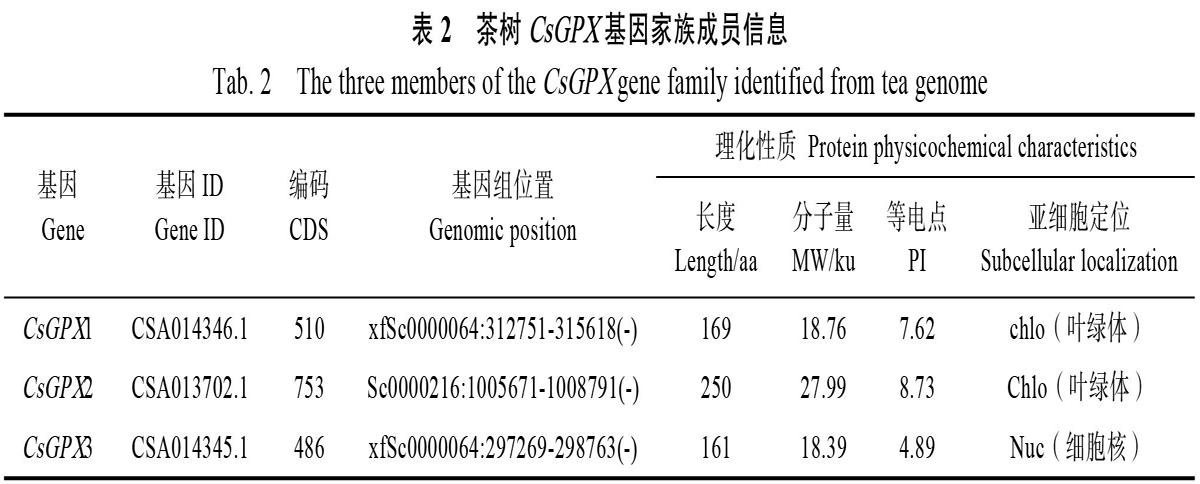
利用HMMER 3.0软件和本地BLAST在茶树基因组数据库中检索出候选蛋白,然后通过Pfam、SMART和DNAMAN比對,确定包含完整GPX结构域以及3个高度保守域(图1A)的CsGPX基因,并依次命名为CsGPX1、CsGPX2和CsGPX3。生物信息学分析表明,CsGPX1编码
169氨基酸残基,分子量为18.76 ku。CsGPX2编码250氨基酸残基,分子量为27.99 ku。CsGPX3编码161氨基酸残基,分子量为18.39 ku(表2)。
2.2 茶树CsGPX蛋白保守结构域和进化分析
利用Pfam和SMART对CsGPX蛋白序列检索发现,这3条CsGPX包含了完整的GSHPx结构域,还包含了GPX家族的PLN02399和GSH_Peroxidase结构域(图1C),主要集中在C端。多序列比对发现,CsGPX含有3段高度保守序列(图1A),分别为GKVLLIVNVASXCG(GPX signature 1),ILAFPCNQ(GPX signature 2)和WNFXKF[27],还有3个半胱氨酸残基(Cys)以及组成预测的催化活性位点的氨基酸残基。
从Phytozome 12数据库其他12个物种的GPX蛋白序列,采用N-J法与拟南芥和茶树CsGPX构建系统进化树,bootstrap检验采用1000次重复。结果显示,CsGPX1、CsGPX2与可可TcGPX6、桃PpGPX6和拟南芥AtGPX6聚为一类,CsGPX3与西红柿SiGPX8、芸薹BrGPX8和拟南芥AtGPX8聚在一起(图1B)。
2.3 茶树CsGPX保守元件和基因结构分析
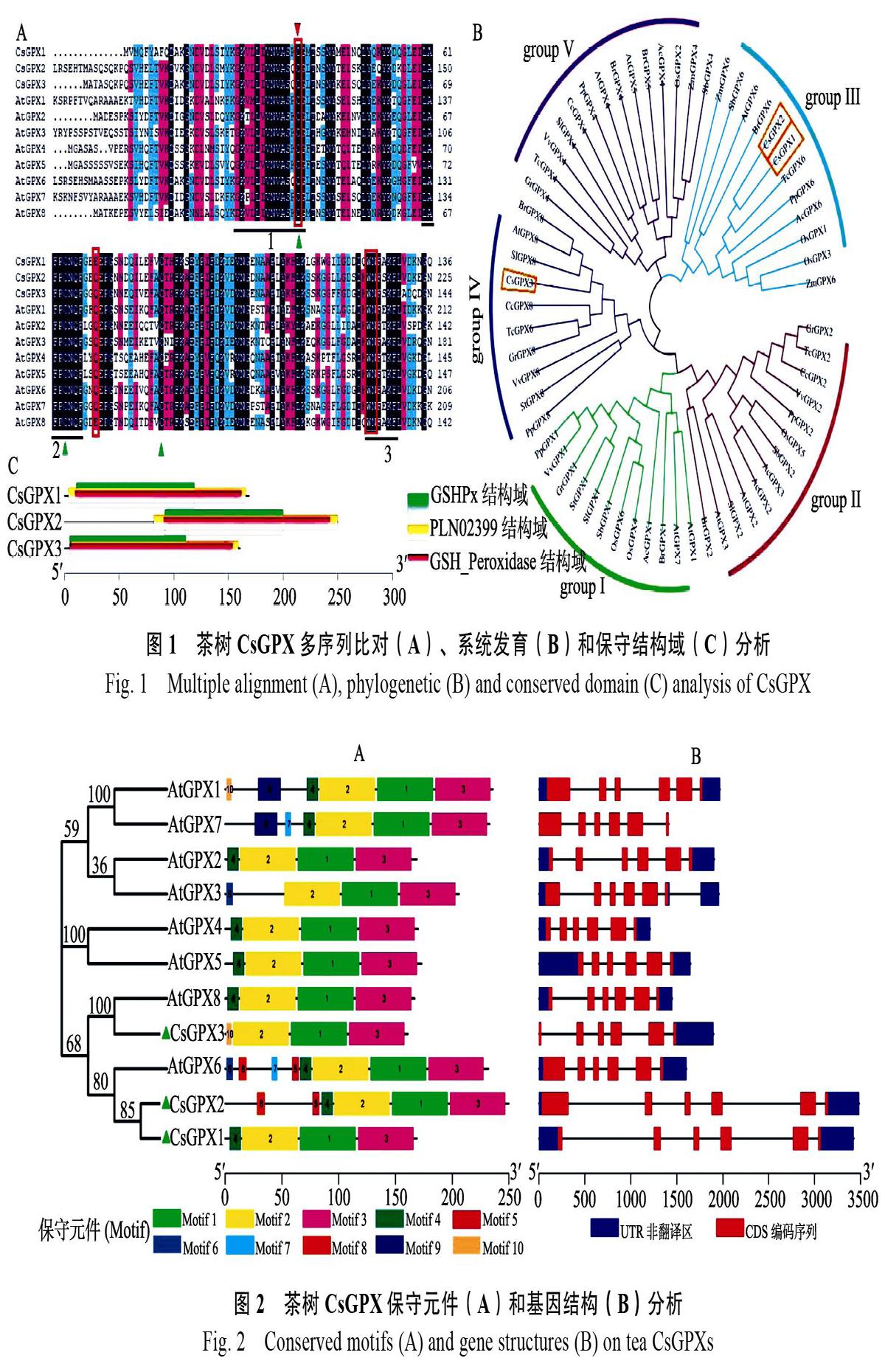
利用MEME在线软件对CsGPX和拟南芥AtGPX蛋白序列分析发现(图2A),所有序列都包含第1、2、3、4总共4个Motif。其中Motif 1、Motif 2和Motif 3组成了CsGPX蛋白GSHPx结构域,Motif 1、Motif 2、Motif 3和Motif 4则构成PLN02399和GSH_Peroxidase结构域,是CsGPX蛋白主要功能区域。
将CsGPX基因CDS和其对应的基因组序列通过GSDS在线分析,进一步了解CsGPX基因结构。结果显示,这些基因结构相似,都包括5个内含子和6个外显子(图2B),与Zhai等人[28]的研究结果一致。
下划线分别表示3个保守结构区域,红色三角形表示哺乳动物中被Sec取代的半胱氨酸残基。绿色三角表示植物GPX蛋白的3个半胱氨酸残基,红色方框表示组成GPX催化部位的氨基酸残基(Cys、Gln、Trp和Asn)。AcGPX:菠萝Ananas comosus;SbGPX:高粱Sorghum bicolor;TcGPX:可可Theobroma cacao;CcGPX:克莱门柚Citrus clementina;StGPX:马铃薯Solanum tuberosum;GrGPX:棉花Gossypium raimondii;AtGPX:拟南芥Arabidopsis thaliana;VvGPX:葡萄Vitis vinifera;OsGPX:水稻Oryza sativa;PpGPX:桃Prunus persica;SlGPX:西红柿Solanum lycopersicum;ZmGPX:玉米Zea mays;BrGPX:芸薹Brassica rapa。
2.4 茶树CsGPX基因顺式作用元件分析
将每条CsGPX起始密码子ATG上游2000 bp作为启动子区,在线软件PlantCARE预测了该区域顺式作用元件。结果显示(表3),3条CsGPX具有的顺式作用元件主要分为植物激素响应元件,如脱落酸响应元件(ABRE)、乙烯响应元件(ERE)、生长素响应元件(AuxRR-core)、茉莉酸甲酯响应元件(CGTCA-motif)、赤霉素响应元件(GARE-motif)等。植物生长发育相关顺式作用元件,如O2-site、Skn-1 motif和circadian。生物和非生物胁迫响应顺式作用元件,如热胁迫响应元件(HSE)、干旱诱导元件(MBS)、逆境防御响应元件(TC-rich)、真菌诱导子响应元件(Box-W1)。预测结果表明茶树CsGPX在调节生长发育、激素信号和应对环境胁迫方面有重要作用。
2.5 茶树CsGPX基因表达分析
通过实时荧光定量PCR分析了CsGPX在铁观音不同组织中的表达,结果显示CsGPX1在根中的表达水平远高地上部组织,而CsGPX2则是地上部的表达量高于地下部根系,CsGPX3则在茎、叶中有较高的表达量(图3)。
茶树CsGPX在ABA、干旱(PEG)、低温(4 ℃)和高盐(NaCl)胁迫下的表达谱见图4,各处理下CsGPX表达的总体趋势是上调,其中ABA诱导CsGPX上调表达的速度快于PEG和NaC。低温处理只对CsGPX1的表达有明显的诱导作用。不同小写字母表示显著差异(P<0.05)。
3 讨论
本研究对茶树全基因组范围内进行了CsGPX基因鉴定,共得到3个CsGPX家族基因。生物信息学分析显示均含有完整GPX保守结构域,以及3段保守蛋白序列GKVLLIVNVASXCG(GPX signature 1),ILAFPCNQ(GPX signature 2)和WNFXKF[29]。植物GPX活性依赖3个保守的半胱氨酸(Cys)残基的前2个[30-31],这与动物的不同,动物GPX的催化活性位点依赖含硒代半胱氨酸(SeCys)残基。此外,茶树CsGPX至少包含4个Motif,构成了CsGPX蛋白主要保守功能区域[32],这也不同于动物GPX。启动子区预测结果显示,茶树CsGPX的启动子上主要有植物激素、生物和非生物胁迫等响应顺式作用元件,暗示它们和其他植物中GPX基因一样会受到多种植物激素的调控[33-35]。
植物中GPX的组织特异性表达分析显示,作为叶绿体蛋白的拟南芥AtGPX1通常在绿色组织中大量表达[31],荷花LjGPX家族成员在不同组织中的表达水平同样差异显著[27]。本研究结果表明根是CsGPX1主要表达部位,CsGPX2在茎、叶、花和果实中的表达量均较高,CsGPX3则是主要在茎、叶中表达。有研究认为抗氧化基因的组织差异性表达模式有利于维持不同组织中的ROS稳态,有利于抗逆性的提高[36]。
作为抗氧化代谢网络中的关键酶,GPX维持细胞ROS水平从而保护细胞膜结构完整,提高植物對胁迫氧化应激的耐受程度[37-38]。过表达拟南芥AtPGX3不仅能够调控H2O2稳态,并且通过Ca2+通道调节气孔参与到ABA代谢途径,提高对干旱胁迫耐受性[35]。在冷害胁迫下,水稻OsGPX参与了根部ROS清除,OsGPX高表达及其酶活性的增加提高了水稻的冷害耐受性[39-40]。Gao等[33]采用蛋白免疫印迹和荧光定量PCR技术发现高盐胁迫在根和叶中调节盐芥TsGPX表达,TsGPX家族成员中TsGPX7对于抵御高盐胁迫最为重要。非生物胁迫下的CsGPX表达谱说明其参与茶树的氧化应激防御机制,本研究结果与已有报道结果相似[8, 12],干旱(PEG)、低温(4 ℃)和高盐(NaCl)胁迫均能诱导提高茶树叶片中不同CsGPX的表达水平,尽管不同的CsGPX对不同的处理的响应程度有明显的差异。
ABA信号传导途径将外界环境的胁迫压力通过一系列的ABA受体传递到下游代谢通道,进而调控抗性基因表达来增强植物耐受性[41]。Dong等[42]对ABA合成突变体拟南芥研究发现,ABA介导的信号网络增强了酶催活性氧清除剂的基因转录水平,提高了耐盐性。本研究中,CsGPX能迅速响应ABA处理,3 h内表达量就提高了3倍,并在处理的中后期保持较高水平。结合启动子序列分析,3条CsGPX均存在响应ABA的顺式作用元件ABRE,使得ABA可以调控CsGPX表达。通过水稻抗寒性转录分析揭示,在低温胁迫下OsGPX表达量被上调,谷胱甘肽过氧化物酶是主要的抗氧化剂,ABA信号转导途径相关基因参与了冷应激反应,并且ABA信号通路起着主导作用[40]。Gaber[43]发现ABA作为胁迫响应基因诱导物可调节拟南芥AtGPX家族基因表达,通过在拟南芥中异源表达小麦TaGPX发现,其在非生物胁迫和ABA信号传导中有重要作用[28]。
茶树基因组数据的公布有利于从全基因组水平上对重要功能基因进行筛选,其结果更为全面。本研究利用生物信息学手段,在茶树全基因组中鉴定的到3条CsGPX基因家族成员,分析其在不同非生物胁迫中的表达模式。明确了该家族基因在茶树逆境氧化应激响应中具有重要功能,丰富了茶树抗逆机理研究,为进一步在模式植物中对CsGPX进行功能验证和优良品种选育提供理论基础。
参考文献
Hu H, Xiong L. Genetic engineering and breeding of drought-resistant crops[J]. Annual Review of Plant Biology 2014, 65: 715-741.
Shakir S K, Irfan S, Akhtar B, et al. Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings[J]. Ecotoxicology, 2018(9): 1-17.
Kumar D, Yusuf M A, Singh P, et al. Modulation of antioxidant machinery in -tocopherol-enriched transgenic Brassica juncea plants tolerant to abiotic stress conditions[J]. Protoplasma, 2013, 250(5): 1079-1089.
Manquián-Cerda K, Cruces E, Escudey M, et al. Interactive effects of aluminum and cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets cultivated in vitro[J]. Ecotoxicology and Environmental Safety, 2018, 150: 320- 326.
Mates J M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology[J]. Toxicology, 2000, 153(1-3): 83-104.
Gill S S, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants[J]. Plant Physiology and Biochemistry, 2010, 48(12): 909-930.
You J, Chan Z. ROS regulation during abiotic stress responses in crop plants[J]. Front Plant Science, 2015, 6: 1092.
Zhou Y, Hu L, Ye S, et al. Genome-wide identification of glutathione peroxidase (GPX) gene family and their response to abiotic stress in cucumber[J]. 3 Biotech, 2018, 8(3): 159.
Herbette S, Menn A L, Rousselle P, et al. Modification of photosynthetic regulation in tomato overexpressing glutathione peroxidase[J]. Biochimica et Biophysica Acta (BBA) - General Subjects, 2005, 1724(1-2): 108-118.
Iqbal A, Yabuta Y, Takeda T, et al. Hydroperoxide reduction by thioredoxin-specific glutathione peroxidase isoenzymes of Arabidopsis thaliana[J]. The Federation of European Biochemical Societies Journal, 2006, 273(24): 5589- 5597.
Milla M A R, Maurer A, Huete A R, et al. Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signaling pathways[J]. The Plant Journal, 2003, 36(5): 602-615.
Islam T, Manna M, Kaul T, et al. Genome-wide dissection of Arabidopsis and rice for the identification and expression analysis of glutathione peroxidases reveals their stress-specific and overlapping response patterns[J]. Plant Molecular Biology Reporter, 2015, 33(5): 1413-1427.
Chen M, Li K, Li H, et al. The glutathione peroxidase gene family in Gossypium hirsutum: genome-wide identification, classification, gene expression and functional analysis[J]. Scientific Reports, 2017, 7: 44743.
Martinez V, Nieves-Cordones M, Lopez-Delacalle M, et al. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin[J]. Molecules, 2018, 23(3): 535.
Xing X, Fang C, Li L, et al. Improved drought tolerance by α-naphthaleneacetic acid-induced ROS accumulation in two soybean cultivars[J]. Journal of Integrative Agriculture, 2016, 15(8): 1770-1784.
Ren L, Zhang D, Chen G, et al. Transcriptomic profiling revealed the regulatory mechanism of Arabidopsis seedlings response to oxidative stress from cryopreservation[J]. Plant Cell Reports, 2015, 34(12): 2161-2178.
Wang X, Fang G, Yang J, et al. A thioredoxin-dependent glutathione peroxidase (OsGPX5) is required for rice normal development and salt stress tolerance[J]. Plant Molecular Biology Reporter, 2017, 35(3): 333-342.
Zhou X, Chen S, Wu H, et al. Biochemical and proteomics analyses of antioxidant enzymes reveal the potential stress tolerance in Rhododendron chrysanthum Pall[J]. Biology Direct, 2017, 12: 10.
Liu S C, Jin J Q, Ma J Q, et al. Transcriptomic analysis of tea plant responding to drought stress and recovery[J]. PLoS ONE, 2016, 11(1): e0147306.
Hao X, Yang Y, Yue C, et al. Comprehensive transcriptome analyses reveal differential gene expression profiles of Camellia sinensis axillary buds at para-, endo-, ecodormancy, and bud flush stages[J]. Frontiers in Plant Science, 2017, 8: 553.
Cheruiyot E K, Mumera L M, Ng'etich W K, et al. High fertilizer rates increase susceptibility of tea to water stress[J]. Journal of Plant Nutrition, 2009, 33(1): 115-129.
Xia E, Zhang H, Sheng J, et al. The tea tree genome provides insights into tea flavor and independent evolution of caffeine biosynthesis[J]. Molecular Plant, 2017, 10(6): 866-877.
Wei C L, Yang H, Wang S, et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality[J]. Proceedings of the National Academy of Sciences, 2018, 115(18): E4151-E4158.
Yue C, Cao H, Wang L, et al. Molecular cloning and expression analysis of tea plant aquaporin (AQP) gene family[J]. Plant Physiology and Biochemistry, 2014, 83: 65-76.
Hao X, Horvath D, Chao W, et al. Identification and evaluation of reliable reference genes for quantitative real-time PCR analysis in tea plant (Camellia sinensis (L.) O. Kuntze)[J]. International Journal of Molecular Sciences, 2014, 15(12): 22155-22172.
Cao H, Wang L, Yue C, et al. Isolation and expression analysis of 18 CsbZIP genes implicated in abiotic stress responses in the tea plant (Camellia sinensis)[J]. Plant Physiology and Biochemistry, 2015, 97: 432-442.
Ramos J, Matamoros M A, Naya L, et al. The glutathione peroxidase gene family of Lotus japonicus: characterization of genomic clones, expression analyses and immunolocalization in legumes[J]. The New Phytologist 2009, 181(1): 103-114.
Zhai C, Zhao L, Yin L, et al. Two wheat glutathione peroxidase genes whose products are located in chloroplasts improve salt and H2O2 tolerances in Arabidopsis[J]. PLoS One, 2013, 8(10): e73989.
Liu W, Zhao C, Wang P, et al. The response of glutathione peroxidase 1 and glutathione peroxidase 7 under different oxidative stresses in black tiger shrimp, Penaeus monodon[J]. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2018, 217: 1-13.
Attacha S, Solbach D, Bela K, et al. Glutathione peroxidase-like enzymes cover five distinct cell compartments and membrane surfaces in Arabidopsis thaliana[J]. Plant, Cell & Environment, 2017, 40(8): 1281-1295.
Navrot N, Collin V, Gualberto J, et al. Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses[J]. Plant Physiology, 2006, 142(4): 1364-1379.
Kim Y, Jang M, Noh H, et al. Molecular characterization of two glutathione peroxidase genes of Panax ginseng and their expression analysis against environmental stresses[J]. Gene, 2014, 535(1): 33-41.
Gao F, Chen J, Ma T, et al. The glutathione peroxidase gene family in Thellungiella salsuginea: genome-wide identification, classification, and gene and protein expression analysis under stress conditions[J]. International Journal of Molecular Sciences, 2014, 15(2): 3319-3335.
Passaia G, Queval G, Bai J, et al. The effects of redox controls mediated by glutathione peroxidases on root architecture in Arabidopsis thaliana[J]. Journal of Experimental Botany, 2014, 65(5): 1403-1413.
Miao Y, Lv D, Wang P, et al. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses[J]. The Plant Cell, 2006, 18(10): 2749-2766.
Yu L, Ma J, Niu Z, et al. Tissue-specific transcriptome analysis reveals multiple responses to salt stress in populus euphratica seedlings[J]. Genes, 2017, 8(12): 372.
Islam T, Manna M, Reddy M K. Glutathione peroxidase of pennisetum glaucum (PgGPx) Is a functional Cd2+ dependent peroxiredoxin that enhances tolerance against salinity and drought stress[J]. PLoS One, 2015, 10(11): e143344.
Koua D, Cerutti L, Falquet L, et al. PeroxiBase: a database with new tools for peroxidase family classification[J/OL]. Nucleic Acids Research, 2009, 37(suppl_1): D261-D266. [2018-10-11]. https://doi.org/10.1093/nar/gkn680
Yoo Y, Anil Kumar N C, Park J, et al. Global analysis of differentially expressed genes between japonica and indica rice roots reveals the molecular basis for enhanced cold tolerance in japonica rice[J]. Plant Biotechnology Reports, 2017, 11(6): 461-473.
Zhao J, Zhang S, Yang T, et al. Global transcriptional profiling of a cold-tolerant rice variety under moderate cold stress reveals different cold stress response mechanisms[J]. Physiologia Plantarum, 2015, 154(3): 381-394.
Lim C W, Baek W, Jung J, et al. Function of ABA in stomatal defense against biotic and drought stresses[J]. International Journal of Molecular Sciences, 2015, 16(12): 15251-15270.
Dong W, Wang M, Xu F, et al. Wheat oxophytodienoate reductase gene TaOPR1 confers salinity tolerance via enhancement of abscisic acid signaling and reactive oxygen species scavenging[J]. Plant Physiology 2013, 161(3): 1217-1228.
Gaber A. The importance of Arabidopsis glutathione peroxidase 8 for protecting Arabidopsis plant and E. coli cells against oxidative stress[J]. GM Crops & Food, 2014, 5(1): 20-26.

