锆簇基纳米分子胶囊的合成、晶体结构和荧光性质
陶艳丽 陈维超*, 王新龙 苏忠民*,,2
(1东北师范大学化学学院,长春 130024)(2长春理工大学,长春 130024)
0 Introduction
Metal-organic molecular capsules(MCs)remains discrete inorganic-organic molecular complexes that are built by the self-assembly of metal ions/clusters and organic linkers[1-3].It has attracted extensively wide investigations as a result of its fascinating structures with adjustable inner cavities and potential applications,such as gas storage-separation[4-6],magnetism[7-10],drug delivery[11-12],catalysis[4,13]together with guest molecule accommodating and release[13-14].As is known to all,the reported secondary building units(SBUs)during the preparation of MCs mainly focuse on early transition metal centers,and the reversible property of such coordination bonds may bring poor water stability as well as limited applications in most research area of MCs.
Recently,Zr-based metal-organic frameworks featuring high stability in both acid and alkaline aqueous solutions owing to the quite strong Zr-Obonds(chemical bond energy:776 kJ·mol-1)have gained extensive interests[15].Inspired by this aspect,in 2013,Yuan and co-workers has developed a facile strategy to access a family of Zr-based metal-organic polyhedra(MOPs)by reaction of bis(cyclopentadienyl)zirconium dichloride (Cp2ZrCl2,Cp=η5-C5H5)with carboxyl ligands[16].Such zirconocene precursor could be easily hydrolyzed in presence of water and carboxylate acid to achieve a trinuclear ligand-bridged Zr-cation moiety,namely Cp3Zr3(μ3-O)(μ2-OH)3,which acts as 3-connected SBUs for the construction of various waterstable coordination polyhedra.Those MOPs show potential applications in catalysis[17],proton conductivity[18]and gas storage-separation[19],etc.So far,only several Zr-based MOPs have been discovered,and the rational design and synthesis of more Zr-based MOPs with specific architecture and functionality still possess a considerable challenge[15-23].
Taking into account the above backgrounds,we herein present the synthesis and crystal structures of two Zr-MCs constructed from trinuclear Cp3Zr3(μ3-O)(μ2-OH)3SBUs and linear dicarboxylate ligands.They were fully characterized by multiple analysis techniques and their photoluminescent properties were also described.
1 Experimental
1.1 Materials and physical measurements
All chemical reagents were derived from commerce and used directly without purification.PXRD patterns were measured from 5°to 50°through a Siemens D5005 diffractometer at 40 kV,30 mA for Cu Kα (λ=0.154 18 nm).Thermogravimetric analysis(TGA)of the crystal samples was tested using a PerkinElmer TG-7 analyzer by heating from 25 to 800℃ (10℃·min-1)under a dry nitrogen environment.Elemental analyses (C,H,N)were performed on a PerkinElmer 2400 CHN Elemental analyzer.The FTIR spectra were measured from 4 000 to 400 cm-1using KBr pellets with an Alpha Centauri FT/IR spectrophotometer.N2gas adsorption-desorption and porosimetry were performed on ASIQM0G002-3.The solid-state photoluminescence (FL)spectra of both crystals and organic ligands were performed at room temperature using F-7000 FL spectrophotometer.A transient spectrofluorimeter(Edinburgh FLSP920)was used to test the excited-state lifetimes (τ)of the two compounds in solid state.
1.2 Preparations of Zr-MC-1 and Zr-MC-2
Zr-MC-1 was synthesized by reacting 4,4′-H2BPDC(0.02 g,0.08 mmol)and zirconocenedichloride(Cp2ZrCl2)(0.03 g,0.1 mmol)in N,N-dimethylformamide(DMF,1.0 mL),CH3OH (1.0 mL)and water(5 drops)at 80℃for 18 hours.After slowly cooling to room temperature,colorless crystals were obtained,washed with DMF and dried in air.Yield:88%based on 4,4′-H2BPDC.Elemental analysis Calcd.for C72H62O20Zr6Cl(%):C 47.33,H 3.29;Found(%):C 47.23,H 3.30.IR(KBr,cm-1):3 420(m),1 654(m),1 608(m),1 574(s),1 524(s),1 415(s),1 180(w),1 097(w),1 013(m),808(w),766(w),695(w),674(m),603(m),536(m),447(s).
Zr-MC-2 was prepared by reacting 2,6-H2NDC(0.02 g,0.08 mmol)and zirconocenedichloride(Cp2ZrCl2)(0.03 g,0.1 mmol)in N,N-dimethylformamide(DMF,1.0 mL),CH3OH (1.0 mL)and water(5 drops)at 80℃for 12 hours.Colorlesscrystalswere isolated(washed with DMF)when the oven was gradually cooled to room temperature.Yield:90%based on 2,6-H2NDC.Elemental analysis Calcd.for C66H60O23Zr6Cl2(%):C 43.11,H 3.27;Found(%):C 43.31,H 3.20.IR(KBr,cm-1):3 198(m),2 930(w),1 657(s),1 599(s),1 553(s),1 494(w),1 419(s),1 365(s),1 247(w),1 197(w),1 092(w),1 017(w),921(w),812(m),787(m),770(w),658(w),607(m),490(m).
1.3 Single-crystal X-ray crystallography
Single-crystal X-ray crystallography data were collected at 298 K on Bruker D8 VENTUREwith microfocus Cu Kα radiation(λ=0.154 178 nm).Absorption corrections were achieved by a multi-scan technique.The structures were solved by direct methods,and further refinement of the structures was performed by using full-matrix least squares techniques with SHELXL-2014 program[24].Hydrogen atoms were fixed geometrically at calculated positions and allowed to ride on the parent non-H atoms.All the non-H atoms were refined ansiotropically during the refinement.Additionally,some restraints were used including SIMU and DFIX in the final refinement.Details of the data are summarized in Table 1.
CCDC:939840,Zr-MC-1;939841,Zr-MC-2.
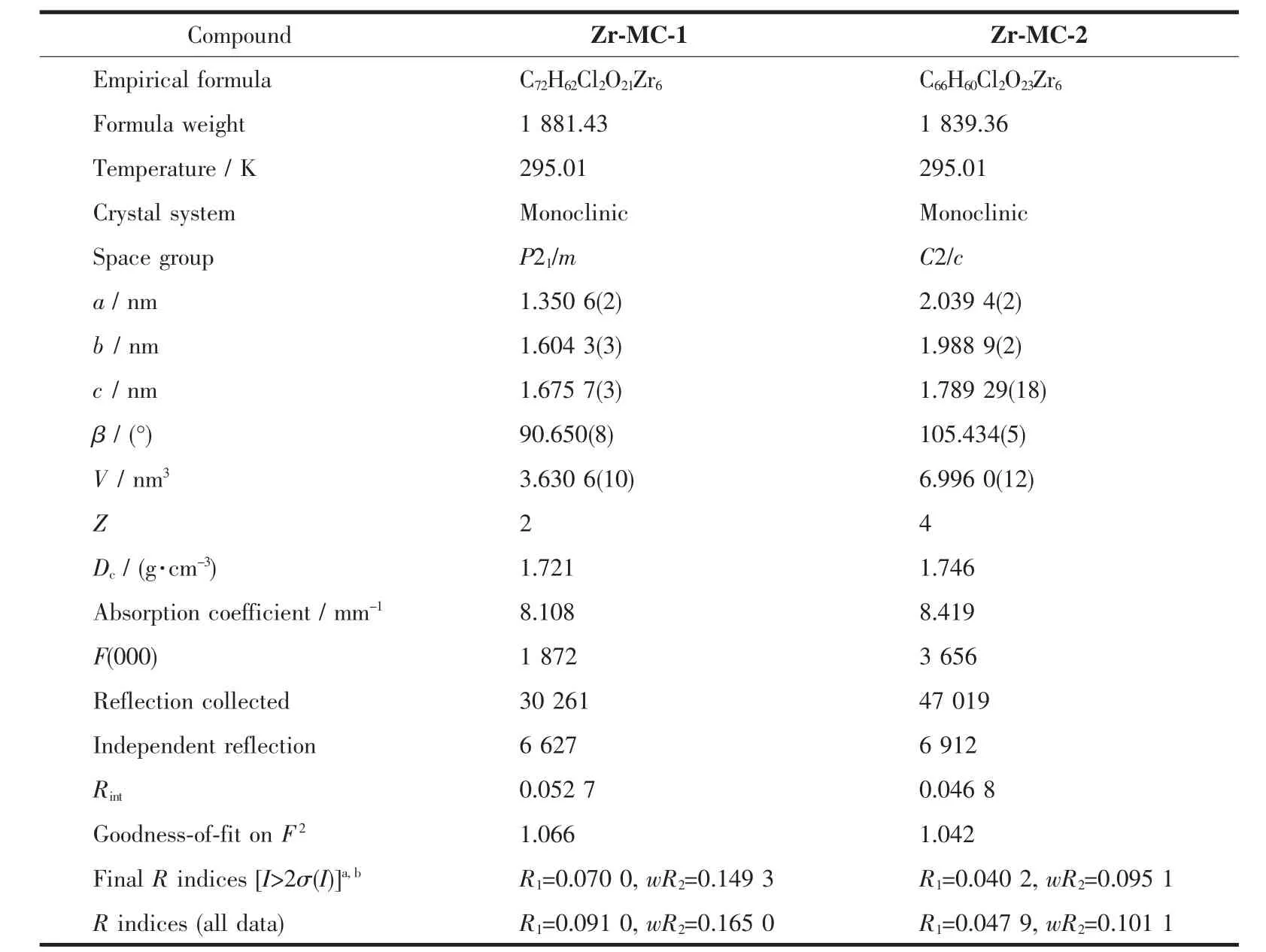
Table 1 Crystal data and structure refinement parameters for Zr-MC-1 and Zr-MC-2
2 Results and discussion
2.1 Structure descriptions of Zr-MC-1 and Zr-MC-2
Reaction of Cp2ZrCl2and linear dicarboxylate ligands(4,4′-H2BPDC for Zr-MC-1 and 2,6-H2NDC for Zr-MC-2)in a mixed solvent system including DMF,CH3OH and distilled water at 80℃gave rise to two nanosized molecular capsules (Zr-MCs).As previously reported by Yuan et al.[16],the cationic Cp3Zr3(μ3-O)(μ2-OH)3SBUs are derived from the slow hydrolysis process of Cp2ZrCl2in the existence of water (Fig.1).The carboxylate ligands in the SBUs(close to C3symmetry)are oriented to one face while theμ-OH species are oriented to the other,where the C2axes in carboxylate ligands create an angle of approximately 60°.In this connection,the substitution of the primary carboxylate in Cp3Zr3(μ3-O)(μ2-OH)3by the linear 4,4′-H2BPDC and 2,6-H2NDC ligands was realized in this system.
Structural analysis (Table 1)indicates that Zr-MC-1 and Zr-MC-2 both crystallize in the monoclinic system with corresponding P21/m and C2/c space group,respectively.Two molecular capsules representing V2E3(V=vertex and E=edge)topology could be regarded as two Cp3Zr3(μ3-O)(μ2-OH)3SBUs as the vertices and three associated ligands as the edges.Similar topology in Zr-MCs including the flexible sulfonate-carboxylate ligand has already been reported by Zang et al[18].Such type of capsules with a well-known linear connection between the metal SBUs and organic species remains excellent candidate in metal-organic supercontainers.As described in Fig.2,the capsule inner (yellow column)pore sizes of the cages are about 1.3 and 1.1 nm(the distance between twoμ3-Oatomsin both zirconocene SBUs),respectively.Just as expected,this distinction is attributed to the lengths of 4,4′-H2BPDC and 2,6-H2NDC ligands.In both crystal structures,hydrogen bonds(O-H…Cl…H-O)among Cl-,μ2-OH on zirconocene and free water not only form the three-dimensional supramolecular networks of MCs but also balance the framework charge.Furthermore,selected bond distances and bond angles of Zr-MC-1 and Zr-MC-2 are listed in Table 2.

Fig.1 Diagrammatic sketch of the formation of trinuclear Cp3Zr3(μ3-O)(μ2-OH)3 SBUs
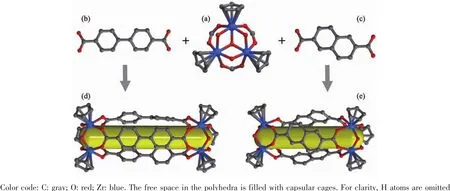
Fig.2 Ball-and-stick representation of[Cp3Zr3(μ3-O)(μ2-OH)3]2 SBUs(a),4,4′-BPDCligand(b),2,6-NDCligand(c),Zr-MC-1(d),and Zr-MC-2(e)
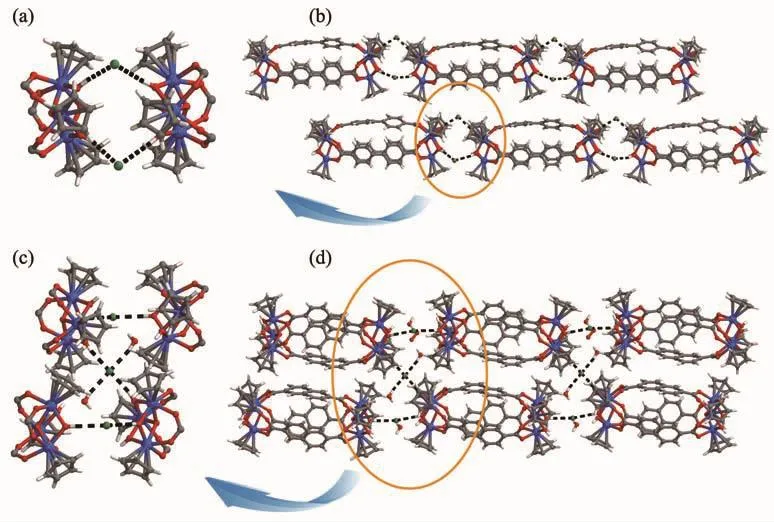
Fig.3 Hydrogen-bonding in Zr-MC-1(a)and 3D stacking of Zr-MC-1(b);Hydrogen-bonding in Zr-MC-2(c)and 3D stacking of Zr-MC-2(d)
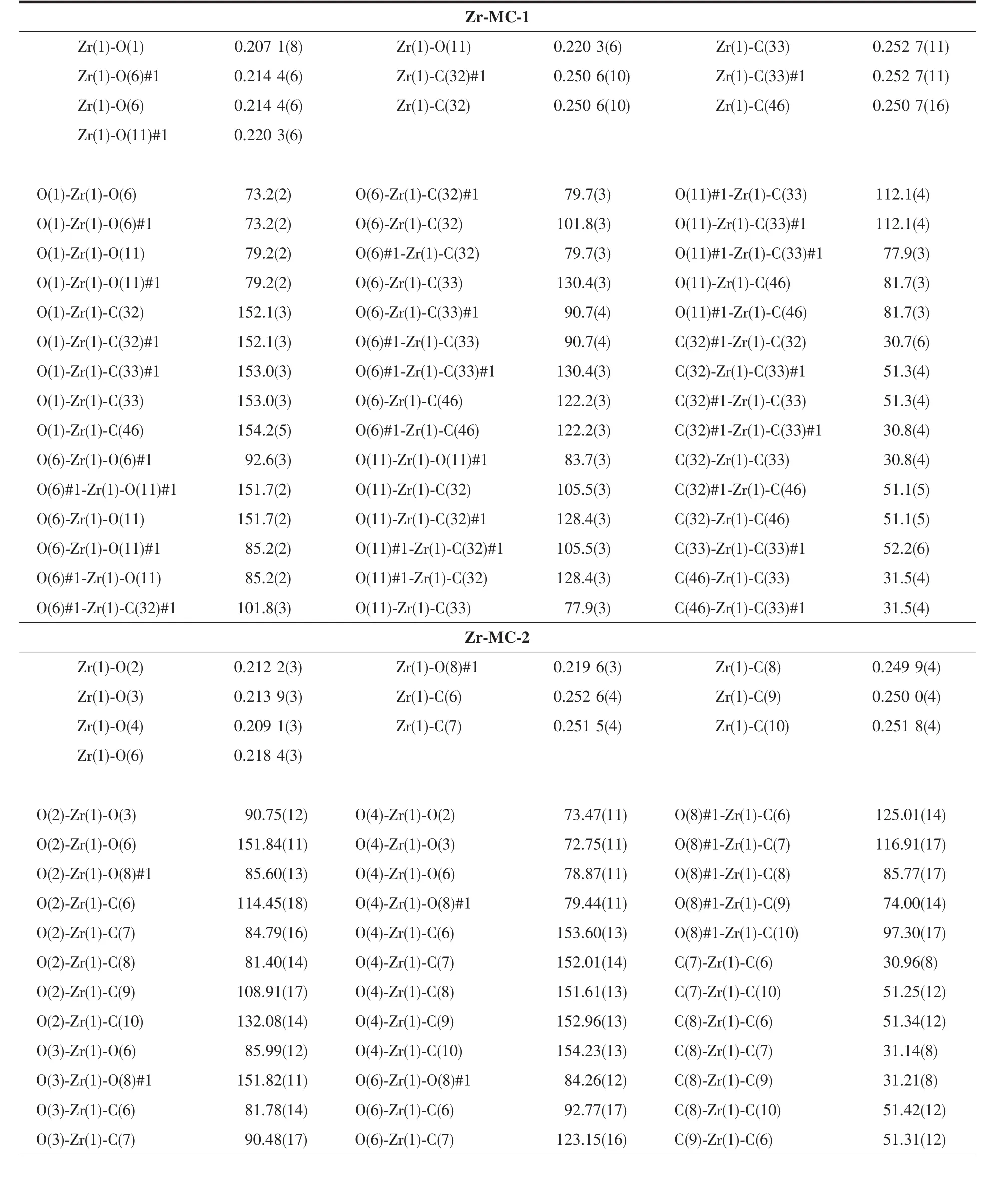
Table 2 Selected bond lengths(nm)and angles(°)for Zr-MC-1 and Zr-MC-2
Continued Table 2
2.2 Powder X-ray diffraction(PXRD)and thermogravimetric analysis(TGA)
The phase purities of Zr-MC-1 and Zr-MC-2 were verified by PXRD.The uniform patterns between the experimental and simulated results give evidence of pure target samples,and the crystalline structures of both MCs remained unchanged after the water treatment (Fig.4).In order to test the thermal stabilities of the two crystals,the TGA experiment was carried out between 25 and 800℃under N2atmosphere.The weight loss below 100℃indicates the loss of physically absorbed water molecules,and chloride ions and lattice water molecules were released afterwards.Finally,Zr-MC-1 and Zr-MC-2 lost weight drastically at~500℃ (Fig.5).

Fig.4 Experimental and simulated powder X-ray diffraction patterns for Zr-MC-1(a)and Zr-MC-2(b)

Fig.5 TG curves for Zr-MC-1(a)and Zr-MC-2(b)
2.3 Infrared spectroscopy(IR)
IR spectroscopy is an effective mean for the analysis of organic ligands and zirconocene SBUs in the crystal structures.For this purpose,IR spectra of two MCs were measured in KBr pellets.As presented in Fig.6,the absorption peaks in 400~1 200 cm-1region belong to zirconium-oxygen characteristic stretching vibrations[25]. The peaks located at 3 420 cm-1for Zr-MC-1 and 3 198 cm-1for Zr-MC-2 could be assigned to the vibration of the OH group,because of the exists ofμ2-OH species and free water molecules in the crystals[26].There was no absorption band in the region from 1 690 to 1 730 cm-1,showing that 4,4′-H2BPDC and 2,6-H2NDC groups in Zr-MC-1 and Zr-MC-2 are deprotonated[26].Furthermore,the peaks of 1 415 cm-1(1 608 cm-1)for Zr-MC-1 and 1 419 cm-1(1 599 cm-1)for Zr-MC-2 areattributed tothesymmetric(asymmetric)C-O stretching vibrations[27].

Fig.6 IR spectra for Zr-MC-1(a)and Zr-MC-2(b)
2.4 N2 adsorption measurements
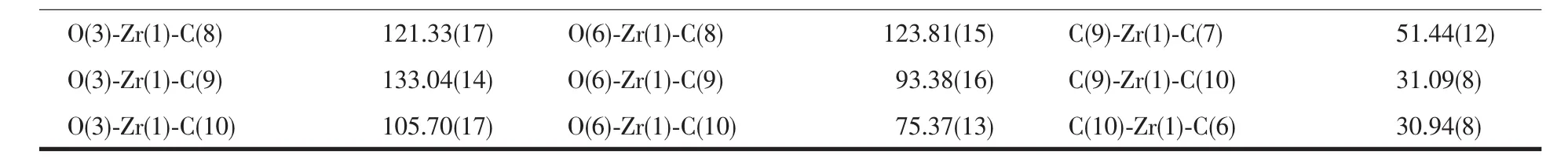
In the light of capsule inner pores and pore connectivity in Zr-MC-1 and Zr-MC-2,N2adsorption experiments were determined at 77 K.Fresh samples(150 mg)were solvent-exchanged with dichloromethane several times for 3 days,and then the soaking samples were further activated at 60℃for 12 hours for next test.As shown in Fig.7a,a hysteresis loop was identified through the relative pressure P/P0ranging from 0.4 to 1.0 for Zr-MC-1.The isotherm could be assigned to the typicalⅣisotherm on the basis of the IUPAC classification[20].Similar observations are also occurred for Zr-MC-2 (Fig.7c).From Fig.7b and 7d,it may be concluded that both MCs are mesoporous materials.The noticeable Brunauer-Emmett-Teller(BET)and Langmuir surface areas of Zr-MC-1 were 28 and 44 m2·g-1,respectively.The pore volume is 0.089 4 nm3and the porosity is 2.5%according to the PLATON routine.Similarly,BET surface areas and Langmuir surface areas of Zr-MC-2 are 90 and 132 m2·g-1,respectively.The pore volume is 0.354 nm3and the porosity is 5.1%based on the calculated results from the PLATON routine[20].
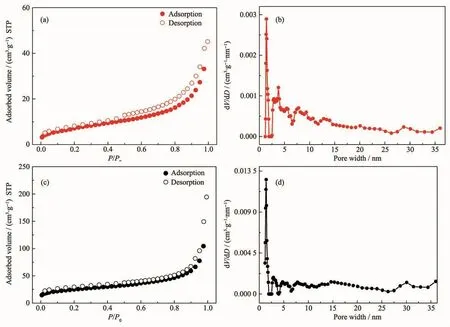
Fig.7 N2 adsorption-desorption isotherms of Zr-MC-1(a)and Zr-MC-2(c)at 77 K;Pore size distributions of Zr-MC-1(b)and Zr-MC-2(d)
2.5 Luminescence property
The solid-state photoluminescence(PL)spectra of Zr-MC-1 and Zr-MC-2 as well as corresponding dicarboxylate ligands were investigated at room temperature.As depicted in Fig.8,on the principle of π*→n transitions of the intraligands in either free ligands,4,4′-H2BPDC ligand (red line,Fig.8a)shows an emission at 407 nm (λex=330 nm)while the strong emission peak of 2,6-H2NDC ligand (red line,Fig.8b)is located at 424 nm(λex=380 nm)[28-29].With respect to Zr-MC-1(black line,Fig.8a),the emission(404 nm,λex=330 nm)is mainly derived from 4,4′-H2BPDC ligand.Conversely,Zr-MC-2 exhibited an apparent red-shifted emission (456 nm,λex=380 nm)compared to 2,6-H2NDC ligand,this phenomenon is not strange and should be assigned to ligand-to-metal charge transfer (LMCT)process.What′s more,these observations indicate that Zr-MC-1 and Zr-MC-2 might be excellent candidates of deep-blue and bluelight-emitting materials,respectively.
Additionally,the luminescence decay curves of Zr-MC-1 and Zr-MC-2 were also measured at room temperature.As shown in Fig.9,the luminescence lifetimes of both MCs are quite different:the lifetime of Zr-MC-1 (λex=330 nm)and Zr-MC-2 (λex=380 nm)are 1.48 and 17.02 ns,respectively.This fact may be attributed to that the rigidity of 2,6-H2NDC ligand is stronger in contrast to 4,4′-H2BPDCligand,thus the much weaker vibrations in Zr-MC-2 effectively reduce the loss of energy by radiationless decay[30].

Fig.8 (a)Emission spectra of 4,4′-H2BPDCand Zr-MC-1,(b)Emission spectra of 2,6-H2NDCand Zr-MC-2
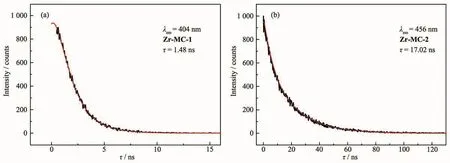
Fig.9 Solid state fluorescence decay curves of Zr-MC-1(a)and Zr-MC-2(b)
3 Conclusions
In summary,two new nanosized Zr-based MCs constructed fromtrinuclear Cp3Zr3(μ3-O)(μ2-OH)3SBUs and linear dicarboxylate ligands have been successfully synthesized under solvothermal conditions.Two crystals were fully characterized by multipleanalysistechniques and their photoluminescent properties indicate that both complexes might be excellent candidates of bluelight-emitting diode devices.The obtained conclusions could inspire further achievements in connection with the exploration of diverse coordination zirconocene molecular capsules with amazing architectures and desired functionalities to be realized.
Acknowledgements:This work was financially supported by the NSFC of China (Grants No.21801038,21471027,21671034),the Science and Technology Research Foundation of the Thirteenth Five Years of Jilin Educational Committee(Grant No.JJKH20190271KJ),the China Postdoctoral Science Foundation funded project(Grants No.2018M630312,2019T120227),and the Fundamental Research Funds for the Central Universities(Grant No.2412018QD003).

