Enzyme-MXene Nanosheets: Fabrication and Application in Electrochemical Detection of H2O2
MA Bao-Kai LI Mian CHEONG Ling-Zhi WENG Xin-Chu SHEN Cai HUANG Qing
Enzyme-MXene Nanosheets: Fabrication and Application in Electrochemical Detection of H2O2
MA Bao-Kai1,2,3, LI Mian3, CHEONG Ling-Zhi2, WENG Xin-Chu1, SHEN Cai3, HUANG Qing3
(1. School of Life and Sciences, Shanghai University, Shanghai 200444, China; 2. Department of Food Science and Engineering, College of Food and Pharmaceutical Sciences, Ningbo University, Ningbo 315211, China; 3. Institute of Materials Technology & Engineering, Chinese Academy of Sciences, Ningbo 315201, China)
Two-dimensional MXene nanosheets with vertical junction structure was employed for easy immobilization of horse radish peroxidase enzymes to fabricate the electrochemical hydrogen peroxide (H2O2) biosensor. The synthesized MXene nanosheets exhibited large specific area, excellent electronic conductivity and good dispersion in aqueous phase. Horse Radish Peroxidase (HRP) enzymes molecules immobilized on MXene/chitosan/GCE electrode demonstrated good electrocatalytic activity toward reduction of H2O2. The fabricated HRP@MXene/chitosan/GCE biosensor exhibited a wide linear range from 5 to 1650 μmol×L-1, a limit of detection of 0.74 μmol×L-1and good operation stability. The fabricated biosensor was successfully employed for detection of trace level of H2O2in both solid and liquid food.
horse radish peroxidase; MXene nanosheets; biosensor; hydrogen peroxide
Hydrogen peroxide (H2O2) is widely used as antimicrobial, oxidizing, reducing and bleaching agents in many fields including pharmaceutical, medical, textile, paper, and food processing[1]. The United States Food and Drug Administration (USFDA) has affirmed the Generally Recognized As Safe (GRAS) status of H2O2for use in food with a maximum permitted concentration in specified foods and residual must be removed by appropriate processing[2]. Excessive amount of H2O2has been reported to have a destructive impact on central nervous system of human body and can result in oxidative stress which is associated with many diseases including neurodegenerative disorders, diabetes, atherosclerosis and cancers[3-4]. Therefore, monitoring H2O2residual in food is of practical significance to both academic and industry. To date, a variety of techniques including fluorometry[5], spectrophotometry[6-7]and electrochemistry[8-9]have been developed for detection and quantification of H2O2.
Electrochemical biosensing technique has generated much interest due to its advantages of simple instrumentation, easy miniaturization, high sensitivity and selectivity, as well as rapid response[10]. At present, very few electrochemical biosensors reached practical application and commercialization mainly due to its inconsistent operational stability[11]. The sensitivity, selectivity and operational stability of electrochemical biosensors are strongly dependent on structure and properties of electrode materials and enzyme immobilization matrixes[1,12-13].
Two-dimensional (2D) transition metal carbides, nitrides and carbonitrides (MXene) are produced by etching layers of sp elements (specifically groups 13 and 14) from their corresponding three-dimensional (3D) MAX phases which correspond to the general formula M+1AX(=1, 2, 3) where M represents early d-block transition metals (Ti, Sc, V, Cr, Ta, Nb, Zr, Mo, Hf), A represents main group sp elements and X is either C or N atom[14-15]. MXenes have generated a lot of interest due to their hydrophilic surfaces, good structural and chemical stabilities, excellent electrical conductivities, and environment- friendly characteristics[16-17]. As MXene surfaces can be used for easy immobilization of enzymes/protein to achieve accelerated reaction kinetics, low detection limits, high sensitivity and selectivity. So it is suitable for use as highly sensitive and selective detection platform for biosensing applications[18-21]. Understanding of the sensitivity, selectivity and long term operational stability of MXene electrochemical biosensors are important for application of MXene biosensors for various purposes.
Present study aims to fabricate a horse radish peroxidase@MXene electrochemical biosensor for detection of H2O2in food. HRP, a heme-containing enzyme, has been widely used to catalyze oxidation of a wide variety of substrates including hydrogen peroxide[22-23]. MXene with vertical junction structure in which MXene sheets are perpendicular to the plane of graphite has been demonstrated to have good electromagnetic absorption properties[24]. We proposed that this vertical junction structure will improve HRP immobilization and demonstrate good electron transfer properties which made it a suitable enzyme immobilization matrix for fabrication of H2O2electrochemical biosensor. We synthesized and characterized MXene using X-ray powder diffraction (XRD), Fourier transform infared spectroscopy (FT-IR) and scanning electron microscopy (SEM). MXene was then used as HRP immobilization matrixes to fabricate HRP@MXene/ chitosan/GCE biosensor. Electrochemical behavior of the fabricated HRP@MXene/chitosan/GCE biosensor was investigated and optimized using cyclic voltammetry (CV) and different pulse voltammetry (DPV). Amperometric method was used to detect concentration of H2O2in real food samples. Selectivity and storage stability of the HRP@MXene/chitosan/GCE were also elucidated.
1 Experimental
1.1 Materials and chemicals
Horseradish peroxide (HRP, activity units×mg–1) was purchased from Sigma Aldrich. Natural flake graphite (48mm), Ti powders (48mm, purity of 99.9%), Al powders (48mm, purity of 99.9%), hydrogen peroxide solution (30wt%), hydroquinone (HQ), chitosan (deacetylation 95%), potassium chloride, acetic acid were obtained from Aladdin, China. Other reagents including NaCl, KCl, sodium hydroxide (NaOH), K3[Fe(CN)6], K4[Fe(CN)6]×3H2O were obtained from Sinoreagent, China. 0.1 mol×L−1phosphate buffer solutions (PBS, pH 7.0) comprising NaH2PO4and Na2HPO4were used as electrolyte. All aqueous solutions were freshly prepared with ultra-pure water (18 MW×cm).
1.2 Synthesis of MXene (Graphite/TiC/Ti3C2)
G(graphite)/TiC/Ti3AlC2were fabricated according to a previously reported method with slight modifications[24]. Graphite powder (48mm), Ti powders (48mm, purity of 99.9%), Al powders (48mm, purity of 99.9%), NaCl and KCl were mixed at a molar ratio of 4 : 4 : 1 : 10 : 10, placed in alumina crucible and packaged in a tube furnace. The tube furnace was heated to 800 ℃at a heating rate of 4 ℃×min−1under argon protection and kept for 300 min. Following that, the mixtures were heated to 1100 ℃ at a heating rate of 4 ℃×min−1, kept for 180 min and finally cooled to room temperature at a cooling rate of 4 ℃×min−1. The resulting product (G/TiC/Ti3AlC2) was washed by deionized water to remove salts and dried at 80 ℃. G/TiC/Ti3C2were obtained by etching process using HF to remove the Al atoms.
1.3 Characterization of MXene
MXene was characterized using XRD, FT-IR and SEM. XRD analysis were conducted at room temperature using Bruker D8 Discover XRD (Cu radiation,=0.l540596 nm) over the 2range of 5°~70° at room temperature. FT-IR spectra was obtained in the range of 500 to 4000 cm–1by using a Fourier-transform infrared (FT-IR) spectroscopy (Nicolet 6700, Thermo, USA).
The microstructures of the powders were examined by a field emission scanning electron microscopy (FEI Quanta FEG 250) equipped with an EDS system and a TEM instrument (FEI Tecnai F20).
1.4 Fabrication of the HRP@MXene/chitosan/ GCE biosensor
Fabrication of the HRP@MXene electrochemical biosensor is illustrated in Fig. 1. Glassy carbon electrodes (GCE, 3 mm) was firstly polished using Al2O3(1.0, 0.3, 0.05 μm), cleaned by ethanol and water for three times, and finally dried under gentle N2stream. Ten microliter of HRP solution [10 mg×mL-1, PBS (0.1 mol×L-1, pH 6.0)] and 20 μL of MXene aqueous solution (5 mg×mL-1) were mixed and shaked at 200 r×min-1for 10 h at low temperature. Following that, 10 μL chitosan solution (6 mg×mL-1, adjusted to pH 6.0 by 10 mg×mL-1NaOH) was added to the mixture and vibrated for 3 min. Chitosan solution has been previously reported to be positively charged and have good electrical conductivity at pH 6.0 due to the protonation of amino groups[8,25]. As MXenes synthesized in present study is negatively charged due to the abundance of hydroxyl or fluoride groups, it could be well adhered in chitosan solutionCoulomb effect and formed a unique film on the surface of GCE[26]. 5 μL of the resultant HRP@MXene/chitosan was dropwisely casted onto the surface of a freshly polished GCE. The prepared electrodes (HRP@MXene/chitosan/GCE) were dried and stored in 0.05 mol×L-1PBS (pH 7.5) in a refrigerator (4 ℃) prior to usage.

Fig. 1 Schematic illustration for fabrication of HRP@MXene (Graphite/TiC/Ti3C2)/chitosan/GCE and H2O2 sensing principle of HRP@MXene/chitosan/GCE
1.5 Electrochemical behavior of the HRP@ MXene/chitosan/GCE biosensor
All electrochemical experiments were carried out using CHI760E electrochemical workstation (Chenhua, Shanghai) with GCE as working electrode, platinum wire as counter electrode and saturated calomel electrode (SCE) as reference electrode. The electrochemical impedance spectroscopy (EIS) and cyclic voltammograms (CVs) of electrodes fabricated using chitosan of different pH was conducted in N2-saturated 0.1 mol×L-1KCl solution containing 5.0 mmol×L-1Fe(CN)63−/4−at open circuit potential in the frequency range from 0.1 Hz to 105Hz with the amplitude 5 mV. The EIS data were analyzed using ZVIEW software.
1.6 Electrochemical biosensing of H2O2 by HRP@MXene/chitosan/GCE biosensor
CVs were carried out in N2-saturated 0.1 mol×L-1PBS (pH 7.5) in the presence of 2.0 mmol×L-1H2O2and 1 mmol×L-1HQ (dissolved in methanol) at a scanning rate of 50 mV×s–1. Differential pulse voltammetry (DPV) was performed in N2-saturated 0.1 mol×L-1PBS (pH 7.5) containing 2 mmol×L-1H2O2and 1 mmol×L-1HQ (dissolved in methanol) with amplitude of 5 mV and pulse width of 0.2 s after five times of CV at a scanning rate of 50 mV×s–1ranging from 0.8 V to –0.8 V. The effects of electrolyte PBS buffer pH (5.5 to 8) and the concentration of MXene were evaluated and optimized in terms of CV and DPV signal.
1.7 Electrochemical detection of H2O2 in spiked dried scallop and milk
Amperometric current-time curves for H2O2were carried out to construct a calibration curve of current response at different H2O2concentration. Measurements were performed in 10 mL of stirring 0.1 mol×L-1PBS (pH 7.5) in the presence of 1 mmol×L-1HQ with successive addition of H2O2at room temperature under an applied peak potential value of –0.1 V. LOD was determined according to the following equation:
LOD = 3SD/K (1)
whereby SD refers to the standard deviation of the control measurement, and K refers to slope of the calibration curve.
Milk and dried scallop were chosen as model of liquid and solid food. Milk sample was used directly for H2O2detection. Dried scallop was pre-treated according to the following procedure to extract H2O2residual. Briefly, 2 g of dried scallop was immersed in 5 mL of H2O2aqueous solution (3%) for 1 h. Following that, the scallop was immersed in 5 mL of water for 0.5 h to extract H2O2residue. H2O2concentration in spiked dried scallop test solution and milk solution (12.5, 50 and 125 μmol×L-1H2O2) were detected using the amperometric current- time curves for H2O2. Recovery of the HRP@MXene/ Chitosan/GCE was calculated.
1.8 Selectivity of the biosensor
Selectivity of the fabricated HRP@MXene/chitosan/ GCE biosensor was evaluated using potentially interfering substances including uric acid, glucose and ascorbic acid [100 μmol×L-1in 0.1 mol×L-1PBS (pH 7.5)].
1.9 Storage stability of the biosensor
Storage stability of the HRP@MXene/GCE was evaluated by monitoring reduction peak in CVs in 0.1 mol×L-1PBS with 1 mmol×L-1HQ and 2 mmol×L-1H2O2during electrodes storage in 0.05 mol×L-1PBS at 4 ℃.
2 Results and discussion
2.1 Characterization of the synthesized MXene and HRP@MXene
XRD patterns of the synthesized MXene (G/TiC/Ti3C2) and G/TiC/Ti3AlC2are showed in Fig. 2(A). G/TiC/Ti3C2demonstrates a dominant phase of graphite (peak at ~26°) and TiC (peaks at 35.9°, 41.8°). This is in agreement with previously reported finding[24]. In addition, after HF etching, the peak at 39° corresponded to the (104) plane of Ti3AlC2disappears compared with the XRD pattern of Ti3AlC2which indicates the elimination of Al during the G/TiC/Ti3C2syntheses process.
As shown in Fig 2(B), FT-IR spectra of MXene do not display any absorption peaks from 3800 to 400 cm−1. Meanwhile, HRP demonstrates characteristic peaks at 2961, 1647, 1541, and 1080 cm−1. The amide I band (1700–1600 cm−1) can be assigned to the-helical conformation of the HRP; meanwhile, the amide II band can be assigned to the-sheet structure of the HRP[3]. Following immobilization of HRP onto the two dimensional MXene nanosheets, the major bands of HRP can be observed on the FT-IR spectra of HRP@MXene indicating successful immobilization process without any conformational change in the secondary structure of HRP.
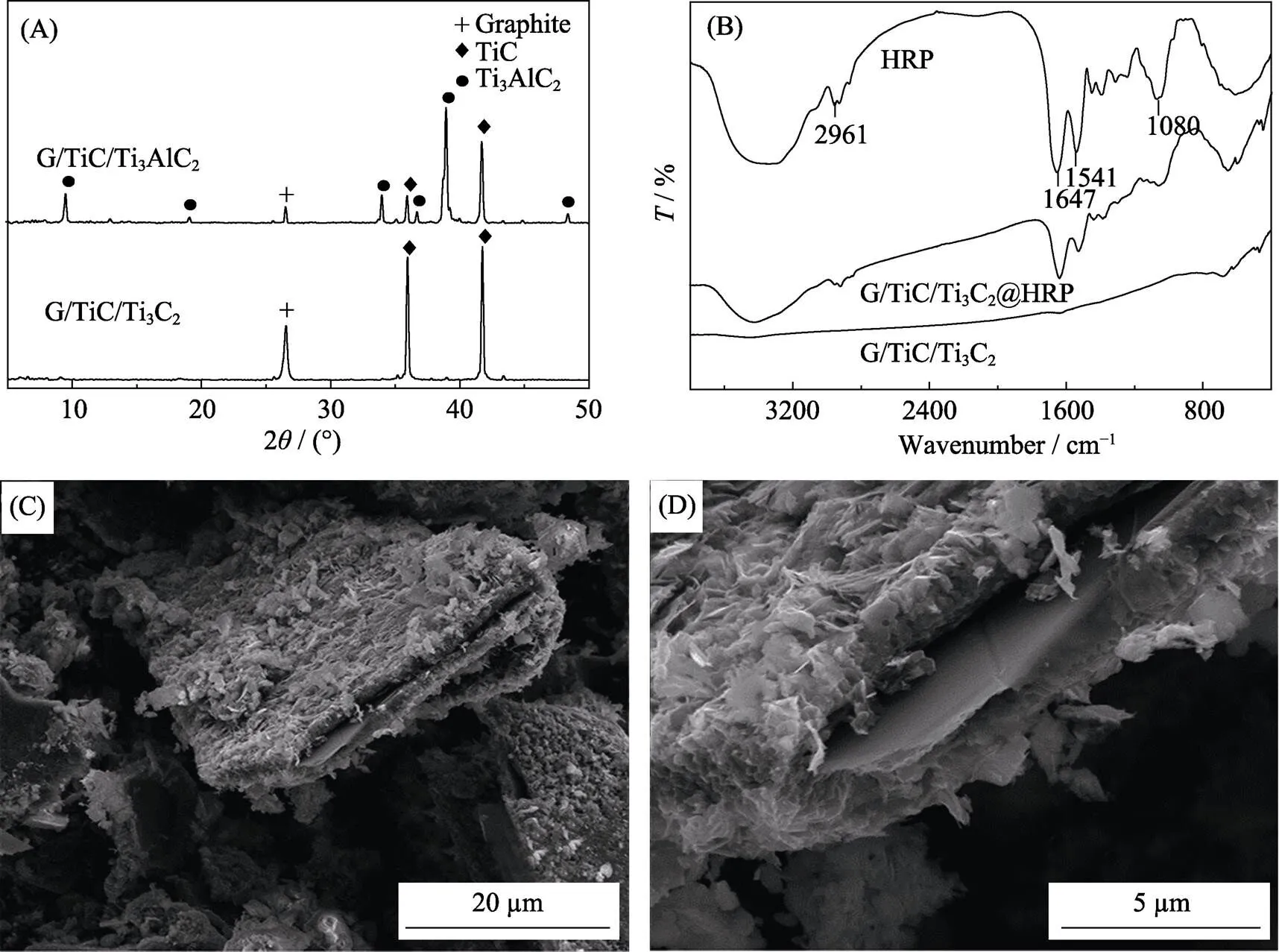
Fig. 2 XRD patterns of G/TiC/Ti3AlC2 and G/TiC/Ti3C2 (A); FT-IR spectra of the MXene, HRP and HRP@MXene (B); SEM images of the MXene G/TiC (C) and Ti3C2 (D)
SEM analysis shows a two dimensional multilayered structured of Ti3C2(<1 μm) standing perpendicular to the plane of G/TiC forming interfacial junctions (Fig. 1(C)). The multilayer Ti3C2also demonstrated typical MXene morphology of two-dimension structure (Fig. 1(D)). This two-dimensional multilayered interfacial junctions structure provides a large specific surface area for efficient enzyme immobilization/entrapment.
2.2 Electrochemical behavior of the fabricated GCE biosensor
Chitosan, a natural film-forming agent, is commonly used in fabrication of enzyme electrodes. It is positively charged at pH<6.3 due to protonation of amino groups[8, 27]. At pH>6.3, chitosan demonstrated decreased solubility in aqueous solution with the decline of adhesion. Fig. S1(A) shows the effects of pH of chitosan solution on charge transfer resistance (ct) of chitosan/GCE electrodes.ctwas found to slightly increase with pH increasing from pH 5.0 to 6.0. However, a dramatic increase inctfrom 0.347 kΩ to 1.304 kΩ can be observed as pH of the chitosan solution increased from 6.0 to 6.5 and reached 4.663 kΩ at pH 7.0.
In addition, according to Fig. S1(B), redox peaks current decreased with increased pH, and peak separation (D) became bigger when pH from 6.0 to 7.0. The increasingctreflected the degressive electrical conductivity of chitosan because of protonation of amino groups, and the increasingDindicated the declined ability of electronic transfer. Considering the film-forming and electrical conductivity of chitosan, in addition, HRP was reported to be most active at nearly neutral[28-29], chitosan solution at pH 6.0 was used in the fabrication of HRP@MXene/ chitosan/GCE biosensor. Fig. 3(A) shows the Nyquist plots of chitosan/GCE, MXene/chitosan/GCE and HRP@MXene/ chitosan/GCE. All three electrodesdemonstrated the electron transfer- limited process in the high frequency area. Chitosan/GCE electrode had anctvalue of 174.40 Ω. Incorporation of MXene onto the chitosan/GCE matrix resulted in a decreasedctvalue of MXene/chitosan/GCE to 52.88 Ω indicating good electron transfer property of MXene from the redox probe of [Fe(CN)6]3−/4−. Nevertheless, immobilization of HRP onto the MXene/chitosan/GCE matrix increase of thectvalue of HRP@MXene/chitosan/ GCE to 542.60 Ω. Increasement of thectvalue is mainly caused by steric hindrance, electrostatic interactions and partial blockage of interfacial electrons by enzyme molecules which has poor conductivity[10]. Cyclic voltammetry(CV) for the different electrodes were carried out in 5.0 mmol×L-1Fe[(CN)6]3-/4-and 0.1 mol×L-1KCl (Fig. 3(B)).
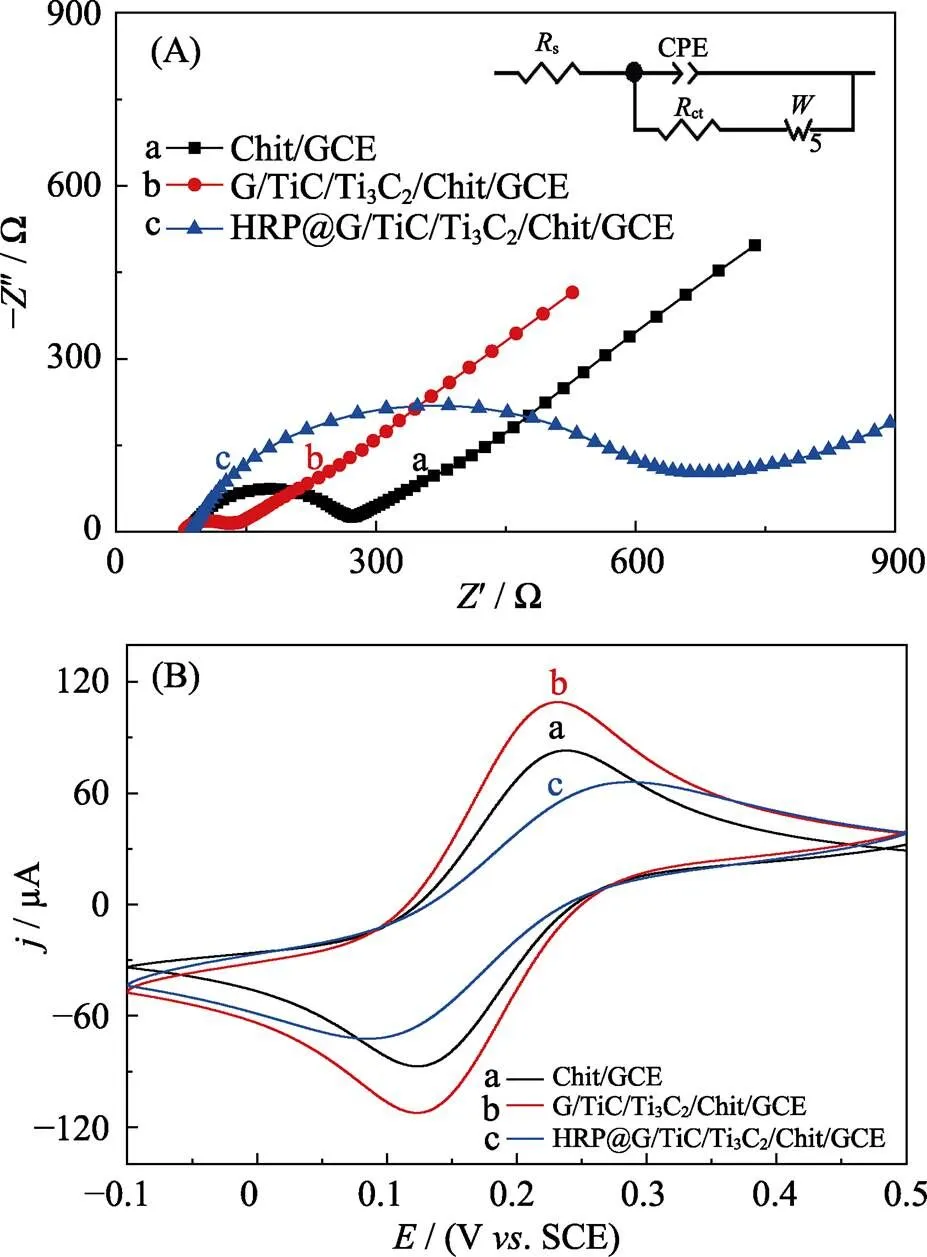
Fig. 3 EIS of Chit(chitosan)/GCE(a), MXene/Chit/GCE(b), HRP@MXene/Chit/GCE (c) electrodes cycled in 0.1 mol×L-1 KCL aqueous solution containing 5 mmol×L-1 [Fe(CN)6]3−/4− (A); CV curves of Chit/GCE (a), MXene/Chit/GCE (b), HRP@MXene/Chit/GCE (c) electrodes cycled in 0.1 mol×L-1 KCL aqueous solution containing 5 mmol×L-1 [Fe(CN)6]3−/4−: (potential window: –0.1–0.5 V vs. SCE) (B)
In comparison with Chitosan/GCE (curve a), MXene/ chitosan/GCE (curve b) demonstrated an increase in current response and similar Δvalue (differences between anodic and cathodic peaks potential) indicating MXene is an excellent electric conducting material. Meanwhile, HRP@MXene/chitosan/GCE (curve c) demonstrated a decrease in current response and an increase in Δvalue indicating HRP hindered the electron conductivity.
2.3 Electrochemical biosensing of H2O2 by the biosensor
Fig. 4 shows the CV of chitosan/GCE, MXene/chitosan/GCE, HRP@chitosan/GCE, and HRP@MXene/chitosan/ GCE electrodes obtained in 0.1 mol×L-1N2-saturated PBS (pH 7.5) containing 1 mmol×L-1HQ and 2 mmol×L-1H2O2. Chitosan/GCE electrode demonstrated a pair of well-defined redox peaks with potentials at about 0.14 and −0.07 V which is characteristic of redox process of HQ and H2O2[30]. In comparison with the signal obtained from chitosan/GCE, modification of the GCE with MXene/chitosan resulted in signal enhanced of the redox peaks. Following HRP immobilization, both HRP@chitosan/ GCE and HRP@MXene/chitosan/GCE demonstrated further enhanced reduction peak with HRP@MXene/chitosan/ GCE showing highest increase in reduction peak’s current(52 μA). Increase in the reduction peak current can be attributed to reduction process of H2O2catalyzed by HRP at its reducing state (HRPRED) (Fig. 1). During this reduction process, the redox centre of HRPREDturned into its oxidizing state (HRPOX). HRPOXwere then regenerated into HRPREDwith the aid of HQ which was oxidized to form benzoquinone. Finally, benzoquinone exchanged electrons with the electrode to electrochemically produced HQ. The redox processes of H2O2and hydroquinone were in agreement with those previously reported findings[30]. The aforementioned findings showed HRP@MXene/chitosan/GCE biosensor can be used for electrochemical biosensing of H2O2and MXene provided a favorable microenvironment to retain the bioactivity of HRP.
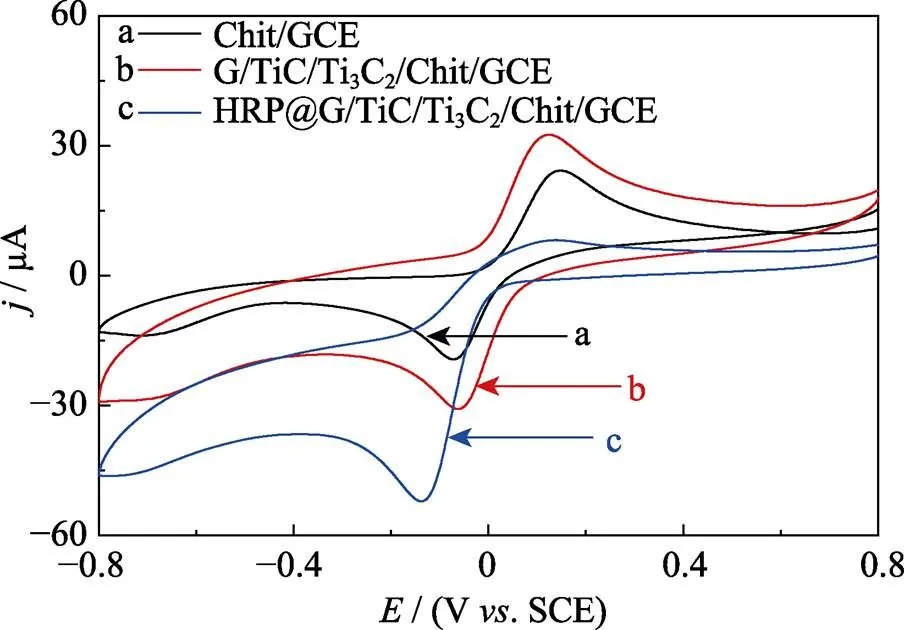
Fig. 4 CV curves of Chit/GCE (curve a, black line), MXene/ Chit/GCE (curve b, red line), HRP/Chit/GCE (curve c, pink line), HRP@MXene/Chit/GCE (curve d, blue line) electrodes cycled in N2–saturated 0.1 mol×L-1 PBS (pH 7.5) containing 1.0 mmol×L-1 HQ and 2.0 mmol×L-1 H2O2 at a scanning rate of 50 mV×s–1(potential window: –0.8–0.8 V vs. SCE).
Fig.S2(A) shows the CV of HRP@MXene/chitosan/GCE obtained in 0.1 mol×L-1N2-saturated PBS (pH 7.5) containing 1 mmol×L-1HQ and 2 mmol×L-1H2O2at various scan rates. The redox peaks of HRP@MXene/chitosan/GCE increased linearlythe square root of scanning rates from 20 to 500 mV×s−1(Fig. S2(B)). The electrochemical behaviors were in accordance with a diffusion-controlled process occurring at the surface of the biosensor[31]. Similar results for different electrodes with mediator were also reported[28, 32].
Based on aforementioned findings, PBS buffer’s pH of 7.5 and MXene concentration of 5 mg×mL-1were used for fabrication of HRP@MXene/chitosan/GCE in the subsequent analysis.
Electrochemical biosensing of H2O2by HRP@MXene/ chitosan/GCE was optimized in terms of electrolyte PBS buffer’s pH (pH 5.5–8.0) and concentration of MXene (0.5–10 mg×mL-1). The pH value of the electrolyte is important for the performance of enzyme electrode as HRP activity is greatly affected by pH. Fig. S3(A) shows that the peak current of HRP@MXene/chitosan/GCE increased from pH 5.5 and reached maximum at pH 7.5. The value of pH was chosen for further study and was also in agreement with previous observations reported[33]. Fig. S3(B) shows the peak currents of cyclic voltammograms of HRP@MXene/chitosan/GCE fabricated with different concentration of MXene. Peak current of HRP@MXene/chitosan/GCE was the highest at 5 mg×mL-1MXene (MXene: HRP ratio IS 1 : 1). At this concentration of MXene, HRP was fully immobilized on the surface of MXene and the biosensor demonstrated most effective performance. In terms of DPV responses (Fig. S3(C)), negative shifts in peak potentials can be observed with increased pH value. This indcated that H+participated in the HRP catalyzed H2O2reduction reaction to produce water. Peak potential was also affected by concentration of MXene with negative shift in peak potential and highest peak current can be observed at 5 mg×mL-1MXene (Fig. S3(D)).
2.4 Electrochemical detection of H2O2 in spiked dried scallop and milk
The current-time curve which is a potential-controlled electrochemical analysis method was used to build a calibration curve of amperometric response at a series of H2O2concentration. Fig. 5(A) shows the amperometric response of HRP@MXene/chitosan/GCE following successive additions of H2O2to PBS buffer (Potential = –0.1 V). The corresponding calibration curves of HRP@MXene/ chitosan/GCE biosensor were presented in Fig. 5(B), which was linear at two concentration ranges (5-190 and 190-1650 μmol×L-1H2O2) with a linear regression equation of=0.02644+0.55914(2=0.999) and=0.01959+1.84114 (2=0.996). Moreover, the fabricated biosensor also showed very low detection limit of 0.74 μmol×L-1. A comparison of linear range and detection limit for H2O2with other H2O2sensors reported in literature are summarized in Table S1. The data demonstrated that both the linear range and detection limit for H2O2are comparable or even better than those detected using sensors recently reported. The excellent biosensing performance of HRP@MXene/chitosan/GCE can be ascribed to the unique vertical junction structure of the two dimensional MXene nanosheets which provided a suitable matrix for HRP immobilization and also platform for H2O2and HQ redox reactions.
Present work used dried scallop and milk as representative of solid and liquid food system to explore the application of HRP@MXene/chitosan/GCE biosensor in detection of H2O2in food samples. Fig. 5(C, D) shows the amperometric response of HRP@MXene/chitosan/GCE following additions of solutions extracted from milk and dried scallop with different concentration of H2O2. The curves show HRP@MXene/chitosan/GCE is a rapid and sensitive method to detect H2O2at different concentraitons. The recovery of H2O2in food samples at different concentrations ranged from (90.24±6.97)% to (109.20±3.33)% (Table 1). The results indicated that the fabricated biosensor is a reliable tool for detection of residual H2O2in food samples.

Fig. 5 Amperometric responses of HRP@MXene/Chit/ GCE at –0.1 V upon successive additions of H2O2 in a stirred 0.1 mol×L-1 PBS (pH 7.5) (A); Calibration curve of amperometric responses at different H2O2 concentrations (B); Amperometric responses of HRP@MXene/Chit/ GCE at –0.1 V upon successive additions of solutions extracted from milk sample (C) and dried scallop (D) spiked with different H2O2 under stirred 0.1 mol×L-1 PBS (pH 7.5)

Table 1 Detection of hydrogen peroxide in real food sample
2.5 Selectivity and stability of the HRP@ MXene/chitosan/GCE
The anti-interference performance of HRP@MXene/ chitosan/GCE biosensor was evaluated by detecting 100 μmol×L-1H2O2in the presence of the same concentration of ascorbic acid, glucose and uric acid as interfering substances. As shown in Fig. S4(A), there were no noticeable amperometric responses from glucose and uric acid. However, amperometric responses can be detected by ascorbic acid (34% H2O2) indicating ascorbic acid has the capability to participate in the redox process of HQ and H2O2; hence, interfering with the measurement of H2O2.
HRP@MXene/chitosan/GCE demonstrated good storage and operational stability. When stored in 0.05 mol×L-1PBS (pH 7.5) at 4 ℃, HRP@MXene/chitosan/GCE was able to retain 84.8% of its initial response to H2O2after a period of 10 d (Fig. S4(B)).This indicated that the vertical junction structure of the MXene (Graphite/ TiC/Ti3C2) were able to act as an effective and stable platform for entrapment enzyme HRP.
3 Conclusion
In summary, we have explored a new type of supporting material for immobilizing HRP and fabricated an electrochemical H2O2biosensor fordetection of H2O2in food products. The synthesized MXene exhibited large specific area, biocompatibility, excellent electronic conductivity, and good dispersion in aqueous phase. HRP enzymes molecules immobilized on MXene/chitosan/GCE electrode showed good electrochemical behaviors and electrocatalytic activity toward reduction of H2O2. The fabricated HRP@MXene/chitosan/GCE biosensor exhibited a wide linear range from 5 μmol×L-1to 1.650 mmol×L-1and a low detection limit of 0.74 μmol×L-1with long-term stability, good reproducibility and high selectivity. The fabricated biosensor has also been successfully employed for detection of trace level of H2O2in real food products (both solid and liquid food). The study provides a good concept for construction of electrochemical H2O2biosensor based on MXene.
Supporting materials
Supporting materials related to this article can be found at https://doi.org/10.15541/jim20190139
[1] ZHANG R, CHEN W. Recent advances in graphene-based nanomaterials for fabricating electrochemical hydrogen peroxide sensors., 2017, 89(Pt1): 249–268.
[2] ADMINISTRATION F D. Code of Federal Regulations, 21CFR184.1366 2018.
[3] DAI H, LU W, ZUO X,. A novel biosensor based on boronic acid functionalized metal-organic frameworks for the determination of hydrogen peroxide released from living cells., 2017, 95: 131–137.
[4] WANG Y, ZHAO K J, ZHANG Z Q,. Simple approach to fabricate a highly sensitive H2O2biosensor by one-step of graphene oxide and horseradish peroxidase co-immobilized glassy carbon electrode.e, 2018, 13(3): 2921–2933.
[5] CHANG M C Y, PRALLE A, ISACOFF E Y,. A selective, cell-permeable optical probe for hydrogen peroxide in living cells., 2004, 126(47): 15392–15393.
[6] SHARMA M, KOTHARI C, SHERIKAR O,. Concurrent estimation of amlodipine besylate, hydrochlorothiazide and valsartan by RP-HPLC, HPTLC and UV–Spectrophotometry., 2014, 52(1): 27–35.
[7] MATSUBARA C, KAWAMOTO N, TAKAMURA K. Oxo[5, 10, 15, 20-tetra(4-pyridyl)porphyrinato]titanium(IV): an ultra-high sensitivity spectrophotometric reagent for hydrogen peroxide., 1992, 117(11): 1781–1784.
[8] ZHOU K, ZHU Y, YANG X,. A novel hydrogen peroxide biosensor based on Au–graphene–HRP–chitosan biocomposites., 2010, 55(9): 3055–3060.
[9] THENMOZHI K, NARAYANAN S S. Horseradish peroxidase and toluidine blue covalently immobilized leak-free Sol-Gel composite biosensor for hydrogen peroxide,, 2017, 70(Pt1): 223–230.
[10] MA B K, CHEONG L Z, WENG X C,. Lipase@ZIF-8 nanoparticles-based biosensor for direct and sensitive detection of methyl parathion., 2018, 283: 509–516.
[11] JOS´E I, REYES-DE-CORCUERA H E O, GARC´ıA-TORRES A R. Stability and Stabilization of Enzyme Biosensors: The Key to Successful Application and Commercialization. 2018.
[12] LIU Y, LIU X, GUO Z,. Horseradish peroxidase supported on porous graphene as a novel sensing platform for detection of hydrogen peroxide in living cells sensitively., 2017, 87: 101–107.
[13] ZHENG J, DIAO J, JIN Y,. An inkjet printed Ti3C2-GO electrode for the electrochemical sensing of hydrogen peroxide., 2018, 165(5): B227– B231.
[14] ZHAO M Q, XIE X, REN C E,. Hollow mxene spheres and 3D macroporous mxene frameworks for Na-ion storage.s, 2017, 29(37): 1702410.
[15] ZHOU J, ZHA X, ZHOU X,. Synthesis and electrochemical properties of two-dimensional hafnium carbide., 2017, 11(4): 3841–3850.
[16] XU B, ZHU M, ZHANG W,. Ultrathin MXene-micropattern- based field-effect transistor for probing neural activity., 2016, 28(17): 3333–3339.
[17] LORENCOVA L, BERTOK T, DOSEKOVA E,. Electrochemical performance of Ti3C2TMXene in aqueous media: towards ultrasensitive H2O2sensing., 2017, 235: 471–479.
[18] LORENCOVA L, BERTOK T, FILIP J,. Highly stable Ti3C2T(MXene)/Pt nanoparticles-modified glassy carbon electrode for H2O2and small molecules sensing applications., 2018, 263: 360–368.
[19] WANG F, YANG C, DUAN M,. TiO2nanoparticle modified organ-like Ti3C2MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances., 2015, 74: 1022–1028.
[20] LIU H, DUAN C, YANG C,. A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2., 2015, 218: 60–66.
[21] RAKHI R B, NAYAK P, XIA C,. Novel amperometric glucose biosensor based on MXene nanocomposite., 2016, 6: 36422.
[22] VEITCH N C. Horseradish peroxidase: a modern view of a classic enzyme., 2004, 65(3): 249–259.
[23] REN Q Q, WU J, ZHANG W C,. Real-timedetection of cellular H2O2under camptothecin stress using horseradish peroxidase, ionic liquid, and carbon nanotube-modified carbon fiber ultramicroelectrode. S, 2017, 245: 615–621.
[24] LI M, HAN M, ZHOU J,. Novel scale-like structures of graphite/TiC/Ti3/C2hybrids for electromagnetic absorption., 2018, 4(5): 1700617.
[25] SHAN C, YANG H, HAN D,. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing., 2010, 25(5): 1070–1074.
[26] WANG F, YANG C, DUAN C,. An organ-like titanium carbide material (MXene) with multilayer structure encapsulating hemoglobin for a mediator-free biosensor., 2014, 162(1): B16–B21.
[27] KANG X B, PANG G C, LIANG X Y,. Study on a hydrogen peroxide biosensor based on horseradish peroxidase/GNPs-thionine/ chitosan., 2012, 62: 327–334.
[28] KOPOSOVA E, LIU X, KISNER A,. Bioelectrochemical systems with oleylamine-stabilized gold nanostructures and horseradish peroxidase for hydrogen peroxide sensor., 2014, 57: 54–58.
[29] YANG S, DING S, LI L,. One-step preparation of direct electrochemistry HRP biosensorelectrodeposition., 2017, 164(13): B710–B714.
[30] CHEN W, YANG W, LU Y,. Encapsulation of enzyme into mesoporous cages of metal–organic frameworks for the development of highly stable electrochemical biosensors., 2017, 9(21): 3213–3220.
[31] BARD A J, FAULKNER L R, LEDDY J,. Electrochemical methods: Fundamentals and Applications. Wiley New York, 1980.
[32] SONG H, NI Y, KOKOT S. Investigations of an electrochemical platform based on the layered MoS2-graphene and horseradish peroxidase nanocomposite for direct electrochemistry and electrocatalysis., 2014, 56: 137–143.
[33] MART N M, SALAZAR P, VILLALONGA R,. Preparation of core–shell Fe3O4@poly(dopamine) magnetic nanoparticles for biosensor construction., 2014, 2(6): 739–746.
酶-二维MXene复合材料的制备及其电化学检测H2O2的应用
马保凯1,2,3, 李勉3, 张绫芷2, 翁新楚1, 沈彩3, 黄庆3
(1. 上海大学 生命科学学院, 上海 200444; 2. 宁波大学 食品与药学学院, 宁波 315211; 3. 中国科学院 宁波材料技术与工程研究所, 宁波 315201)
本研究合成了具有垂直栅栏结构的二维MXene材料, 与辣根过氧化物酶进行固定, 构筑了过氧化氢电化学酶传感器。合成的MXene纳米栅栏具有大的比表面积, 优良的电子传导特性和在水溶液中的良好分散特性; 固定化在酶电极上的辣根过氧化物酶分子表现出了优良的过氧化氢催化效果。结果表明HRP@MXene/chitosan/GCE酶电化学传感器在过氧化氢浓度为5~1650 μmol/L范围内表现出很好的线性关系, 最低检测限为0.74 μmol/L, 且具有很好的操作稳定性, 该生物传感器被成功地应用于固态与液态食品中过氧化氢残留检测。
辣根过氧化物酶; MXene纳米片; 生物传感器; 过氧化氢
TS207
A
1000-324X(2020)01-0131-08
10.15541/jim20190139
2019-03-28;
2019-04-16
Ningbo University(421401560); National Natural Science Foundation of China (21706137, 21671195)
MA Bao-Kai (1992-), male, Master candidate. E-mail: mabaokai@nimte.ac.cn
马保凯(1992-), 男, 硕士研究生. E-mail: mabaokai@nimte.ac.cn
CHEONG Ling-Zhi, associate professor. E-mail: lingzhicheong@yahoo.com
张绫芷, 副教授. E-mail: lingzhicheong@yahoo.com

