D2DR影响运动疲劳后皮层信息输出的作用机制
王晓昕 李科 陈孟娇 李佳欣 乔德才 侯莉娟
摘 要:通過观察运动疲劳后大鼠黑质致密区DA能神经元局部场电位(local field potential, LFPs)及D2DR干预后皮层M1-纹状体通路电活动变化情况,分析黑质DA能神经元各频段放电及D2DR介导的皮层M1-纹状体通路信息传递的变化,探讨D2DR介导的黑质-纹状体DA系统在皮层M1区信息输出中的作用机制。方法:采用Wistar大鼠建立运动疲劳模型,随机分为电生理组(n=6)、D2DR激动剂干预组(n=6)及TH蛋白表达组(n=9),TH蛋白表达组内又根据不同的运动阶段分为对照组(CG)、7 d力竭运动即刻组(7FG)及7 d重复力竭运动24 h恢复组(24RG)。采用在体多通道实时同步记录技术,结合实时视频录像记录M1-纹状体及黑质LFPs,分析黑质不同频段在运动疲劳前后放电变化、D2DR激动剂作用后各频段放电情况;采用免疫组化检测纹状体背外侧区TH蛋白在运动疲劳前后的表达情况。结果:1)与CG比较,7FG纹状体背外侧区TH蛋白表达降低,差异具有显著性(P<0.05)。2)与CG比较,7FG黑质致密区θ频段(4~7 Hz)、α频段(7~13 Hz)及β频段(15~30 Hz)PSD值升高;与7FG比较,24RG的PSD值降低,且差异均具有显著性(P<0.05);3)D2DR激动剂干预后,大鼠皮层M1区及纹状体在7 d重复力竭运动后,α频段与β频段PSD值均出现升高,且与CG相比,差异具有显著性(P<0.05);与7FG比较,恢复24 h以后PSD值显著降低(P<0.05)。结论:D2DR作为DA信号系统的关键受体,调节黑质-纹状体DA通路电活动,影响皮层综合信息输出,可视为延缓运动疲劳现象产生的药物干预靶点。
关键词:运动疲劳;D2DR;皮层-纹状体;黑质致密区;神经元电活动
中图分类号:G 804.2 学科代码:040302 文献标识码:A
Abstract:Objective: The Local field potential (LFPs) was observed in the substantia nigra compact and electrical activity change in corticostriatal pathway after D2DR intervention in exercise-induced fatigue rats. We analyzed the changes of DA neuron discharge and D2DR mediated corticostriatal pathway information transmission. To explore the mechanism of D2D2 mediated DA system in the information output of cortical M1 region. Methods: Wistar rats were used to establish the model of exercise-induced fatigue. The rats were divided into control group (CG), 7 days fatigue group (7FG) and 24 hour recovery group (24RG). We used in vivo multichannel recording technology to record electrical activity in the M1, striatum and substantia nigra compact of rats and observed the electrophysiological changes after D2DR intervention. We also detected the expression of TH proteins in the dorsolateral striatum before and after exercise-induced fatigue by immunohistochemistry. Results: 1) Compared with group CG, the expression of TH protein in the dorsolateral area of striatum was significantly decreased in group 7FG (P<0.05). 2) Compared with the CG group, the power spectral density of the θ, α and β band of the SNc was increased after seven days of exhaustion exercise (P < 0.05). After 24 hours of recovery, the PSD value decreased significantly compared with the 7FG group(P<0.05). 3) Compared with the CG group the power spectral density of alpha (7-13Hz) and beta (15-30Hz) bands in the M1 region and striatum was increased in 7FG after injection D2DR agonist (P < 0.05). Conclusion: As a key receptor for the DA signal system, D2DR regulates the electrical activity of the nigrostriatal DA pathway and affects the comprehensive information output of the cortex, which can be regarded as a target for exercise-induced fatigue.
Keywords:exercise-induced fatigue; D2DR; corticostriatal; SNc; neural activity
基底神经节是皮层下一群相互联系的核团的总称,包括纹状体、黑质、苍白球、丘脑底核,对运动的调节及学习认知功能起到重要的作用[1-3]。其中黑质致密区投射到纹状体的DA能神经元构成了中脑DA神经元上行通路中的一条,末梢支配纹状体,从而协调小脑及基底神经节功能,对运动控制起到关键的调控作用。DA通过位于纹状体的D1DR和D2DR平衡锥体外系运动功能,对直接通路起到兴奋效应,对间接通路起到去抑制效应,均能起到易化运动区的作用。研究表明,在正常的衰老过程中,运动行为协调性显著下降[4-5],纹状体D2DR明显减少[6],而提高老年人DA能神经活动则可以改善这种变化[7-9],这表明DA系统损伤可能导致运动功能障碍。
笔者所在实验室的前期研究发现,运动疲劳后,大鼠黑质致密区DA能神经元电活动兴奋性和活动规律性降低[10],新纹状体神经元自发放电频率发生改变,高频放电神经元数量明显增加[11];且纹状体中DA代谢水平显著上升[12],D2DR水平有明显升高,D1DR则没有变化[13],DA能神经元主要通过D2DR对纹状体的电活动进行调节[14],表明黑质DA神经元电活动的变化对纹状体功能的调节起到重要作用,且通过纹状体影响皮层M1区运动信息的输出。2005年,Shi [15]使用频谱分析技术处理多巴胺神经元的自发电信号时,发现近半数自发放电存在低频振荡现象,且这种低频振荡活动还能触发神经元的爆发式放电,且此现象必须在完整的神经回路中才可以观察到,所以低频振荡也被认为是研究脑区之间功能联系和信息交流的新电生理学方法[16]。因此,本研究拟采用在体多通道阵列梯度微电极,结合同步记录技术,分析大鼠黑质致密区低频振荡活动及D2DR干预后皮层M1和纹状体神经元集群的电活动变化,从DA神经振荡及皮层M1—纹状体通路电变化入手探讨运动疲劳后D2DR介导的皮层M1信息输出变化在运动疲劳中枢机制中的调节作用。

1 材料与方法
1.1 实验动物与分组
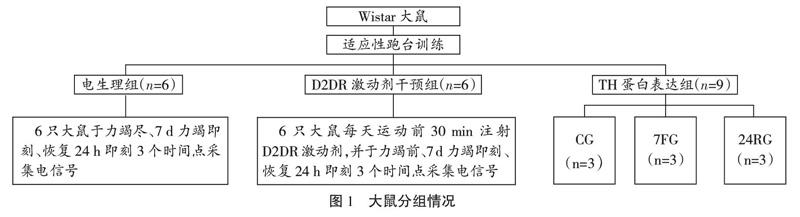
雄性Wistar大鼠(240~260 g)由北京维通利华实验动物科技有限公司(SCXK(京)2012-0001)提供,分笼饲养、自由饮食,动物房温度20~25 ℃,湿度45 %~50 %。大鼠适应性饲养2~3 d后,随机分为电生理组(n=6)、D2DR激动剂干预组(n=6)及TH蛋白表达组(n=9),TH蛋白表达组内单独进行相应实验。针对实验不同运动时期,TH蛋白表达组内分为对照组(control group,CG)、7 d力竭运动即刻组(7-day fatigue group,7FG)及7 d力竭运动24 h恢复组(24-hour recovery,24RG)3个实验组,如图1所示。

1.2 实验技术与方法
1.2.1 大鼠運动疲劳跑台模型建立
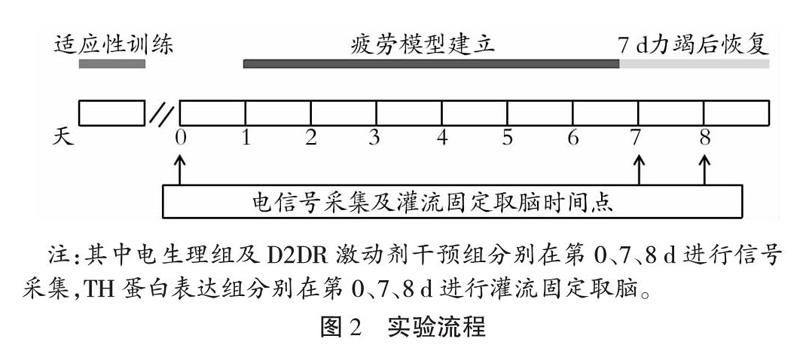
实验前大鼠进行为期3 d的适应性跑台训练,20 min/d。CG大鼠在跑台中自由运动与FG大鼠一次力竭等长时间,排除由于跑台因素对大鼠脑区电活动产生的干扰;7FG大鼠完成7 d重复力竭运动记录原始信号;24RG在7 d力竭运动之后恢复24 h记录原始信号。大鼠疲劳模型通过本实验室改良过后的Bedford递增负荷运动方案建立[17],如图2所示。

运动方案分成三级递增负荷[18-19]:1)一级负荷:运动速度为8.2 m/min,运动时间15 min;2)二级负荷:运动速度为15 m/min,运动时间15 min;3)三级负荷:运动速度为20 m/min,运动至力竭。
1.2.2 大鼠皮层-纹状体及黑质部位阵列微丝电极植入
适应性饲养3 d后的大鼠腹腔注射10%水合氯醛(0.35 g/kg体质量)进行麻醉,固定于双臂数显脑立体定位仪上,对大鼠颅顶范围进行剃毛、消毒、剪皮,根据George Paxinos大鼠脑立体定位图谱[20]确定皮层M1-纹状体坐标范围(AP:0.5~1.5 mm,R:2.3~3.5 mm)、黑质坐标(AP:-4.8~-5.8 mm,R:1.5~2.5 mm),并完成开窗手术,植入16导梯度阵列微丝电极(Stablohm 675,直径35 μm,间距200 μm)。暴露的脑组织处用生物硅胶封口,牙科水泥固定。术后大鼠单笼饲养,自由饮食,腹腔注射青霉素防止感染。
1.2.3 大鼠皮层-纹状体及黑质部位在体电信号的记录
电生理组及D2DR激动剂干预组大鼠在力竭运动后,即刻通过Cerebus-128信号采集装置记录脑电信号,采样频率定为2 kHz;通过lowpass250 Hz滤波得到LFPs原始电信号,Neuromotive系统实时跟踪大鼠自主活动,30 min/次。
1.2.4 组织切片定位
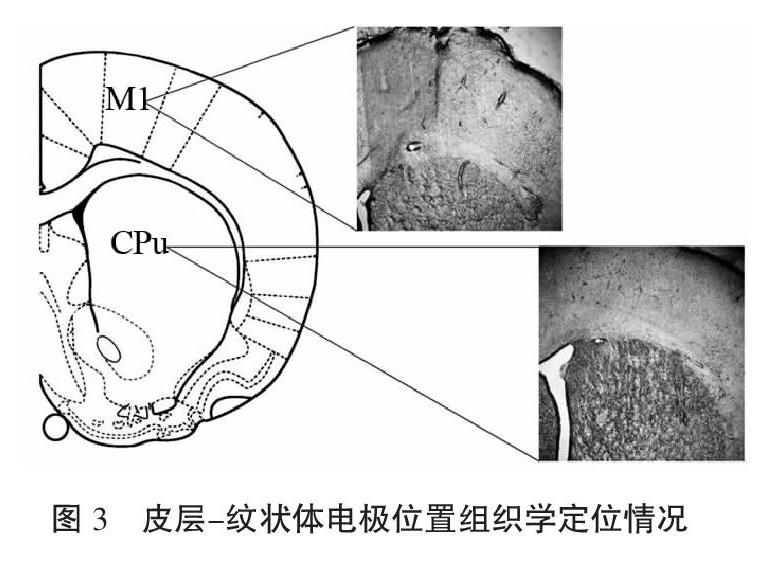
信号采集结束后,植入阵列电极的大鼠用4%多聚甲醛进行灌流、取脑,对皮层M1、STR、SNc所在脑区进行冠状切片(40 μm),Olympus显微镜拍片并观察电极位置(如图3所示)。剔除电极没有落入规定区域的大鼠信号,进入电生理数据统计分析的大鼠共11只合计176通道(电生理组5只80通道,D2DR激动剂干预组6只96通道)。
1.2.5 纹状体背外侧 TH蛋白免疫组织化学检测
TH蛋白表达组大鼠力竭运动即刻采用4%多聚甲醛溶液灌流固定,断头取脑,根据大鼠脑立体定位图谱取纹状体背外侧部脑组织,置于4%PFA中固定24 h;然后脱水、包埋并修块、切片、贴片;一抗、二抗孵育,使用PBS缓冲液冲洗;DAB(二氨基联苯胺)显色,苏木素复染,中性树胶加盖盖玻片封片。
1.3 数据处理
利用Neuro Explorer 5 x86 & Matlab 2015a平台分析整理采集到的原始电生理数据;Olympus显微镜采集免疫组化切片图像,Image Pro Plus 6.0统计分析阳性细胞平均光密度(average opticaldensity,AOD)值;采用SPSS 20.0软件进行统计学分析,Sigmaplot 12.5软件做图,各组实验结果都以平均数±标准差来进行表示。电生理及免疫组化指标组间均数均采用单因素方差分析进行比较,均值差异统一选择LSD/Tamhanes T2检验进行分析;以P<0.05表示差异之间具有显著性。
2 结果
2.1 运动疲劳对大鼠纹状体背外侧TH表达影响
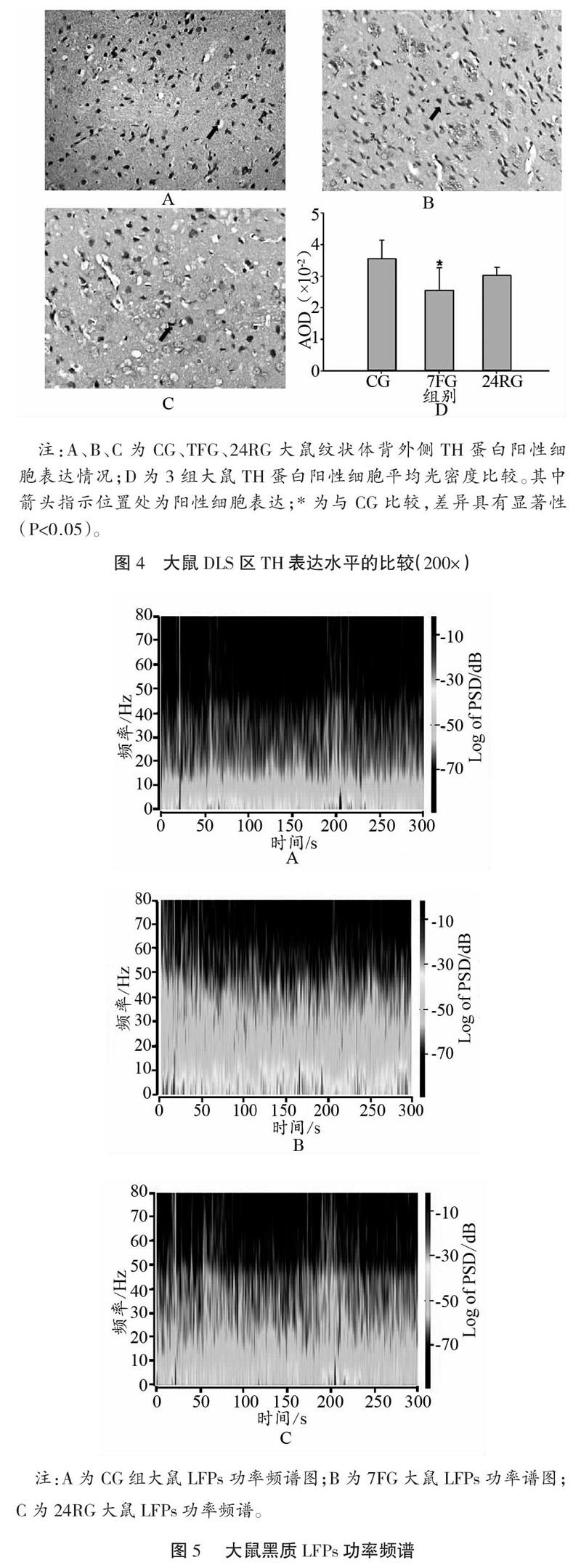
取3组大鼠纹状体背外侧区进行免疫组织化学检测,TH蛋白表达水平如图4所示,免疫组化切片的染色结果当中阳性细胞呈现褐色。与CG比较,7FG大鼠纹状体背外侧TH蛋白表达水平下调,差异具有显著性(P<0.05),恢复24 h后表达上调,且较7FG差异具有统计学意义(P<0.05)。
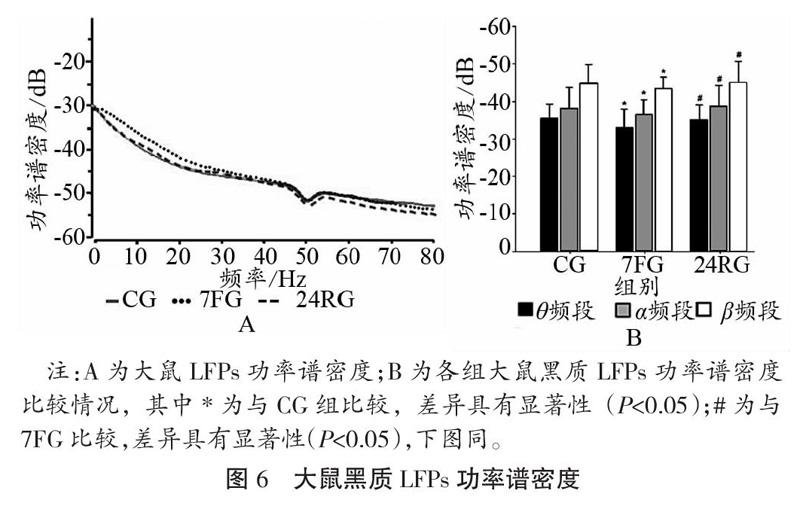
2.2 运动疲劳对大鼠黑质电信号的影响
大鼠安静、7 d重复力竭运动及24 h恢复状态黑质致密区LFPs的功率频谱、功率谱密度如图5、图6所示,在重复力竭运动后各频段的能量值均有所升高,恢复24 h后又出现一定程度的降低(如图5A-C所示);在7 d重复力竭运动后,θ频段、α频段及β频段PSD值升高(如图4 D所示)。与CG相比,7FG大鼠黑质致密区θ频段、α频段及β频段PSD值升高,与7FG比较,24RGPSD值降低,且差异均具有统计学意义(P<0.05)。
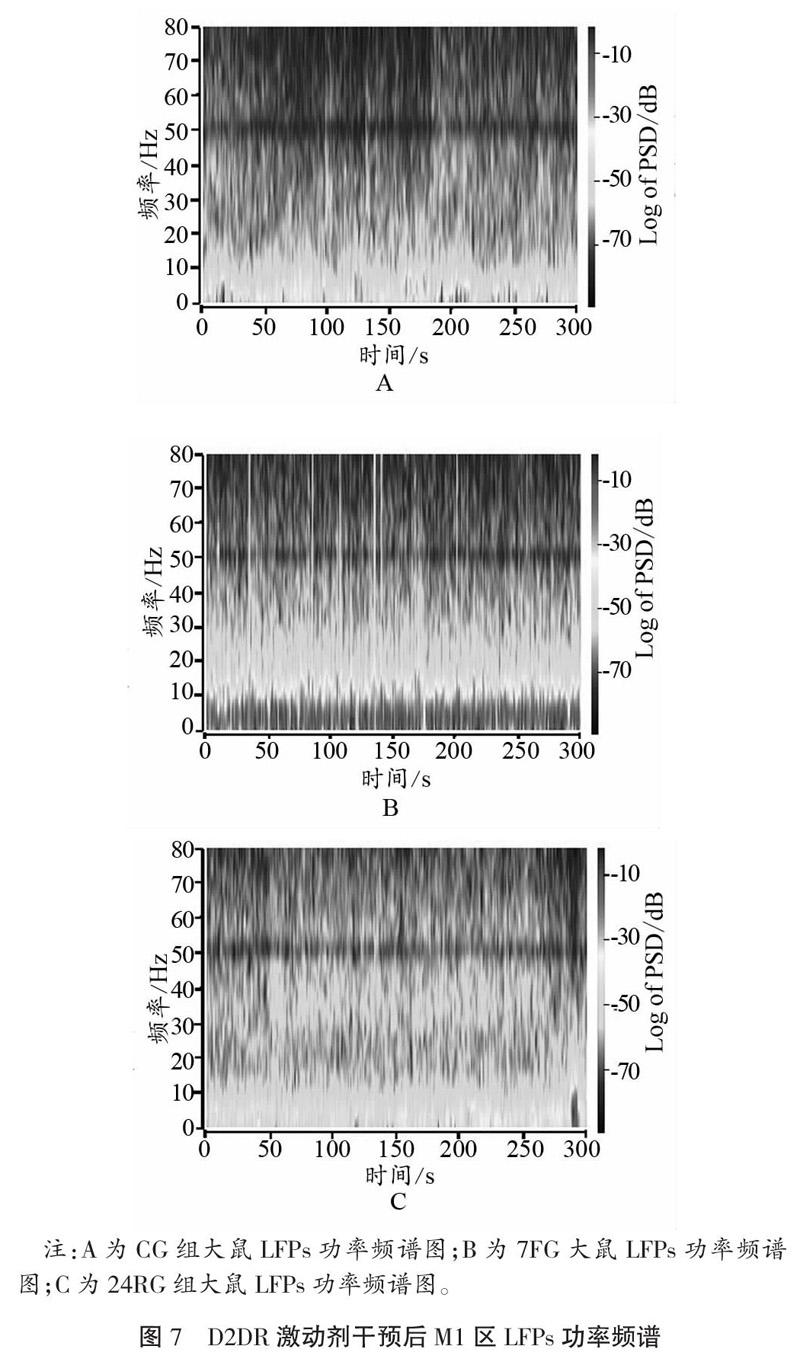
2.3 D2DR激動剂干预对运动疲劳大鼠电活动的影响
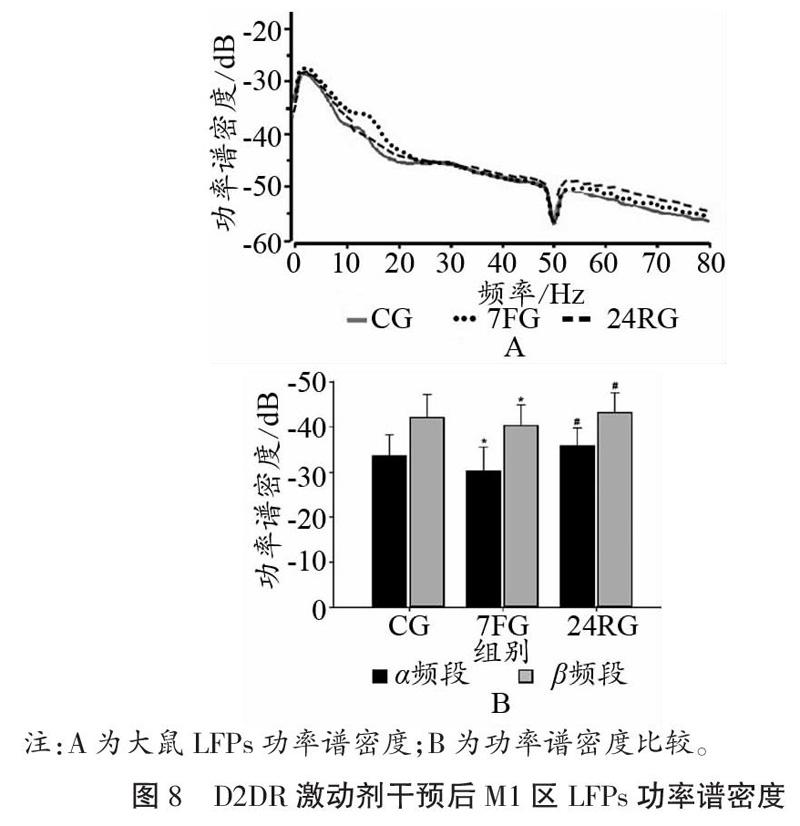
2.3.1 D2DR激动剂干预对运动疲劳大鼠皮层M1区LFPs的影响
如图7、图8所示,D2DR激动剂干预后,大鼠皮层M1区在7 d重复力竭运动后,α频段、β频段PSD值均出现升高,且与CG相比,差异具有显著性(P<0.05);与7FG相比,恢复24 h以后PSD值显著降低(P<0.05)。
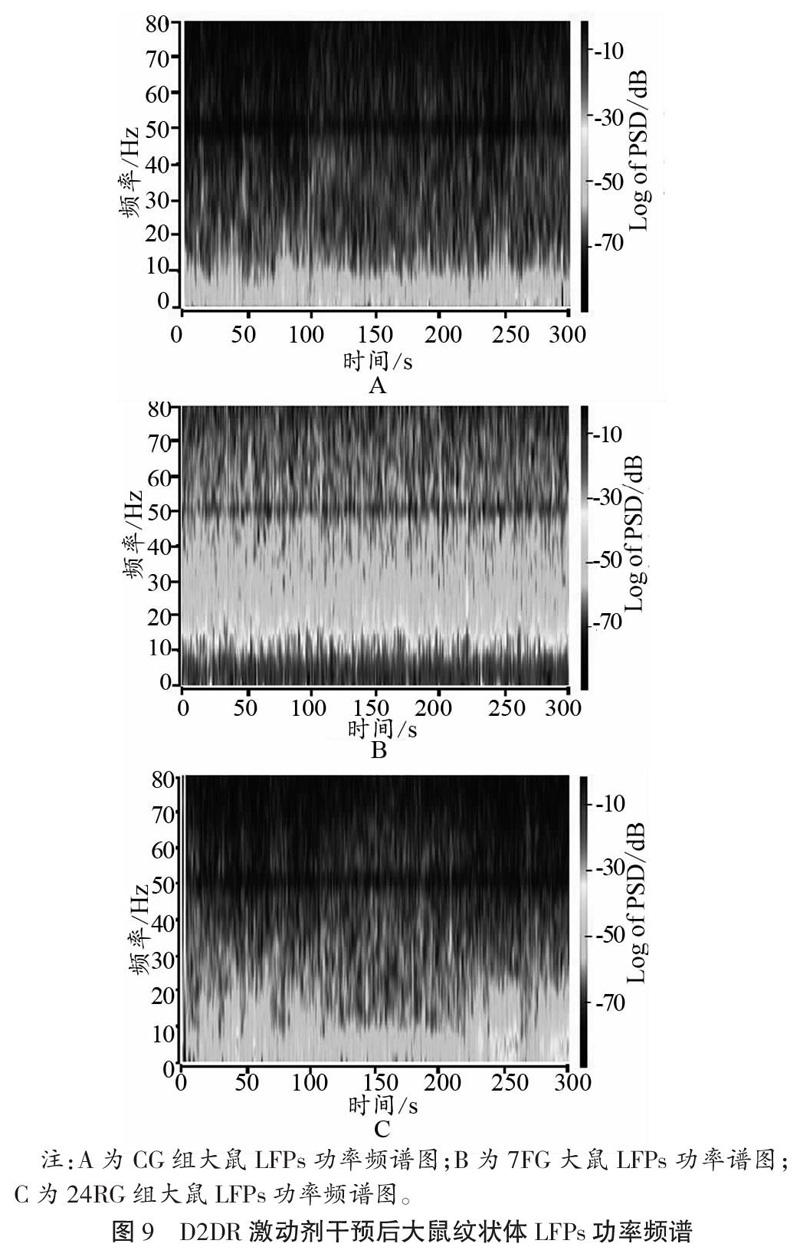
2.3.2 D2DR激动剂干预对运动疲劳大鼠纹状体LFPs的影响
如图9、图10所示,D2DR激动剂干预后,大鼠纹状体在7 d重复力竭运动后,α频段和β频段PSD值均出现升高,且与CG相比,差异具有显著性(P<0.05);与7FG相比,恢复24 h以后PSD值显著降低(P<0.05)。
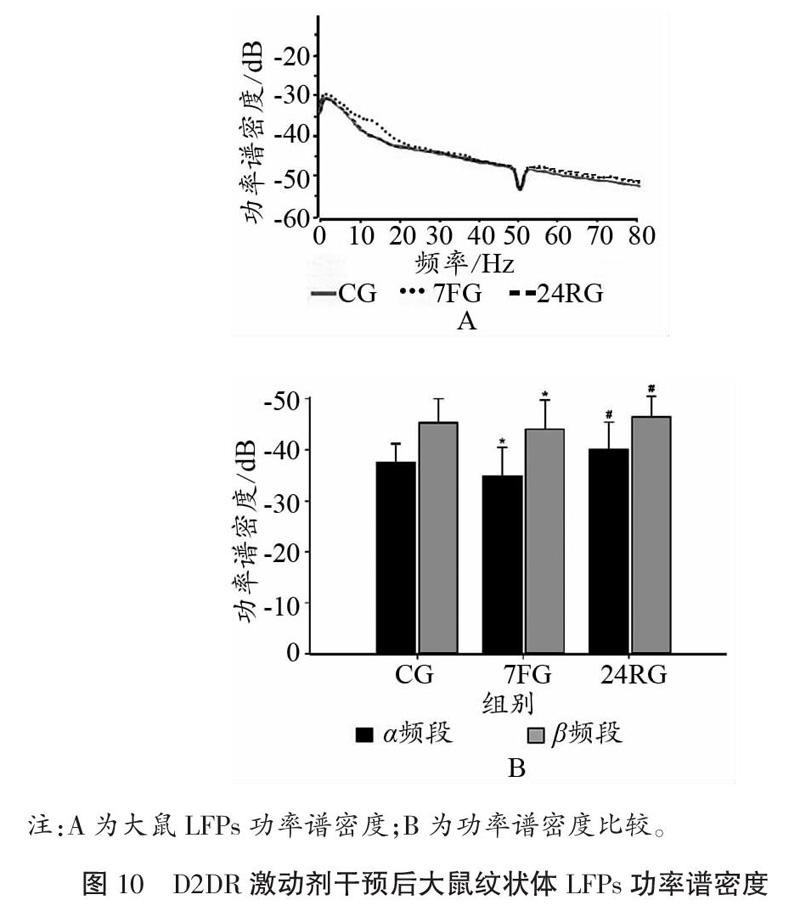
2.3.3 D2DR激动剂干预前后大鼠皮层M1区、纹状体电活动变化
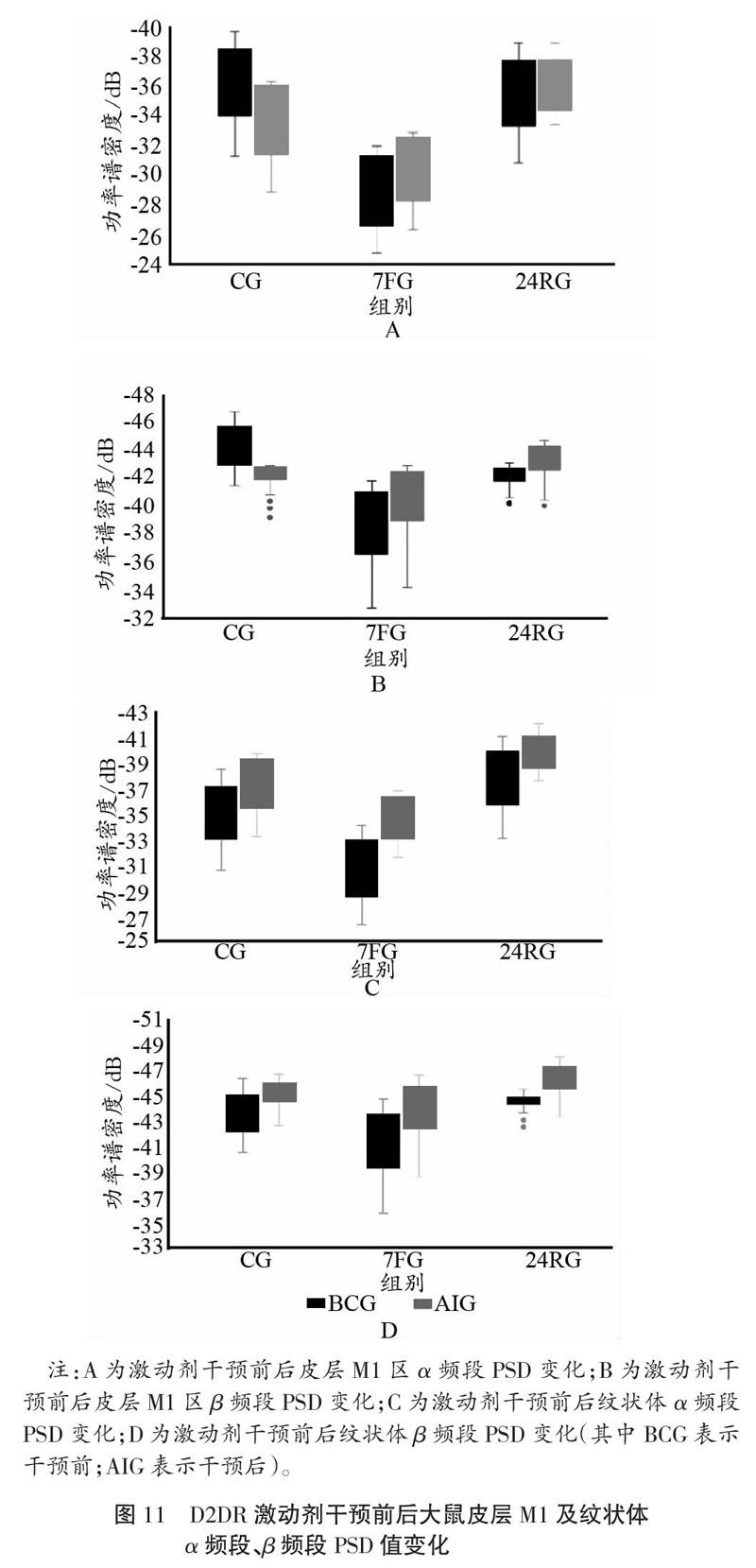
大鼠腹腔注射了D2DR激动剂后,与BCG比较可知,7 d重复力竭运动过后,皮层M1及纹状体α频段、β频段PSD值均出现降低,且纹状体α频段、β频段PSD值在安静、7 d重复力竭运动及24 h恢复状态阶段均低于对照组(如图11所示)。
3 讨论
4 结论
运动疲劳后,大鼠黑质致密部θ频段、α频段及β频段PSD值升高;而D2DR激动剂干预后,与对照组相比,大鼠皮层M1区及纹状体α频段、β频段PSD值均出现降低。表明D2DR作为DA信号系统的关键受体,调节黑质—纹状体DA通路电活动,影响皮层综合信息输出,可视为延缓运动疲劳产生的药物干预靶点。
参考文献:
[1] GRAYBIEL A M. Building action repertoires: memory and learning functions of the basal ganglia[J]. Current Opinion in Neurobiology, 1995, 5(6): 733.
[2] HAMMOND C, BERGMAN H, BROWN P. Pathological synchronization in parkinsons disease: networks, models and treatments[J]. Trends in Neurosciences, 2007, 30(7): 357.
[3] WICHMANN T, DELONG M R. Deep-brain stimulation for basal ganglia disorders[J]. Basal Ganglia, 2011, 1(2): 65.
[4] CUNNINGHAM R L, MACHEDA T, WATTS L T, et al. Androgens exacerbate motor asymmetry in male rats with unilateral 6-hydroxydopamine lesion[J]. Hormones & Behavior, 2011, 60(5): 617.
[5] MCCORMACK A L, DI M D, DELFANI K, et al. Aging of the nigrostriatal system in the squirrel monkey[J]. Journal of Comparative Neurology, 2004, 471(4): 387.
[6] MACRAE P G, SPIRDUSO W W, WILCOX R E. Reaction time and nigrostriatal dopamine function: the effects of age and practice[J]. Brain Research, 1988, 451(1): 139.
[7] ESTEBAN S, GARAU C, APARICIO S, et al. Improving effects of long-term growth hormone treatment on monoaminergic neurotransmission and related behavioral tests in aged rats[J]. Rejuvenation Research, 2010, 13(6): 707.
[8] HURLEY P J, ELSWORTH J D, WHITTAKER M C, et al. Aged monkeys as a partial model for parkinsons disease[J]. Pharmacology Biochemistry & Behavior, 2011, 99(3): 324.
[9] RAZGADO-HERNANDEZ L F, ESPADAS-ALVAREZ A J, REYNA-VELAZQUEZ P, et al. The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson's disease[J]. Plos One, 2015, 10(2): 117391.
[10] 喬德才,吴迪,侯莉娟,等.运动疲劳对大鼠黑质致密区DA能神经元电活动的影响[J]. 上海体育学院学报,2010,34(1):43.
[11] 乔德才,侯莉娟,何德富,等.运动疲劳对大鼠新纹状体神经元电活动的影响[J].中国运动医学杂志,2005,24(6):676.
[12] 杨东升,刘晓莉,乔德才.力竭运动及恢复期大鼠纹状体5-HT、DA及其代谢物浓度的动态变化研究[J].中国应用生理学杂志,2011,27(4):432.
[13] 侯莉娟,刘晓莉,乔德才.运动疲劳对大鼠纹状体单胺类递质含量及多巴胺受体表达的影响[J].中国康复医学杂志,2010,25(7):639.
[14] 刘晓莉,吴迪,乔德才,等.电刺激前脑内侧束对运动疲劳大鼠纹状体神经元诱发电活动的影响[J].西安体育学院学报,2012,29(1):78.
[15] SHI W X. Slow oscillatory firing: a major firing pattern of dopamine neurons in the ventral tegmental area[J]. Journal of Neurophysiology, 2005, 94(5): 3516.
[16] PIERRO M L, SASSAROLI A, BERGETHON P R, et al. Phase-amplitude investigation of spontaneous low-frequency oscillations of cerebral hemodynamics with near-infrared spectroscopy: a sleep study in human subjects[J]. Neuroimage, 2012, 63(3): 1571.
[17] BEDFORD T G, TIPTON C M, WILSON N C, et al. Maximum oxygen consumption of rats and its changes with various experimental procedures[J]. Journal Applied Physiology, 1979, 47(6): 1278.
[18] 侯莉娟,成佳俐,王曉昕,等.运动疲劳引起纹状体突触超微结构变化及D2DR介导的行为学干预研究[J].体育科学,2017,37(6):62.
[19] 刘晓莉,罗勇,乔德才.大鼠一次性力竭跑台运动模型的建立及动态评价[J].中国实验动物学报,2012,20(3):25.
[20] PAXINOS G, WATSON C. The rat brain in stereotaxic coordinates, Fourth Edition[M]. London: Academic Press, 1998: 80-113.
[21] BECKSTEAD M J, FORD C P, PHILLIPS P E M, et al. Presynaptic regulation of dendrodendritic dopamine transmission[J]. European Journal of Neuroscience, 2010, 26(6): 1479.
[22] CRAGG S J. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum[J]. Journal of Neuroscience, 2003, 23(10): 4378.
[23] RICE M E, PATEL J C, CRAGG S J. Dopamine release in the basal ganglia[J]. Neuroscience, 2011(198): 112.
[24] MOGENSON G J, JONES D L, YIM C Y. From motivation to action: functional interface between the limbic system and the motor system[J]. Progress in Neurobiology, 1980, 14(2-3): 69.
[25] 侯莉娟,刘晓莉,乔德才.DA受体对运动疲劳后纹状体神经元信号转导调节作用的研究[J].西安体育学院学报,2011,28(1):79.
[26] KREITZER A C. Physiology and pharmacology of striatal neurons[J]. Annual Review of Neuroscience, 2009, 32(1): 127.
[27] NISENBAUM E S, WILSON C J. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons[J]. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 1995, 15(6): 4449.
[28] CARR D B, DAY M, CANTRELL A R, et al. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity[J]. Neuron, 2003, 39(5): 793.
[29] SURMEIER D J, EBERWINE J, WILSON C J, et al. Dopamine receptor subtypes colocalize in rat striatonigral neurons[J]. Proceedings of the National Academy of Sciences of the United States of America, 1992, 89(21): 10178.
[30] CARTER A G, SABATINI B L. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons[J]. Neuron, 2004, 44(3): 483.
[31] FLORES H J, CEPEDA C E E, CALVERT C R, et al. Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32[J]. Journal of Neurophysiology, 2002, 88(6): 3010.
[32] HALLETT P J, SPOELGEN R, HYMAN B T, et al. Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking[J]. Journal of Neuroscience, 2006, 26(17): 4690.
[33] SURMEIER D J, BARGAS J, JR H H, et al. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons[J]. Neuron, 1995, 14(2): 385.
[34] DI F M, PICCONI B, TANTUCCI M, et al. Short-term and long-term plasticity at corticostriatal synapses: implications for learning and memory[J]. Behavioural Brain Research, 2009, 199(1): 108.
[35] CALABRESI P, PISANI A, MERCURI N B, et al. Long-term potentiation in the striatum is unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels[J]. European Journal of Neuroscience, 1992, 4(10): 929.
[36] KERR J N, WICKENS J R. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro[J]. Journal of Neurophysiology, 2001, 85(1): 117.
[37] KREITZER A C, MALENKA R C. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum[J]. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 2005, 25(45): 10537.
[38] SHEN W, FLAJOLET M, GREENGARD P, et al. Dichotomous dopaminergic control of striatal synaptic plasticity[J]. Science, 2008, 321(5890): 848.
[39] MACRAE P G, SPIRDUSO W W, WALTERS T J, et al. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolites in presenescent older rats[J]. Psychopharmacology, 1987, 92(2): 236.
[40] 侯莉娟,劉晓莉,乔德才.运动疲劳对大鼠纹状体单胺类递质含量及多巴胺受体表达的影响[J].中国康复医学杂志,2010,25(7):639.
[41] TRIFILIEFF P, MARTINEZ D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity[J]. Neuropharmacology, 2014, 76(1): 498.
[42] LEMOS J C, FRIEND D M, KAPLAN A R, et al. Enhanced GABA transmission drives bradykinesia following loss of dopamine D2 receptor signaling[J]. Neuron, 2016, 90(4): 824.
[43] SCIAMANNA G, BONSI P, TASSONE A, et al. Impaired striatal D2 receptor function leads to enhanced GABA transmission in a mouse model of DYT1 dystonia[J]. Neurobiology of Disease, 2009, 34(1): 133.
[44] JONES G. Caffeine and other sympathomimetic stimulants: modes of action and effects on sports performance[J]. Essays in Biochemistry, 2008(44): 109.

